Clinical History
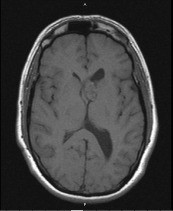
A 40‐year‐old male with a one year clinical history of vertigo and intermittent headaches was admitted for evaluation at a tertiary university hospital. Magnetic Resonance Image (MRI) showed a heterogenously enhancing mass with cystic and calcific components in the anterior horn of the left lateral ventricle measuring 2.5 × 2.2 × 1.6 cm (Figure 1). The patient underwent partial resection of the intraventricular tumor through a left frontal craniotomy with transfrontal approach to the left ventricle. The patient was discharged four days later and has done well since that time with no neurological deficits.
Figure 1.
Pathological Findings
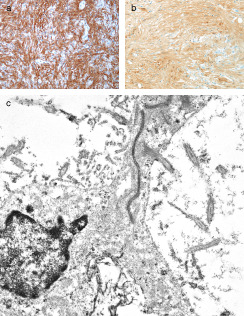
The H&E sections showed a neoplasm which in most areas was composed of elongated spindle cells arranged in a fascicular pattern (Figure 2). Some areas were more cellular and showed mild nuclear pleomorphism, while others were hypocellular and composed mainly of fibrillary cell processes. Some of the hypocellular areas showed clustered nuclei surrounded by a fibrillary stroma and merged into foci with a microcystic appearance (Figure 3). In addition there were rare collections of large pleomorphic glial cells with a ganglionic appearance (see image in web case). The Ki‐67 immunostain showed a low proliferative index. The tumor cells were strongly immunoreactive for glial fibrillary acidic protein (GFAP) (Figure 4a) and S‐100 protein (Figure 4b), but showed no immunoreactivity for epithelial membrane antigen (EMA). The synaptophysin immunostain showed focal entrapment of pre‐existing axons, but there was not too much diffuse infiltration of pre‐existing parenchyma. Electron microscopy was helpful (Figure 4c). What is the diagnosis?
Figure 2.
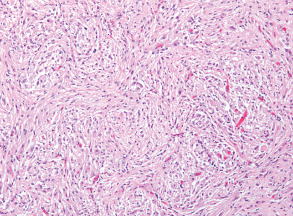
Figure 3.
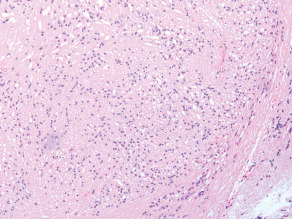
Figure 4.
Diagnosis
Combined tanycytic ependymoma and subependymoma, (WHO grade II).
Discussion
Tanycytic ependymoma (TE) (WHO grade II) is a rare subtype of ependymoma arising from tanycytes and is composed of clusters of elongated cells forming nuclear dense zones and streaming cell processes forming fibrillary zones 7. Subependymomas (WHO grade I) are slow growing, benign neoplasms typically attached to a ventricular wall composed of clusters of glial tumor cells embedded in an abundant fibrillary matrix with frequent microcystic change 3.
A tumor showing features of both TE and subependymoma raises an interesting question regarding its histogenesis. The following hypotheses have been proposed: (A) these two components can reflect a “collision” phenomenon between two separate neoplastic clones, (B) both elements can be derived from a single totipotent stem cell of a common progenitor “combination phenomenon”, (C) or one component can reflect metaplasia or dedifferentiation from another “conversion phenomenon”.
The origin of ependymoma is a matter of debate. There seems to be an agreement that the ontogeny of ependymoma is radial glia, which is the stem cell of tanycyte and glia. Tanycytes are interspersed among the columnar ependymal cells. Studies suggested that tumors with histomorphologic and ultrastructural characteristics similar to tanycyte also include pilocytic astrocytoma, myxopapillary ependymoma, astroblastoma, and subependymoma 7. Interestingly the precursor cells of subependymomas have not been conclusively identified although some candidates have been proposed: subependymal glia, astrocytes of the subependymal plate, ependymal cells and a mixture of astrocytes and ependymal cells 4.
Subependymomas represent only 0.2 to 0.7% of intracranial tumors and are often clinically silent 3. In 1978, Scheithauer reviewed a total of 95 cases from the literature, the largest study to date, including 21 symptomatic cases from his personal files 6. In a clinicopathological evaluation of 83 cases 5, 43 tumors arose in the posterior fossa, 37 in lateral ventricles, 2 in spinal cord and one in the temporal horn. 18% (15/83) of subependymomas exhibited a mixed histologic pattern: that is, subependymoma together with another glial tumor. The most common mixture (13/83) was subependymoma and ependymoma and the remaining two cases were subependymoma mixed with astrocytoma.
TE generally do not show typical ependymal rosettes and these have been defined as “pure type” 2. Another type was defined as “mixed type” and this contained both true rosettes and perivascular pseudorosettes. In Friede and Pollak's initial report of 11 tumors 6 of them were likely mixed type with the remaining 5 being pure type 1.
TE must be differentiated from other tumors which are composed of elongated piloid cells mainly pilocytic astrocytomas, diffuse astrocytomas and schwannomas. Electron microscopy helps in this differential diagnosis with neoplastic tanycytes showing microvilli, cilia, cell junctions, intracytoplasmic filaments and absence of basal lamina. Pilocytic astrocytomas, diffuse astrocytomas and schwannomas are devoid of microvilli and cilia, whereas in schwannomas tumor cells are surrounded by basal lamina.
Thus, the present case is an unusual example of TE. The occurrence of SEGA‐like pleomorphic cells was an additional unusual feature.
Abstract
Combined tumors showing histologic features of both ependymoma and subependymoma have been described. In this report we present a case of combined tanycytic ependymoma with foci of subependymoma (WHO grade II), occurring in a 40 year‐old male, which arose in the wall of the lateral ventricle. The tanycytic ependymoma component showed elongated fibrillary cells with a fascicular pattern of growth, while the subependymoma component showed clustered cell bodies surrounded by a fibrillary stroma with a microcystic appearance. We consider the present case to be an unusual example of tanycytic ependymoma; which to the best of our knowledge has not been associated with a subependymoma.
References
- 1. Friede RL, Pollak A (1978) The cytogenetic basis for classifying ependymomas. J Neuropathol Exp Neurol 37(2):103–118. [DOI] [PubMed] [Google Scholar]
- 2. Kawano N, Yagishita S, Oka H, Utsuki S, Kobayashi I, Suzuki S, Tachibana S, Fujii K (2001) Spinal tanycytic ependymomas. Acta Neuropathol 101(1):43–48. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO Classification of Tumours of the Central Nervous System. Springer‐Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maekawa M, Fujisawa H, Iwayama Y, Tamase A, Toyota T, Osumi N, Yoshikawa T (2010) Giant subependymoma developed in a patient with aniridia: analyses of PAX6 and tumor‐relevant genes. Brain Pathol 20(6):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rushing EJ, Cooper PB, Quezado M, Begnami M, Crespo A, Smirniotopoulos JG, Ecklund J, Olsen C, Santi M (2007) Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol 85(3):297–305. [DOI] [PubMed] [Google Scholar]
- 6. Scheithauer BW (1978) Symptomatic subependymoma. Report of 21 cases with review of the literature. J Neurosurg 49(5):689–696. [DOI] [PubMed] [Google Scholar]
- 7. Zhang S, Wang X, Zhang Z, Chen Y (2008) Tanycytic ependymoma arising from the right lateral ventricle: a case report and review of the literature. Neuropathology 28(4):427–432. [DOI] [PubMed] [Google Scholar]


