Abstract
Medulloblastoma (MB) is a clinically and biologically heterogeneous group of tumors, and currently classified into four molecular subgroups (Wnt, Shh, Group 3 and Group 4). Intracellular signaling of the Wnt pathway has been divided into two classes: the “canonical” and the “non‐canonical” signaling pathway. The canonical signaling pathway is a well‐established, β‐catenin‐dependent signaling pathway in MB. In contrast, very little research about the non‐canonical WNT signaling pathway in MB exists. In order to identify the roles of Wnt‐5a and Ror2, two non‐canonical WNT pathway‐related genes, we studied 76 cases of MB with immunohistochemistry and quantitative real‐time PCR and correlated the results with clinicopathological and other molecular parameters and prognosis. Wnt5a and Ror2 were immunopositive in 20 (29.4%) and 35 (51.5%) of 68 cases, respectively. There were positive associations among protein expressions of Wnt5a, Ror2 and β‐catenin. Ror2 mRNA levels were well correlated with immunoexpression. Ror2 mRNA expression was significantly associated with CTNNB1 mutation. High Ror2 mRNA expression was an independent favorable prognostic factor. In conclusion, our study demonstrates the first attempt to identify Wnt5a and Ror2 as additional mechanisms contributing to dysregulation of the non‐canonical WNT signaling pathway in MB. Ror2 may play a role as an oncosuppressor in MB.
Keywords: medulloblastoma, prognostic factor, Ror2, Wnt 5a, WNT pathway
Introduction
Medulloblastoma (MB) is the most common malignant embryonal neuroepithelial tumor of the central nervous system (CNS) occurring in childhood. This disease entity is a clinically and biologically heterogeneous group of tumors composed of distinct molecular subgroups that have different pathways of tumorigenesis. The identification of signaling pathways that play a critical role in tumor pathogenesis provides an opportunity to understand the biological causes of various tumors and a valuable chance for diagnostic or therapeutic implementation 21.
MBs are currently classified into four molecular subgroups (Wnt, Shh, Group 3 and Group 4). The Wnt and Shh (sonic hedgehog) are the signaling pathways thought to play roles in the pathogenesis of these subgroups. The four groups of MB showed clearly distinct demographics, histology, DNA copy number aberrations, and clinical outcome on an international meta‐analysis 12. Nearly all of the Wnt MBs have classic histology, frequent CTNNB1 mutation, nuclear staining for β‐catenin, monosomy 6 and excellent prognosis. Shh subgroup of MBs can have all three histologic types: PTCH1/SMO/SUFU mutation, immunostaining for GAB1, and intermediate prognosis. Although less is known about the biology of groups 3 and 4, MYC (but not MYCN amplification) appears to be almost always limited to group 3 tumors, and group 3 tumors have a high incidence of large cell/anaplastic (LC/A) histology, very frequent metastasis and poor prognosis. Group 4 tumors have classic or LC/A histology, isochromosome 17q and intermediate prognosis 12.
Wnt proteins are a large family of cysteine‐rich secreted molecules that function through activation of distinct intracellular signaling pathways in development 16. Intracellular signaling of the Wnt pathway has been divided into two classes: those that signal through the “canonical” pathway and those that signal through the “non‐canonical” pathway 19. The canonical signaling pathway is a well‐established intracellular signaling pathway that is a β‐catenin‐dependent signaling pathway 16. Activation of canonical Wnt signaling downregulates degradation of intracellular β‐catenin, allowing nuclear translocation and accumulation of β‐catenin where it binds T‐cell factor (TCF)/lymphoid enhancer factor (LEF). Activation of this pathway also positively regulates transcription of various target genes including MYCC, Cyclin D1, matrix metalloproteinase‐7 (MMP‐7), gastrin and immunoglobulin transcription factor‐2 (ITF‐2) 8, 26.
Approximately two‐thirds of MB with the Wnt pathway harbors mutations of CTNNB1, a gene encoding β‐catenin. Mutations in other pathway elements, such as APC and AXIN1, have been recorded in the absence of CTNNB1 mutation, but these are much less frequent.
In MB, an additional mechanism with excessive signaling in the non‐canonical WNT pathway through secreted frizzled‐related protein (SFRP) gene silencing was recently suggested 11. Non‐canonical Wnt signaling is defined as Wnt‐ or Fz‐initiated signaling that is independent of β‐catenin transcriptional function. Non‐canonical Wnt pathways are diverse and less well characterized than the canonical Wnt signaling pathway. Wnt‐5a is one of the most highly investigated non‐canonical Wnts and has been shown to be involved in almost all aspects of non‐canonical Wnt signaling 8. The role of Wnt‐5a in cancer has shown opposing results in that it may have a tumor suppressive or an oncogenic effect depending on the cancer type 8. The receptor tyrosine kinase‐like orphan receptor 2 (Ror2) is part of a family of orphan RTKs 1, 25. Ror2 is characterized by an intracellular tyrosine kinase domain 18 and an extracellular frizzled‐like cysteine‐rich domain. It has been shown to act as a Wnt‐binding domain 23 and to mediate non‐canonical Wnt signaling 9, 24, 27. This large protein family is involved in regulating diverse cellular processes such as the cell cycle, cell migration, proliferation and differentiation 5. Similar to Wnt5a, the role of Ror2 appears to be different depending on the various cancer types. Recently, Wnt5a/Ror2 has been shown to activate the planar cell polarity (PCP) pathway and to inhibit the β‐catenin/TCF pathway 20, 28, 31.
Still, very little research about the non‐canonical WNT signaling pathway has been performed on MB. In this study, we focused our analyses on the non‐canonical WNT signaling pathway, namely, the β‐catenin‐independent pathway in MB. Moreover, to our knowledge, the role of Wnt5a/Ror2 in the non‐canonical pathway in MB has not yet been investigated. In order to identify its role, we performed an immunohistochemical and qRT‐PCR study for the expression of Wnt‐5a and Ror2 using tumor samples from 76 patients with MB. We correlated our findings with clinicopathological and other molecular parameters and prognosis.
Materials and Methods
Patient selection and data collection
This study included 76 patients who were histologically diagnosed as having MB and underwent surgery at the Samsung Medical Center from 1995 to 2011. We retrospectively reviewed hematoxylin‐and‐eosin (H&E) slides, clinical charts, and pathologic reports, and the diagnosis of MB was established independently by two pathologists (SEL and YLS).
Tissue microarrays (TMAs)
All 76 H&E‐stained slides were reviewed, and representative tumor tissue samples were selected from each case. The corresponding formalin‐fixed, paraffin‐embedded (FFPE) tissue blocks were retrieved. Selected areas were circled on the slide with a marker pen for TMA construction. Two 3.0‐mm tissue cores were taken from the representative region of each paraffin block. Of the 76 cases, only 68 cases were available for TMA cores because of specimens with insufficient tissue in the block.
Histology and immunochemistry
A total of 76 MBs were classified histopathologically into three types, namely, classic, desmoplastic/nodular (D/N), and LC/A, using the 2007 World Health Organization (WHO) CNS tumor classification. The D/N of MB, including the paucinodular D/N variant, was characterized by the histological presence of nodules, which are well‐circumscribed, pale regions of tumor with fibrillar cytoplasm and a network of internodular collagen fibers. Although the size and shape of nodules were variable, the presence of nodules was designated as this subtype. In addition, internodular desmoplasia, the background around nodules, was required for diagnosis. Reticulin preparations were used to confirm internodular desmoplasia. The Ki‐67 labeling index of intranodular and internodular cells was assessed and found to show a much higher index in internodular regions. All things considered, 15 cases were classified into the D/N subtype. The anaplastic MB shows marked cytological pleomorphism in association with high mitotic and apoptotic counts 3, 17. The large cell MB is defined by groups of uniform, large round cells with a single nucleolus, which are mixed with groups of anaplastic cells in most cases 7. Large cell and anaplastic tumors were combined in study datasets as LC/A tumors. Although all MBs showed some degree of atypia, the LC/A type was designated when more than 50% of the area was anaplastic. In the anaplastic subtype, the degree of anaplasia was graded as moderate or severe, and the extent of anaplasia was assessed as either focal or diffuse. Four tumors had moderate anaplasia (29%), and 10 tumors had severe anaplasia (71%). Anaplasia (all grades) was focal in 3 patients (21%) and diffuse in 11 patients (79%). The range and mean number of mitoses and apoptosis per 5 high power fields were 3–44 (mean, 18) in mitosis and 1–42 (mean, 12) in apoptosis.
Immunohistochemistry was performed on the TMA (GAB1, Wnt5a and Ror2) and representative paraffin‐embedded tumor tissue (β‐catenin) sections using the avidin–biotin complex method. The primary antibodies included β‐catenin (mouse monoclonal, dilution 1:200, Novocastra, Newcastle, UK) and GAB1 (rabbit monoclonal, dilution 1:100, epitomics). For Ror2 immunohistochemistry, sections of TMAs with MBs were cut to a thickness of 4 μm, deparaffinized in xylene and hydrated in a graded series of alcohols. The deparaffinized slides were then boiled by microwave for 12 minutes in citrate buffer, pH 6. A primary mouse antihuman ROR2 monoclonal antibody was used at a 1:25 dilution 2. The immunohistochemical reaction was visualized using the EnVision+ system (Dako, Carpinteria, CA, USA) with diaminobenzidine. Wnt5a immunohistochemistry was performed using the automatic Ventana ES IHC staining machine (Ventana Medical Systems, Tucson, AZ, USA) and Wnt5a (mouse monoclonal, dilution 1:3000, Novus Biologicals, Littleton, CO, USA). Positive control tissues for the immunohistochemistry were: β‐catenin, fibromatosis with known β‐catenin expression; GAB1, tonsil; Wnt5a, gastric mucosa; Ror2, TMA containing various types of samples with Ror2 expression. For negative control, the primary antibody was replaced by normal goat serum.
For the interpretation of β‐catenin, only nuclear staining was considered to be a positive result. For the interpretation of GAB1, cytoplasm staining was considered to be a positive result. The immunoreactivity for β‐catenin and GAB1 was scored as positive or absent. Wnt‐5a and Ror2 expression appeared in the form of predominantly membranous and occasionally cytoplasmic staining (Figure 1). The staining for Wnt‐5a and Ror2 was classified according to the percentage of positively stained cancer cells. To score the samples based on the percentage of positive cells, the following criteria were used: samples with <1% positive neoplastic cells were given a score of 0; samples with 1%–9% positive neoplastic cells were given a score of 1; samples with 10%–49% positive neoplastic cells were given a score of 2; and samples with >50% positive neoplastic cells were given a score of 3 (Figure 2).
Figure 1.
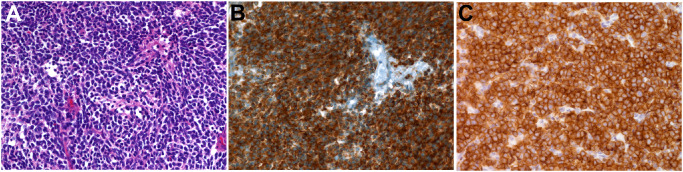
Histological and immunohistochemical findings of classic type medulloblastoma. A. The tumor consists of sheets of poorly differentiated cells that lack distinctive features [hematoxylin‐and‐eosin (H&E), ×400]. B. Tumor cells show strong Wnt5a expression in the form of membranous and cytoplasmic staining (Wnt5a antibody, ×400). C. Similarly, tumor cells show strong Ror2 expression in the form of membranous and cytoplasmic staining (Ror2 antibody, ×400).
Figure 2.
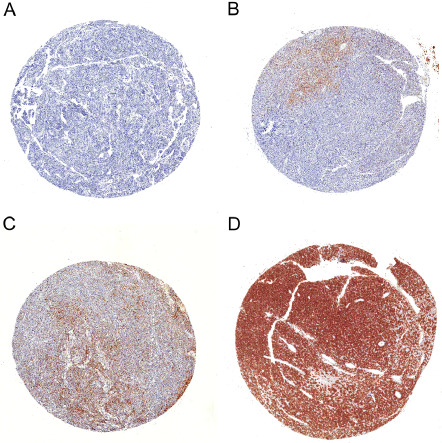
Four tissue microarray (TMA) cores with Ror2 immunohistochemistry. The immunoreactivity is semiquantitatively scored as (A) score 0, <1% positive neoplastic cells; (B) score 1, 1%–9%; (C) score 2, 10%–49%; (D) score 3, >50% (Ror2 antibody, ×20).
CTNNB1 DNA sequence analysis
Genomic DNA was extracted from ten 1‐μm‐thick sections of 10% neutral FFPE tumor tissue blocks using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). The mutational analysis of the CTNNB 1 (exon 3) was performed using directional sequencing of PCR fragments amplified from genomic DNA. PCR was performed in a 20 μL volume containing 100 ng template DNA, 10 X PCR buffer, 0.25 mM dNTPs, 10 pmol primers and 1.25 U Taq DNA polymerase (iNtRON, Sungnam, Korea). PCR products were electrophoresed on 2% agarose gel and purified with a QIAquick PCR purification kit (Qiagen). Bidirectional sequencing was performed using the BigDye Terminator v1.1 kit (Applied Biosystems, Foster City, CA, USA) on an ABI 3130xl genetic analyzer (Applied Biosystems). Sequencher version 4.10.1 (Gene Codes Corporation, Ann Arbor, MI, USA) was used with manual chromatogram reviews for sequence analysis. The results were marked as mutation positive if a mutation was detected in both the forward and reverse DNA strand.
MYCC and MYCN quantitative PCR analysis
To validate the copy number change, quantitative TaqMan® copy number variant (CNV) assays (Applied Biosystems) for the MYCC exon 2 (Assay ID: Hs00834648_cn) and MYCN exon 3 (Assay ID: Hs02718426_cn) were performed by the 2‐ΔΔCt (RQ) method using TaqMan Copy Number Reference Assay RNaseP (Applied Biosystems, Part No. 4403326) as an endogenous control and human genomic reference DNA (Roche, Indianapolis, IN, USA; Cat. No. 11 691 112 001) as a calibrator. Extractions of gDNA were performed using the Qiagen gDNA extraction kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. All reactions with TaqMan CNV assays were performed in triplicate. qPCR was performed with 20 ng gDNA according to the manufacturer's protocol in an Applied Biosystems (ABI PRISM 7500HT Fast real‐time PCR). The One Step Plus Real‐Time PCR System was used with the default universal cycling conditions. TaqMan universal PCR master mix and No AmpErase® UNG were used in real‐time PCR with default universal cycling conditions. Thermal cycling commenced with DNA polymerase activation at 95°C for 10 minutes and continued with 45 cycles of denaturation at 95°C for 15 s, followed by annealing and extension at 60°C for 1 minute. The cut‐off value for the presence of amplification was 2.0 for MYCC and MYCN.
RNA extraction and quantitative real‐time RT‐PCR
The expression levels of Wnt5a and ROR2 messenger RNA (mRNA) were measured by quantitative real‐time RT‐PCR using TaqMan Gene Expression Assays (Applied Biosystems, Assay ID: Hs00896176_m1 for ROR2; Hs00998537_ml for WNT5a). The GAPDH gene (Applied Biosystems, Assay ID: Hs99999905_m1) was used as an endogenous control. Total RNA extractions were isolated from FFPE tumor samples using the RNeasy Mini kit (Qiagen) according to the manufacturer's instructions. RT‐PCR was conducted using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Cat. No. 4368814) according to the manufacturer's instructions. A total of 5 μL of each complementary DNA sample was analyzed in triplicate with ABI PRISM 7500HT Fast Real‐time PCR (Applied Biosystems). Ct, the threshold cycle number at which the amount of amplified target reached a fixed threshold, was determined, and the mRNA expression levels of each gene were measured using the 2‐ΔCt (ΔCt = ΔCttarget gene−ΔCtGAPDH) method. The cut‐off value for low and high expressions was 3.0 for Ror2 and Wnt5a.
Statistical analyses
The χ2‐test or Fisher's exact test was used to examine associations between histologic subtypes and clinicopathologic parameters. P‐values reported in this manuscript were adjusted for multiplicity. The difference in expression levels of Ror2 and Wnt5a according to IHC score and the difference in expression levels of MYCC and MYCN among three histologic subtypes were evaluated by the Kruskall–Wallis non‐parametric test.
The correlation between the immunoreactive score of Wnt5a and Ror2 was investigated using Spearman's rank correlation test. Correlations were evaluated by the Spearman's rank correlation coefficient. We used the Cochran–Armitage test to evaluate the trend among Wnt5a, Ror2 and β‐catenin expressions.
Overall survival (OS) was calculated from the date of diagnosis to the date of death or last follow‐up. Progression‐free survival (PFS) was defined from the day of first surgery until tumor progression, death or end of follow‐up. Survival analysis was estimated using the Kaplan–Meier method and compared between two or more groups of patients using the log‐rank test. Univariate analysis was performed, and the significance of differences in survival between the groups was determined using the log‐rank test. Cumulative survival curves and OS for groups were computed according to the Kaplan–Meier method. To evaluate the hazard ratios and the independent prognostic relevance, multivariate analysis using the Cox proportional hazard model was performed using the following covariates: age, histologic type, mRNA expression level of Wnt5a and Ror2, CTNNB1 mutation status, MYCC and MYCN amplification status, initial surgery type, and history of postoperative treatment coded as they were in the univariate analysis.
Results
Clinical demographics according to histologic subtypes of MB (Table 1)
Table 1.
Clinical characteristics of 76 patients with medulloblastoma according to histologic subtypes. Abbreviations: D/N = desmoplastic/nodular; F/U = follow‐up; LC/A = large cell/anaplastic
| Characteristics |
Total (n = 76) |
Classic (n = 47) |
D/N (n = 15) |
LC/A (n = 14) |
P‐value |
|---|---|---|---|---|---|
| Male : Female | 47:29 | 29:18 | 8:7 | 10:4 | 0.673 |
| Age | |||||
| <5 years | 14 (18%) | 5 | 7 | 2 | 0.017 |
| 5–15 years | 47 (62%) | 30 | 8 | 9 | |
| >15 years | 15 (20%) | 12 | 0 | 3 | |
| Recurrence (%) | 22 (28.9) | 12 (25.5) | 5 (33.3) | 5 (35.7) | 0.741 |
| Metastasis (%) | 15 (19.7) | 6 (12.8) | 4 (26.7) | 5 (35.7) | 0.130 |
| Death (%) | 25 (32.9) | 16 (34.0) | 3 (20.0) | 6 (42.9) | 0.520 |
| F/U [months] (Mean ± SD) | 59.1 ± 44.0 | 61.7 ± 44.7 | 63.9 ± 36.5 | 45.3 ± 44.1 | 0.428 |
The study was comprised of 76 patients, 47 male and 29 female, with a mean age at diagnosis of 12 years (range, 2–53 years). Fourteen patients (18%) were younger than 5 years, and 15 patients (20%) were older than 15 years. Of the 76 patients, recurrence occurred in 22 patients and death occurred in 25 patients. Sixty‐one patients (80.3%) had localized disease (stage M0), and 15 patients (35%) had metastatic disease (M1‐4). Of 15 patients with metastasis, 14 had metastases at initial presentation, and the remaining one developed metastatic relapse at 2.3 years after initial diagnosis.
Metastatic staging included six patients (7.9%) with positive cerebrospinal fluid (CSF) cytology only (stage M1), three patients (3.9%) with intracranial leptomeningeal spread only (stage M2), five patients (6.6%) with metastatic disease in both the brain and the spine (stage M3), and one patient (1.3%) with extraneural disease (stage M4).
All patients underwent surgical resection. As first surgical treatment gross total resection (GTR) was performed in 48 patients (63.2%) and subtotal or near total resection (non‐GTR) was conducted in 28 patients (36.8%). Concurrent chemotherapy and radiotherapy were administered to 66 patients (86.8%). Five patients received only postoperative chemotherapy, whereas one patient received only postoperative radiotherapy. No adjuvant treatment was administered to four patients after surgical resection.
The follow‐up period ranged from 1.0 to 179 months (mean: 59.1 months, SD: 44.0 months). According to histopathologic evaluation, 47 tumors were classified as classic, 15 tumors were classified as D/N, and 14 tumors were classified as LC/A type. Statistical analyses revealed no significant differences among histologic types according to sex, recurrence rate, metastasis rate, death rate or follow‐up (F/U) duration (P = 0.673, P = 0.741, P = 0.130, P = 0.520 and P = 0.428, respectively).
Different expression of canonical pathway‐related genes: β‐catenin immunohistochemistry and CTNNB1 mutation analysis
As shown in Table 2, nuclear only staining of β‐catenin was seen in 17.1% (13/76) of cases, with the majority of positive cases (84.6%) observed in the classic type. However, there was no statistical difference in immunoreactivity of β‐catenin among the three histologic subtypes (P = 0.085).
Table 2.
Association of immunophenotypes with histologic subtypes of medulloblastoma. Abbreviations: D/N = desmoplastic/nodular; LC/A = large cell/anaplastic
| Variables |
Total (n = 76) |
Classic (n = 47) |
D/N (n = 15) |
LC/A (n = 14) |
P‐value |
|---|---|---|---|---|---|
| β‐catenin | 13 | 11 | 0 | 2 | P = 0.085 |
| Wnt5a | 20 | 16 | 1 | 3 | P = 0.033 |
| Ror2 | 35 | 22 | 6 | 7 | P = 0.553 |
| GAB1 | 14 | 2 | 9 | 3 | P < 0.001 |
Sequence analysis of the CTNNB1 exon 3 was performed on 76 tumors, of which 73 had successful results. Ten out of 73 tumors (13.7%) had missense point mutations. These mutations were C98T (S33F) in four cases, C110T (S37F) in four cases, G94T (D32Y) in one case, and C98G (S33C) in one case. All mutations were identified exclusively in the classic type. The mutation of CTNNB1 was statistically different among the three histologic subtypes (P = 0.033). Comparison of β‐catenin immunoreactivity and sequence analyses indicated that seven (70%) of the β‐catenin positive cases harbored CTNNB1 mutations, whereas three cases were CTNNB1 wild type. Unfortunately, DNA sequencing analysis for CTNNB1 mutation was unsuccessful in 3 out of 13 cases with β‐catenin nuclear staining.
Different expression of non‐canonical pathway‐related genes: Ror2 and Wnt5a immunohistochemistry and mRNA qPCR
Immunohistochemically, when the positive cases were considered as from score 1+ to score 3+, positivity of Wnt5a and Ror2 was seen in 20/68 (29.4%) and 35/68 (51.5%) cases, respectively. Of 20 cases with Wnt5a immunoreactivity, 16 (80.0%) were observed in the classic type. The protein expression of Wnt5a was statistically different among the three histologic subtypes (P = 0.033). Similarly, of 35 cases with Ror2 immunopositivity, 22 (62.9%) were observed in the classic type, but the protein expression of Ror2 showed no significant difference among the three histologic subtypes. Associations among the protein expression levels of Wnt5a, Ror2 and β‐catenin are shown in Table 3. Ror2 expression was positively associated with Wnt5a expression (r = 0.596, P < 0.001) in MB. Furthermore, there was a positive association among the protein expressions of Wnt5a, Ror2 and β‐catenin (P = 0.001 and P = 0.006, respectively).
Table 3.
Correlation among the protein expressions of Wnt 5a, Ror2 and β‐catenin
| Variable | Wnt 5a | β‐catenin | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | P‐value (ρ) | Negative | Positive | P‐value | |
| Ror2 | ||||||||
| 0 | 30 | 0 | 1 | 2 | <0.001a | 31 | 2 | 0.006b |
| 1+ | 10 | 0 | 0 | 0 | (0.596) | 10 | 0 | |
| 2+ | 3 | 0 | 0 | 1 | 4 | 0 | ||
| 3+ | 5 | 1 | 2 | 13 | 13 | 8 | ||
| β‐catenin | 0.001b | |||||||
| Negative | 46 | 1 | 2 | 9 | ||||
| Positive | 2 | 0 | 1 | 7 | ||||
≤1% positive neoplastic cells, score 0; 1%–9%, score 1; 10%–49%, score 2; ≥50%, score 3.
Spearman correlation analysis
Cochran–Armitage trend test.
The Wnt5a and Ror2 mRNA expression levels were quantitatively determined by qRT‐PCR in 76 FFPE samples. Wnt5a and Ror2 mRNA expression assay was successfully performed in 64 cases. The remaining cases failed to yield a reliable quality and quantity of RNA from the available limited quantities of tumor sections. Variable Wnt5a and Ror2 mRNA expression levels were observed, ranging from 0.00 to 156.59, and 0.00 to 123.98, respectively. The median values of Wnt5a and Ror2 mRNA were 0.54 and 0.04, respectively. We compared the mRNA levels of Wnt5a and Ror2 with the protein expressions of Wnt5a and Ror2. The Ror2 mRNA level was significantly correlated with Ror2 immunoexpression (P < 0.001). Tumors with immunohistochemically high expressions of Ror2 had higher mRNA levels than tumors with low expression. However, no correlation was observed between the Wnt5a mRNA levels and Wnt5a immunoexpression (P = 0.302). For mRNA levels, the expression of Wnt5a and Ror2 did not reveal a correlation between the three subtypes (P = 0.729 and P = 0.729, respectively).
The association between Ror2 mRNA expression and presence of the CTNNB1 mutation showed that 80% (8/10) of tumors harboring CTNNB1 mutations showed a high expression of Ror2 mRNA (Table 4). There was a significant association between Ror2 expression of mRNA and presence of the CTNNB1 mutation (P = 0.041), but there was not a significant association between Wnt5a expression of mRNA and presence of the CTNNB1 mutation (P = 0.302).
Table 4.
Association between the mRNA expression of Wnt5a, Ror2 and CTNNB1 mutation status
| Variable | Ror2 | Wnt5a | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Low | High | P‐value | n | Negative | Positive | P‐value | |
| CTNNB1 | ||||||||
| Wild | 54 | 31 | 23 | 0.041 | 54 | 32 | 22 | 0.302 |
| Mutant | 10 | 2 | 8 | 10 | 4 | 6 | ||
Additional analysis of SHH and non‐Wnt/SHH molecular subgroup: GAB1 immunohistochemistry and MYCC and MYCN amplification status
We chose the anti‐GAB1 antibody as a SHH molecular group marker. Fourteen out of 68 cases (20.6%) demonstrated positive immunoreactivity for GAB1. Of the 14 GAB1‐positive cases, 9 (64.3%) cases were observed in the D/N type. GAB1 expression was statistically different among the three histologic subtypes (P < 0.001) (Table 2). The GAB1 expression was exclusively correlated with CTNNB1 mutation and β‐catenin expression.
The MYCC amplification level was statistically different among the three histologic subtypes (P = 0.004) (Figure 3). The LC/A subtype showed higher levels of MYCC amplification by qPCR compared with the classic and D/N types. The mean amplification level was 3.79 ± 2.6 in the classic type, 1.74 ± 1.6 in the D/N type, and 5.80 ± 1.6 in the LC/A type. However, MYCN amplification level among the three histologic subtypes was not statistically different (P = 0.403).
Figure 3.
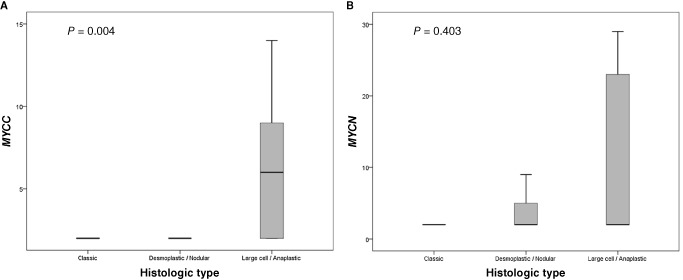
Amplification level of MYCC/MYC according to histologic subtypes of medulloblastoma. A. MYCC amplification level. B. MYCN amplification level.
Relationship of clinicopathological and biological factors with OS and PFS (Table 5)
Table 5.
Clinicopathological and biological factors affecting OS and PFS rates of patients with medulloblastoma by univariate and multivariate analyses. Abbreviations: CI = confidence interval; D/N = desmoplastic/nodular; GTR = gross total resection; HR = hazard ratio; LC/A = large cell/anaplastic; OS = overall survival; PFS = progression‐free survival
| OS | PFS | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.015 (0.969–1.063) | 0.526 | 0.968 (0.902–1.038) | 0.359 | 1.009 (0.965–1.056) | 0.686 | 0.968 (0.910–1.031) | 0.313 |
| Histologic type | 0.287 | 0.140 | 0.729 | 0.202 | ||||
| Classic | ||||||||
| D/N | 0.541 (0.132–2.218) | 0.392 | 0.138 (0.011–1.774) | 0.164 | 0.746 (0.279–1.991) | 1.000 | 0.280 (0041–1.910) | 0.274 |
| LC/A | 1.632 (0.554–4.805) | 0.310 | 1.688 (0.276–10.328) | 1.000 | 1.205 (0.483–3.005) | 1.000 | 1.548 (0.301–7.960) | 1.000 |
| CTNNB1 | ||||||||
| Mutant (vs. wild) | 0.247 (0.037–2.028) | 0.205 | 0.627 (0.065–6.019) | 0.627 | 0.410 (0.097–1.721) | 0.223 | 0.892 (0.169–4.721) | 0.893 |
| Ror2 mRNA | ||||||||
| High expression (vs. low) | 0.388 (0.152–0.995) | 0.049 | 0.179 (0.036–0.879) | 0.034 | 0.360 (0.152–0.851) | 0.020 | 0.213 (0.054–0.845) | 0.213 |
| Wnt5a mRNA | ||||||||
| High expression (vs. low) | 0.759 (0.309–1.863) | 0.547 | 2.755 (0.524–14.488) | 0.232 | 0.748 (0.337–1.661) | 0.476 | 1.556 (0.410–5.902) | 0.516 |
| MYCC | ||||||||
| Amplification (vs. no amplification) | 1.039 (0.386–2.800) | 0.939 | 0.838 (0.131–5.362) | 0.852 | 1.039 (0.386–2.800) | 0.939 | 0.973 (0.231–4.099) | 0.970 |
| MYCN | ||||||||
| Amplification (vs. no amplification) | 1.558 (0.662–3.664) | 0.310 | 2.550 (0.873–7.447) | 0.087 | 1.824 (0.864–3.849) | 0.761 | 4.157 (0.906–10.757) | 0.083 |
| Metastasis | ||||||||
| Metastasis (vs. no metastasis) | 2.756 (1.143–6.642) | 0.024 | 3.489 (0.968–12.574) | 0.056 | 0.870 (0.355–2.131) | 0.296 | 1.700 (0.508–5.685) | 0.389 |
| Primary surgery type | ||||||||
| Non‐GTR (vs. GTR) | 2.522 (1.142–5.570) | 0.022 | 0.966 (0.526–3.652) | 0.966 | 2.887 (1.410–5.912) | 0.004 | 1.469 (0.500–4.317) | 0.484 |
| Postoperative treatment | ||||||||
| Yes (vs. No) | 0.062 (0.019–0.205) | <0.001 | 0.047 (0.008–0.269) | 0.001 | 0.105 (0.034–0.325) | <0.001 | 0.147 (0.032–0.678) | 0.014 |
When the patients were divided into two groups based on the level of Ror2 expression in qPCR, high Ror2 expression group showed significantly better OS (P = 0.040) than the low expression group. Although the expression level of Wnt5a tended to show increased OS, this difference was not significant (P = 0.543). Kaplan–Meier survival curves and corresponding P‐values are shown in Figure 4. The 5‐year and 10‐year OS rates were 65.3% and 29.3% respectively for patients with low expression levels of Ror2 and 77.6% and 77.6% for patients with high expression levels of Ror2, respectively.
Figure 4.
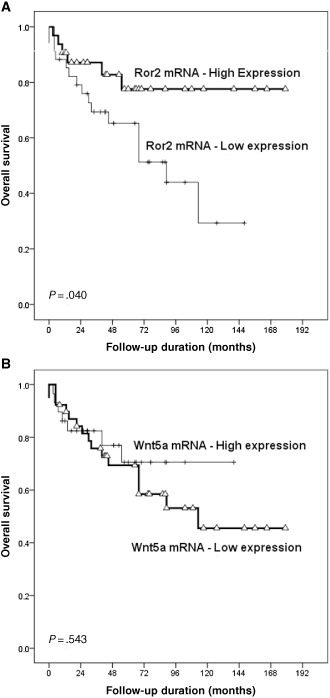
Kaplan–Meier survival curves with log‐rank test of overall survival (OS) according to Ror2 and Wnt5a expression level. A. High expression of Ror2 is associated with longer OS (P = 0.040). B. The expression level of Wnt5a tends to be associated with increased OS (P = 0.543).
We analyzed the prognostic relevance of clinicopathological and biologic parameters on OS and PFS of patients with MB. OS was significantly associated with Ror2 mRNA expression (P = 0.049), metastasis (P = 0.024), initial surgery type (P = 0.022) and the history of postoperative treatment (P < 0.001) in a univariate analysis. In Cox multivariate analysis with stepwise selection, high Ror2 mRNA expression and the history of postoperative treatment were independent prognostic factors in patients with MB [hazard ratio = 0.179, 95% CI, 0.036–0.879, P = 0.034; hazard ratio = 0.047, 95% CI, 0.008–0.269, P = 0.001, respectively]. Similarly, PFS was significantly associated with high Ror2 mRNA expression and the history of postoperative treatment [hazard ratio = 0.360, 95% CI, 0.152–0.851, P = 0.020; hazard ratio = 0.147, 95% CI, 0.032–0.678, P = 0.014, respectively].
Discussion
Understanding the molecular biology of MB as a first step is required to risk stratify groups for targeted therapy. Although aggressive multimodal therapy has improved the prognosis for children with MB, a significant portion of high‐risk patients are currently incurable. In addition, survivors from low‐risk group that respond well to therapy often suffer from significant adverse effects of treatment, including neurocognitive deficits related to radiation therapy. In an attempt to better classify MB, many groups have turned to high‐dimensional profiling studies; however, the validity of many biomarkers reported by various groups has been questioned. Therefore, their clinical utility is limited by conflicting results.
The purpose of the present study was to investigate new biomarkers that have not yet been reported in MB and their prognostic significance. Furthermore, we showed an association between these biomarkers and other known immunohistochemical and molecular biomarkers that are used to classify molecular subgroups of MB. Taking it a step further, we suggested additional mechanisms contributing to the pathogenesis of MB.
The Wnt pathway plays a crucial role in CNS development, particularly in cerebellar development, tissue homeostasis and cancer 15. In MB, dysregulation of WNT signaling has been identified in up to 20% of tumors 11. Of those, the canonical Wnt/β‐catenin pathway is aberrantly activated in approximately 10%–15% 4. In this study, the canonical Wnt/β‐catenin signaling pathway in MB was identified by nuclear immunoreactivity for β‐catenin and mutation detection of CTNNB1, the gene encoding β‐catenin. The nuclear staining of β‐catenin was seen in 17.1% (13/76) of total cases, and the majority of positive cases (84.6%) were observed in the classic type. The CTNNB1 mutation was identified in 13.7% (10/73) of total cases, and the mutations were exclusively identified in the classic type. These frequencies of the Wnt/β‐catenin molecular subgroup are consistent with the results observed in previous studies. However, β‐catenin immunoreactivity and mutation of CTNNB1 were only identified in 27.7% (13/47) and 21.3% (10/47) respectively of the classic type. The Wnt pathway in MB is nearly always seen in the classic type, but the classic type of MB also includes a considerable portion of the non‐Wnt/SHH as well as the Wnt molecular subgroups 3. There are overlapping features between morphologic and molecular groups of MB. Therefore, many portions of the classic type of MB cannot be accounted for with the pathogenesis of the Wnt pathway, the presence of downstream activating CTNNB1 mutations or rare APC and AXIN1/2 mutations. In other words, all Wnt pathway tumors do not have CTNNB1 mutations. Meanwhile, the mRNA expression of Ror2 was identified in 34.2% of patients (26/76) and Wnt5a was identified in 30.3% of patients (23/76) in this study. It comprises a larger portion than cases of β‐catenin immunoreactivity and CTNNB1 mutation. Aberrant signaling in the non‐canonical WNT pathway probably is firmly related to the pathogenesis of MB. Recently, Kongkham et al 11 reported that excessive signaling in the non‐canonical WNT pathway through gene silencing of SFRP, Wnt inhibitors, is an additional mechanism of MB pathogenesis.
Interestingly, we observed Ror2 genes to be significantly highly expressed in CTNNB1‐mutated MBs compared to the wild‐type cases. This finding can be accounted for by the fact that the canonical and non‐canonical pathways are not distinct in regard to the pathogenesis of MB. Wnt5a can activate or inhibit the canonical Wnt signaling depending on the availability of specific receptors 19. Wnt‐5a can also inhibit the activation of the canonical signaling pathway (β‐catenin/TCF‐dependent transcription) by either calcium signaling through CamKII 29 or through the Ror2 signaling pathways 20. However, there is evidence to suggest that in the presence of FZ‐4 and LRP‐5 and the absence of Ror2, Wnt‐5a can stimulate β‐catenin transcriptional activation 20. Furthermore, Li et al 14 demonstrated that Ror2 also positively modulates Wnt3a‐activated canonical signaling in a lung cancer cell line. As one of the potential mechanisms of Ror2 in modulating canonical Wnt signaling, Ror2 may interact with Fzd2 which couples with Lrp5/6 in the presence of Wnt3a. They showed that the cysteine‐rich domain (CRD) and the tyrosine kinase domains, but not the proline‐rich domain (PRD) and tyrosine kinase activity of Ror2, are required for mediating the Wnt3a activation of canonical Wnt signaling. These findings demonstrate the multifunctional properties of Ror2 in canonical and non‐canonical Wnt signaling, and Wnt signaling can be regulated by cooperative functions of multiple mediators.
Ror2, a member of the Ror family of receptor tyrosine kinases, acts as a receptor or coreceptor for Wnt5a 24. Several previous studies on the function of Ror2 have shown opposite results in various tumor types. For example, Ror2 is associated with tumor invasiveness and metastasis in melanoma, renal cell carcinoma and squamous cell carcinoma 10, 22, 30. In contrast, the expression of Ror2 was reduced in colon cancer tissues 13, and decreased Ror2 expression had a poorer prognosis in hepatocellular carcinoma (HCC) patients 6.
Although we found that high expression of Wnt5A was not correlated with OS (P = 0.543), high expression of Ror2 was correlated with increased OS (P = 0.040). Furthermore, in multivariate analysis, high expression of Ror2 showed a good survival prognostic effect. Therefore, patients with tumors having a low expression of Ror2 had a poorer prognosis than those with tumors that had high Ror2 expression. Our findings suggest that Ror2 may play a role as an oncosuppressor.
In conclusion, we have focused on the prognostic significance of signaling pathway abnormalities, which are likely to be involved in the pathogenesis of MB. Our study demonstrates the first attempt to identify Wnt5a and Ror2 as additional mechanisms contributing to dysregulation of the non‐canonical WNT signaling pathway in MB. Although additional functional studies are needed to confirm and more fully explore the role of Ror2, our results suggest that Ror2 is an independent prognostic factor in MB.
Sang Bum Suh is a student of Whimoon High School.
References
- 1. Deloukas P, Schuler GD, Gyapay G, Beasley EM, Soderlund C, Rodriguez‐Tome P et al (1998) A physical map of 30,000 human genes. Science 282:744–746. [DOI] [PubMed] [Google Scholar]
- 2. Edris B, Espinosa I, Muhlenberg T, Mikels A, Lee CH, Steigen SE et al (2012) ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol 227:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ellison DW (2010) Childhood medulloblastoma: novel approaches to the classification of a heterogeneous disease. Acta Neuropathol 120:305–316. [DOI] [PubMed] [Google Scholar]
- 4. Fattet S, Haberler C, Legoix P, Varlet P, Lellouch‐Tubiana A, Lair S et al (2009) Beta‐catenin status in paediatric medulloblastomas: correlation of immunohistochemical expression with mutational status, genetic profiles, and clinical characteristics. J Pathol 218:86–94. [DOI] [PubMed] [Google Scholar]
- 5. Forrester WC (2002) The Ror receptor tyrosine kinase family. Cell Mol Life Sci 59:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geng M, Cao YC, Chen YJ, Jiang H, Bi LQ, Liu XH (2012) Loss of Wnt5a and Ror2 protein in hepatocellular carcinoma associated with poor prognosis. World J Gastroenterol 18:1328–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giangaspero F, Rigobello L, Badiali M, Loda M, Andreini L, Basso G et al (1992) Large‐cell medulloblastomas. A distinct variant with highly aggressive behavior. Am J Surg Pathol 16:687–693. [PubMed] [Google Scholar]
- 8. Giles RH, van Es JH, Clevers H (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653:1–24. [DOI] [PubMed] [Google Scholar]
- 9. Katoh M (2005) WNT/PCP signaling pathway and human cancer (review). Oncol Rep 14:1583–1588. [PubMed] [Google Scholar]
- 10. Kobayashi M, Shibuya Y, Takeuchi J, Murata M, Suzuki H, Yokoo S et al (2009) Ror2 expression in squamous cell carcinoma and epithelial dysplasia of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 107:398–406. [DOI] [PubMed] [Google Scholar]
- 11. Kongkham PN, Northcott PA, Croul SE, Smith CA, Taylor MD, Rutka JT (2010) The SFRP family of WNT inhibitors function as novel tumor suppressor genes epigenetically silenced in medulloblastoma. Oncogene 29:3017–3024. [DOI] [PubMed] [Google Scholar]
- 12. Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA et al (2012) Molecular subgroups of medulloblastoma: an international meta‐analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol 123:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lara E, Calvanese V, Huidobro C, Fernandez AF, Moncada‐Pazos A, Obaya AJ et al (2010) Epigenetic repression of ROR2 has a Wnt‐mediated, pro‐tumourigenic role in colon cancer. Mol Cancer 9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF et al (2008) Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu C, Tu Y, Sun X, Jiang J, Jin X, Bo X et al (2011) Wnt/beta‐catenin pathway in human glioma: expression pattern and clinical/prognostic correlations. Clin Exp Med 11:105–112. [DOI] [PubMed] [Google Scholar]
- 16. Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810. [DOI] [PubMed] [Google Scholar]
- 17. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Masiakowski P, Carroll RD (1992) A novel family of cell surface receptors with tyrosine kinase‐like domain. J Biol Chem 267:26181–26190. [PubMed] [Google Scholar]
- 19. McDonald SL, Silver A (2009) The opposing roles of Wnt‐5a in cancer. Br J Cancer 101:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta‐catenin‐TCF signaling depending on receptor context. Plos Biol 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakagawara A (2001) Trk receptor tyrosine kinases: a bridge between cancer and neural development. Cancer Lett 169:107–114. [DOI] [PubMed] [Google Scholar]
- 22. O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC et al (2010) The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene 29:34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oishi I, Sugiyama S, Liu ZJ, Yamamura H, Nishida Y, Minami Y (1997) A novel drosophila receptor tyrosine kinase expressed specifically in the nervous system. Unique structural features and implication in developmental signaling. J Biol Chem 272:11916–11923. [DOI] [PubMed] [Google Scholar]
- 24. Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B et al (2003) The receptor tyrosine kinase Ror2 is involved in non‐canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654. [DOI] [PubMed] [Google Scholar]
- 25. Oldridge M, Fortuna AM, Maringa M, Propping P, Mansour S, Pollitt C et al (2000) Dominant mutations in ROR2, encoding an orphan receptor tyrosine kinase, cause brachydactyly type B. Nat Genet 24:275–278. [DOI] [PubMed] [Google Scholar]
- 26. Pinto D, Clevers H (2005) Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res 306:357–363. [DOI] [PubMed] [Google Scholar]
- 27. Saldanha J, Singh J, Mahadevan D (1998) Identification of a frizzled‐like cysteine rich domain in the extracellular region of developmental receptor tyrosine kinases. Protein Sci 7:1632–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Topol L, Jiang X, Choi H, Garrett‐Beal L, Carolan PJ, Yang Y (2003) Wnt‐5a inhibits the canonical Wnt pathway by promoting GSK‐3‐independent beta‐catenin degradation. J Cell Biol 162:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Torres MA, Yang‐Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT (1996) Activities of the Wnt‐1 class of secreted signaling factors are antagonized by the Wnt‐5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol 133:1123–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S et al (2009) Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene 28:2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamanaka H, Moriguchi T, Masuyama N, Kusakabe M, Hanafusa H, Takada R et al (2002) JNK functions in the non‐canonical Wnt pathway to regulate convergent extension movements in vertebrates. EMBO Rep 3:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]


