Abstract
Surgical correction of congenital cardiac malformations mostly implies the use of cardiopulmonary bypass (CPB). However, a possible negative impact of CPB on cerebral structures like the hippocampus cannot be neglected. Therefore, we investigated the effect of CPB on hippocampus CA1 and CA3 regions without or with the addition of epigallocatechin‐3‐gallate (EGCG) or minocycline. We studied 42 piglets and divided them into six experimental groups: control without or with EGCG or minocycline, CPB without or with EGCG or minocycline. The piglets underwent 90 minutes CPB and subsequently, a 120‐minute recovery and reperfusion phase. Thereafter, histology of the hippocampus was performed and the adenosine triphosphate (ATP) content was measured. Histologic evaluation revealed that CPB produced a significant peri‐cellular edema in both CA regions. Moreover, we found an increased number of cells stained with markers for hypoxia, apoptosis and nitrosative stress. Most of these alterations were significantly reduced to or near to control levels by application of EGCG or minocycline. ATP content was significantly reduced within the hippocampus after CPB. This reduction could not be antagonized by EGCG or minocycline. In conclusion, CPB had a significant negative impact on the integrity of hippocampal neural cells. This cellular damage could be significantly attenuated by addition of EGCG or minocycline.
Keywords: cardio‐pulmonary bypass, EGCG, hippocampus, histology, minocycline
Introduction
The invention of the heart–lung machine was a milestone in the development of cardiac surgery. This technique of extracorporeal circulation allowed the correction of very complex heart operations and although, several cardiac operations can be carried out without cardiopulmonary bypass (CPB), the heart–lung machine is indispensable for corrective surgery of most inborn cardiac diseases. However, negative effects of CPB on brain function in children undergoing cardiac surgery cannot be ignored. The neurologic deficits are subtle and neurologic defects clinically manifest often years after successful correction of the inborn heart defect 34 and might include various cognitive impairments like learning disabilities, memory deficits, hyperactivity or behavioral disorders 40. The causes of neurologic deficits following CPB are multiple and complex and might involve the artificial laminar flow of CPB, hypothermia, temporary hypoperfusion associated with low blood pressure, inflammatory and microembolic processes 40. All these factors might therefore contribute to an impairment of cognitive performance, which is detectable in up to 10% of the operated children. The pathophysiologic reasons why the developing brain is sensible to ischemic lesions are diverse, but it is accepted that the immature brain is especially sensitive to hypoxic and low flow conditions because of the immature fragile vasculature with less autoregulative capabilities 11, 23.
Several children studies report on reduced abilities in language and speech after successful correction of the congenital heart defect. Moreover, behavioral and academic problems, seizures and deficits in motor skills have been described. The children's neurologic problems seem to be associated with bypass duration and were more prevalent in patients operated with circulatory arrest compared with low flow conditions 6, 7, 17, 22.
In contrast, adult patients––frequently operated because of ischemic heart or degenerative valve diseases––are for the most part affected by reversible neuropsychological deficits like temporary psychosyndromes or transient ischemic attacks although stroke and intracerebral bleeding are also known and severe complications 28, 33. However, it needs to be noted that children have a high risk to receive permanent neurologic deficits during cardiac operations with long life impairment of their skills.
Therefore, protection of the brain during cardiac surgery is especially important and can be achieved through various strategies such as moderate hypothermia, selective brain perfusion or pulsatile flow 9, 13. Moreover, there are several hints that the antioxidant catechin epigallocatechin‐3‐gallate (EGCG)––the main ingredient of green tea––and the antibiotic and anti‐apoptotic drug minocycline might be beneficial against ischemia and reperfusion injury 1, 4. Especially from minocycline neuroprotective effects have been demonstrated in brain injury models. For example, Drabek et al 14 demonstrated in their animal study that minocycline significantly attenuated neuroinflammation after hypothermic circulatory arrest. Moreover, a less recent study on neonatal rats revealed that minocycline also has prolonged protective effects on the hippocampus 15.
Thus, the aim our study was to evaluate whether CPB might injure hippocampal structures, and whether EGCG or minocycline might have positive effects. Therefore, 4‐week‐old piglets underwent CPB with or without medical treatment, and the hippocampus was histolopathogically studied with respect to ischemic lesions. According to previous work of our study group, we analyzed transcriptions factors and molecules involved in processes of hypoxic tissue damage and early induction of apoptosis (hypoxia‐inducible factor‐1α [HIF‐1α], apoptosis‐inducing factor [AIF], cleaved caspase‐3 [cC3], poly‐adenosine diphosphate‐ribose [PAR] and nitrotyrosine) 42, 43.
Methods
The following procedures were reviewed and approved by the Animal Care Committee of the German Regional Council Leipzig, which ensured humane treatment of all animals as indicated by the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (NIH Publication No. 85‐23, revised 1996).
Anesthesia and perioperative management of the domestic pigs were carried out by veterinarians, thoracotomy and connection to the CPB by experienced heart surgeons and operation of the heart–lung machine was done by cardio technicians.
The experimental setup is analogous to that reported previously from our working group 42.
In brief, 42 piglets of 4 weeks of age weighting between 8 and 12 kg received a premedication of midazolam 0.5 mg/kg and ketamine 25 mg/kg of body weight (b.w.). They were intubated and general anesthesia was induced with 2–3% isoflurane and maintained with 1.5–2.0% isoflurane and sufentanil‐dihydrogenecitrate (bolus 3 μg/kg b.w. followed by 1–2 μg/h/10 kg b.w.). Ventilation of the piglets was performed with 50% air and 50% O2 using the Cato anesthesia apparatus (Drägerwerk, Lübeck, Germany). During CPB the piglets were anesthetized with propofol (20–30 mg/kg b.w.) and after CPB anesthesia was continued with isoflurane. All experimental groups (controls without or with drugs and CPB without or with drugs) received the same anesthesia protocol.
Oxygen saturation, pH, lactate, blood gases as well as central venous pressure were controlled. The mean systemic arterial pressure was >50 mmHg.
21 of the 42 piglets underwent CPB for 90 minutes and 28°C (without or with either EGCG or minocycline, respectively) followed by a 120‐minute reperfusion and recovery interval, the other 21 piglets (without or with either EGCG or minocycline, respectively) served as time and drug controls and were also thoracotomized, but not connected to the CPB.
Thus, with both the CPB and the control group, three different experimental designs were performed: no drug administration, administration of EGCG or administration of minocycline.
The following time scale was used:
time 0: clamping of the aorta and start of CPB or start of controls;
time 90: opening of aortic cross clamp and reperfusion (weaning from CPB);
time 120: disconnection of CPB; and
time 210: end of the reperfusion time and also end of the controls.
In piglets receiving medication EGCG (10 mg/kg b.w.) or minocycline (4 mg/kg b.w.) were administered 15 minutes before time 0 and again at time 120, a second drug administration was performed (EGCG 10 mg/kg b.w. or minocycline 2 mg/kg b.w.).
Detailed study protocol
After thoracotomy and cannulation of the right auricle and the aortic bow heparin was administered to achieve an activated clotting time (ACT) of 400 s and the CPB was connected (SIII pump, Stöckert, Munich, Germany; priming volume was 350 mL whole‐blood). Pump flow rate was maintained at 100 mL/kg/min and ACT was controlled to monitor heparin therapy. Aorta was cross‐clamped and cardiac arrest was initiated with 350 mL cold cardiolplegic solution (Custodiol, Koehler Chemie, Bensheim, Germany). At the end of CPB heparin was antagonized stepwise with protamine. The piglets were randomly assigned to each experimental group.
During CPB, all piglets were subjected to a moderate hypothermia (28°C for 60 minutes followed by a rewarming step). After a total of 90 minutes, the aortic cross clamp was reopened to start the reperfusion period (30 minutes) during which the flow of CPB was consecutively reduced to zero. CPB was disconnected and a 90‐minute recovery interval followed (i.e., a total of 120 minutes reperfusion and recovery).
All piglets were successfully weaned from CPB. If necessary, during reperfusion and recovery catecholamines were administered to stabilize the circulatory system. The control piglets received thoracotomy, but were not connected to CPB. In the experimental drug groups EGCG or minocycline were applied at the time points described above.
At the end of the investigation (time 210 minutes) the cranium was opened rapidly in deep anesthesia, the brain was removed and the hippocampi were either fixed in neutral buffered 4% formalin solution for histologic examination or snap frozen in liquid nitrogen for adenosine triphosphate (ATP) analysis.
ATP measurement by high‐pressure liquid chromatography (HPLC)
Tissue ATP levels were determined by HPLC as previously published 42. Briefly, tissue samples were homogenized on ice with perchloric acid and precipitated with KOH. Thereafter, probes were clarified by centrifugation and 20 μL of each supernatant were injected at a flow of 1 mL/min onto a pre‐equilibrated RP18 column (Lichrocart, Merck, Darmstadt, Germany). For detection of ATP an ultraviolet detector (PDA Detector 2800, Knauer, Berlin, Germany) and a HPLC‐apparatus from Knauer (Berlin, Germany) was used. Peaks were measured at 254 nm. For calibration we injected three concentrations of ATP (2, 20 and 60 μg/mL). Each sample (standard and probe) was injected three times, and the concentration was determined as the mean of these three detections.
Histology
Preparation and staining of the samples were done as previously published 43. Briefly, specimens of the hippocampus were embedded in paraffin and 2 μm sections were cut. Thereafter, the various stains were performed. All specimens were viewed and analyzed using the Axioimager M1 from Zeiss and the Zen Pro 2012 software (Jena, Germany). Pictures were taken at 400× magnification and at least 10 pictures per piglet were evaluated by a blinded observer.
Testing of antibody specificity was carried out by Western blotting (see Supporting Information) and revealed that the antibodies used in our histologic analysis were specific for our target proteins.
Hematoxylin‐eosin (HE) staining
To evaluate cellular edema HE staining was performed following classical protocols. Cells with pericellular edema were evaluated and for analysis at least 150–200 cells of the CA1 and 150–200 cells of the CA3 region were counted. The percentage of edematous cells was evaluated separately for each region.
Immunohistology
HIF‐1α
The transcription factor HIF‐1α responds to changes in ambient oxygen with translocation from the cytoplasm into the nucleus 39. Thus, we aimed to evaluate HIF‐1α positivity within the CA1 and CA3 regions of the hippocampus. Therefore, histologic samples were blocked with 1% bovine serum albumin to reduce unspecific background and stained with rabbit anti‐HIF‐1α primary antibody (1:100, Santa Cruz, Heidelberg, Germany) at 4°C over night. After washing secondary horseradish peroxidase (HRP)‐labeled antibody was applied (1 h, room temperature) and visualized using the red chromogen AEC (3‐amino‐9‐ethylcarbazol, DAKO, Hamburg, Germany). Again, cells of the CA1 and CA3 region were counted and the percentage of positive (i.e., red) nuclei was calculated for each region separately.
cC3, AIF and PAR
Caspase‐3, which is involved in the programmed cell death and AIF a protein, which triggers the caspase‐independent pathway of apoptosis were also evaluated in our organ samples 36, 45. Moreover, as PAR is also involved in cell death via stimulation of mitochondrial AIF‐release we aimed to investigate PAR as well 46. Therefore, histologic specimen were stained with either anti‐cC3 primary antibody (1:100 New England Biolabs, Frankfurt, Germany), or anti‐AIF primary antibody (1:100, Santa Cruz, Heidelberg, Germany) or with anti‐PAR primary antibody (1:200, BD Biosciences, Heidelberg, Germany). The immunohistologic staining and evaluation of hippocampus was carried out as described earlier.
Nitrotyrosine staining
Nitrogen species such as peroxynitrite induce nitrosylation of tyrosine residues. The resulting product nitrotyrosine is responsible for protein dysfunction. Peroxynitrite itself induces DNA strand brakes, which then in turn activate the DNA repair enzyme PAR polymerase (PARP). This enzyme restores DNA integrity in an ATP‐consuming process thereby releasing PAR 30.
Thus, we also stained our samples for nitrotyrosine‐positive structures using an anti‐nitrotyrosine primary antibody (1:200, Millipore, Darmstadt, Germany) followed by secondary HRP‐labeled antibody and AEC‐staining. To analyze nitrotyrosine‐positivity we measured the percentage of nitrotyrosine‐positive cells in the CA1 and CA3 region of the hippocampus.
Materials
Secondary HRP‐labeled antibodies and all other chemicals were purchased from Sigma‐Aldrich (Steinheim, Germany).
Statistic analysis
For statistic analysis, analysis of variance (ANOVA) was performed, and if ANOVA indicated significant differences (P < 0.05), data were additionally analyzed with the post hoc Tukey's honestly significant difference test. Statistic analysis was carried out using the software Systat for Windows, version 11 (Systat Inc., Evanston, IL, USA).
All data are given as means ± standard error of the mean of n = 7 experiments per group.
Results
All piglets in all groups could be successfully weaned from CPB. In Table 1, heart rate, mean arterial pressure, arterial O2 saturation and hematocrit are depicted. In both the CPB and CPB + EGCG groups hematocrit were slightly lower at the end of bypass and also at the end of reperfusion.
Table 1.
Heart rate, mean arterial pressure, hematocrit and arterial pO 2 saturation at 0′ (baseline), 120′ (disconnection of CPB) and 240′ (end of reperfusion)
| Heart rate (1/min) | Mean arterial pressure (mmHg) | Arterial O2 saturation (%) | Hematocrit (%) | |
|---|---|---|---|---|
| Control 0′ | 115 ± 10 | 57 ± 1 | 99.3 ± 0.3 | 23.0 ± 2.0 |
| Control 120′ | 122 ± 8 | 61 ± 2 | 99.1 ± 0.4 | 23.0 ± 2.4 |
| Control 240′ | 122 ± 5 | 64 ± 3 | 99.1 ± 0.5 | 26.0 ± 3.3 |
| Control + EGCG 0′ | 118 ± 5 | 64 ± 4 | 99.7 ± 0.3 | 23.0 ± 1.8 |
| Control + EGCG 120′ | 124 ± 4 | 70 ± 4 | 99.7 ± 0.1 | 26.0 ± 1.2 |
| Control + EGCG 240′ | 125 ± 5 | 65 ± 2 | 99.6 ± 0.2 | 30.0 ± 2.2 |
| Control + minocycline 0′ | 129 ± 12 | 65 ± 3 | 99.9 ± 0.1 | 25.0 ± 1.0 |
| Control + minocycline 120′ | 124 ± 5 | 67 ± 5 | 99.1 ± 0.4 | 26.0 ± 1.9 |
| Control + minocycline 240′ | 121 ± 5 | 61 ± 3 | 99.0 ± 0.3 | 25.0 ± 2.1 |
| CPB 0′ | 115 ± 5 | 55 ± 3 | 99.6 ± 0.3 | 24.0 ± 1.5 |
| CPB 120′ | 128 ± 5 | 54 ± 2 | 97.6 ± 0.5 | 18.0 ± 1.7a, b |
| CPB 240′ | 126 ± 7 | 63 ± 3 | 97.5 ± 0.7 | 17.0 ± 1.4a, b |
| CPB+EGCG 0′ | 119 ± 5 | 65 ± 11 | 99.5 ± 0.3 | 23.0 ± 1.8 |
| CPB+EGCG 120′ | 114 ± 7 | 57 ± 6 | 98.7 ± 0.5 | 18.0 ± 1.7a, b |
| CPB+EGCG 240′ | 127 ± 11 | 60 ± 8 | 98.8 ± 0.4 | 16.0 ± 1.9a, b |
| CPB + minocycline 0′ | 136 ± 5 | 59 ± 2 | 99.5 ± 0.3 | 26.0 ± 1.6 |
| CPB + minocycline 120′ | 123 ± 10 | 57 ± 5 | 98.6 ± 0.7 | 27.0 ± 1.4 |
| CPB + minocycline 240′ | 131 ± 7 | 67 ± 4 | 99.0 ± 0.5 | 28.0 ± 2.9 |
CPB = cardio‐pulmonary bypass; EGCG = epigallocatechin‐3‐gallate.
Significance versus control (P < 0.05).
Significance versus 0′ (P < 0.05).
Tissue ATP levels and lactate
Blood lactate concentration significantly increased nearly threefold during CPB. Application of either EGCG or minocycline had no influence on this increase.
In contrast, hippocampal ATP levels decreased to nearly 25% in the CPB group. Again, neither EGCG nor minocycline could prevent the ATP decline (Figure 1A,B).
Figure 1.
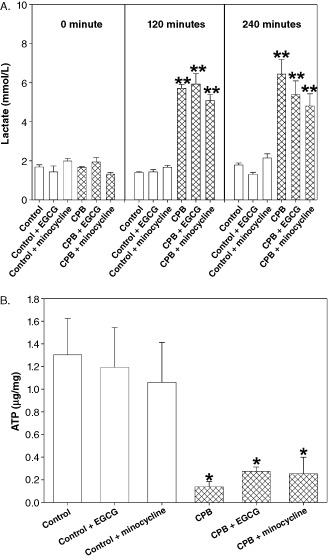
A. Blood lactate concentration at 0 minute (baseline), 120 minutes (disconnection of cardiopulmonary bypass [CPB] ) and 240 minutes (end of reperfusion). B. Hippocampus adenosine triphosphate (ATP) concentration. All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks (P < 0.05).
Cellular edema
HE staining of CA1 and CA3 regions of the hippocampus revealed that––compared with control conditions––CPB resulted in a significant pericellular edema within the CA1 and CA3 regions. Pretreatment of the piglets with EGCG significantly diminished edema formation although control levels were not reached. However, minocycline application completely prevented from peri‐cellular edema in both CA areas (Figure 2A,B). Under control conditions drug administration had no influence on pericellular edema.
Figure 2.
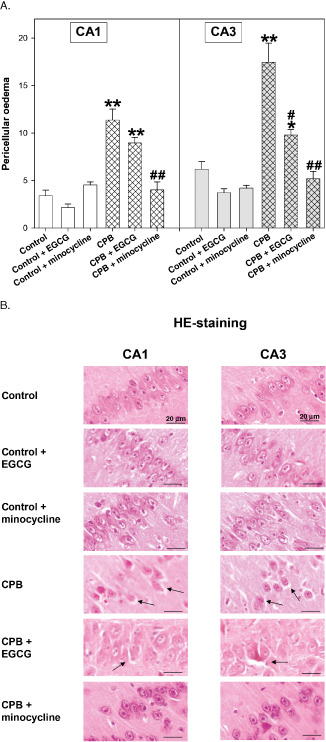
A. Hematoxylin‐eosin (HE) staining of hippocampus CA1 and CA3 regions of control piglets without or with epigallocatechin‐3‐gallate (EGCG) or minocycline and piglets after cardiopulmonary bypass (CPB) without or with EGCG or minocycline, respectively. Bar graphs depict the number of cells (in %) with pericellular edema. All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks (P < 0.05), (P < 0.01); significant differences to CPB by a hash # (P < 0.05), # # (P < 0.01). B. Original image showing HE staining of hippocampus. Arrows indicate pericellular edema of CA1 and CA3 regions.
HIF‐1α staining
During CPB HIF‐1α positivity was significantly increased in cell nuclei of both CA areas, indicating formation of HIF‐1α and translocation of this transcription factor from the cytoplasm into the nucleus. Pretreatment with EGCG could diminish the CPB‐induced rise in nuclear HIF‐1α, and minocycline completely inhibited HIF‐1α translocation (Figure 3A,B). Both drugs had no effect on HIF‐1α translocation in hippocampi of control piglets.
Figure 3.
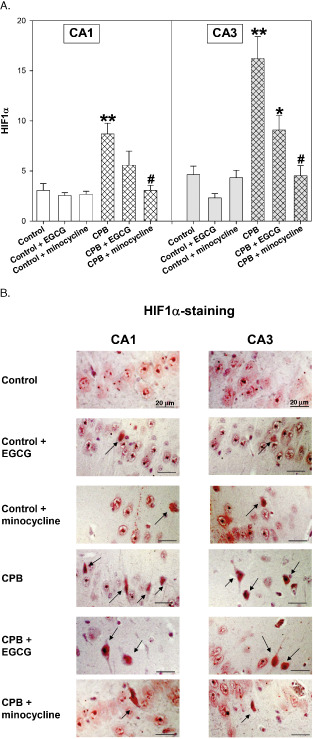
A. Hypoxia‐inducible factor‐1α (HIF‐1α) staining of hippocampus CA1 and CA3 regions of control piglets without or with epigallocatechin‐3‐gallate (EGCG) or minocycline and piglets after cardiopulmonary bypass (CPB) without or with EGCG or minocycline, respectively. Bar graphs depict the number of positive cell nuclei (in %). All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks * (P < 0.05), ** (P < 0.01); significant differences to CPB by a hash # (P < 0.05). B. Original image showing HIF‐1α‐staining of hippocampus CA1 and CA3 regions. Arrows indicate cell nuclei positive for HIF‐1α (stained in red).
AIF, cC3 and PAR staining
Both apoptosis‐inducing factors AIF and cC3 were elevated during CPB. Application of EGCG decreased AIF translocation, although control levels were not reached (Figure 4A,B). In contrast, minocycline completely inhibited AIF translocation.
Figure 4.
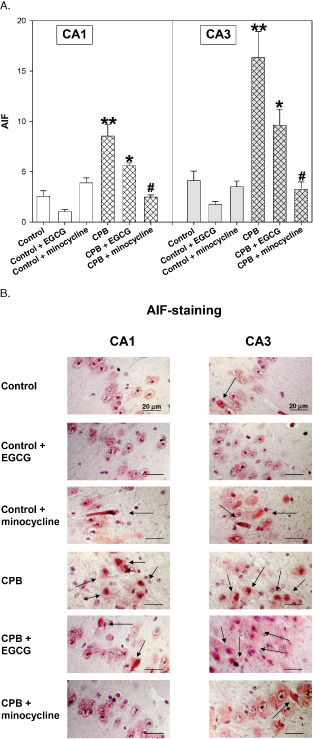
A. Apoptosis‐inducing factor (AIF)‐staining of hippocampus CA1 and CA3 regions of control piglets without or with epigallocatechin‐3‐gallate (EGCG) or minocycline and piglets after cardiopulmonary bypass (CPB) without or with EGCG or minocycline, respectively. Bar graphs depict the number of positive cell nuclei (in %). All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks * (P < 0.05), ** (P < 0.01); significant differences to CPB by a hash # (P < 0.05). B. Original image showing AIF‐staining of hippocampus CA1 and CA3 regions. Arrows indicate cell nuclei positive for AIF (stained in red).
Interestingly, in the cC3 stains we saw a reverse picture: EGCG completely inhibited cC3 activation, whereas minocycline significantly reduced cC3 translocation without reaching control levels (Figure 5A,B).
Figure 5.
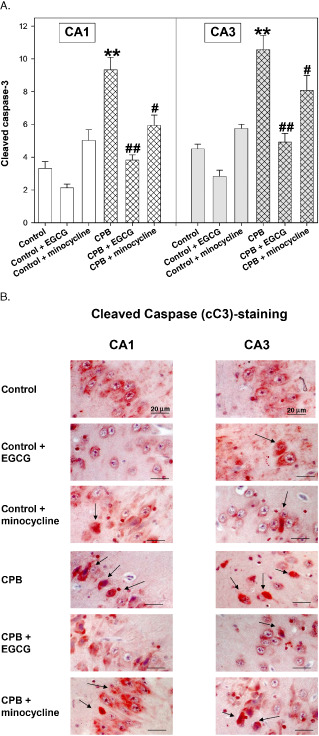
A. Cleaved caspase 3 (cC3) staining of hippocampus CA1 and CA3 regions of control piglets without or with epigallocatechin‐3‐gallate (EGCG) or minocycline and piglets after cardiopulmonary bypass (CPB) without or with EGCG or minocycline, respectively. Bar graphs depict the number of positive cell nuclei (in %). All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks ** (P < 0.01); significant differences to CPB by a hash # (P < 0.05), # # (P < 0.01). B. Original image showing cleaved caspase 3 (cC3)‐staining of hippocampus CA1 and CA3 regions. Arrows indicate cell nuclei positive for cC3 (stained in red).
During CPB, PAR formation was significantly enhanced, which was significantly reduced by both drugs (Figure 6A,B).
Figure 6.
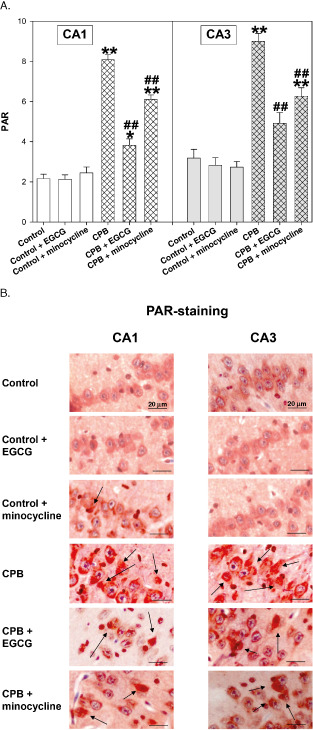
A. Poly‐adenosine diphosphate‐ribose (PAR) staining of hippocampus CA1 and CA3 regions of control piglets without or with epigallocatechin‐3‐gallate (EGCG) or minocycline and piglets after cardiopulmonary bypass (CPB) without or with EGCG or minocycline, respectively. Bar graphs depict the number of positive cell nuclei (in %). All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks * (P < 0.05), ** (P < 0.01); significant differences to CPB by a hash # (P < 0.05), # # (P < 0.01). B. Original image showing PAR staining of hippocampus CA1 and CA3 regions. Arrows indicate cell nuclei positive for PAR (stained in red).
Again under control conditions EGCG and minocycline had no impact on translocation of apoptosis‐inducing factors or PAR.
Nitrotyrosine staining
During CPB we observed a significant increase in nitrotyrosine within the cytoplasm of cells of the CA1 and CA3 regions. This CPB‐dependent rise in nitrosylated proteins was significantly inhibited by pretreatment with both EGCG and minocycline (Figure 7A,B). In the control piglets, both drugs exhibited no influence on nitrotyrosin formation.
Figure 7.
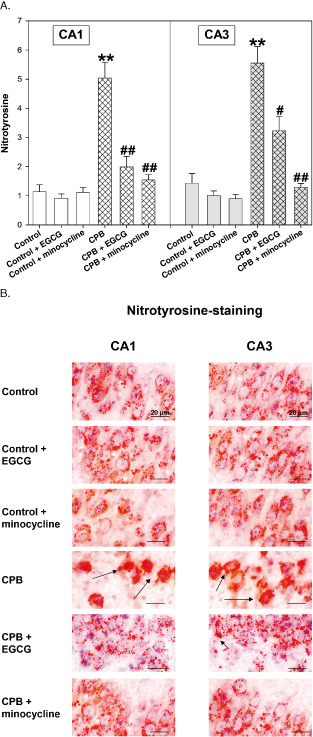
A. Nitrotyrosine staining of hippocampus CA1 and CA3 regions of control piglets without or with epigallocatechin‐3‐gallate (EGCG) or minocycline and piglets after cardiopulmonary bypass (CPB) without or with EGCG or minocycline, respectively. Bar graphs depict the number of cells positive for nitrotyrosine (in %). All values of are given as means ± standard error of the mean (SEM). Significant differences to control are indicated by asterisks ** (P < 0.01); significant differences to CPB by a hash # (P < 0.05), # # (P < 0.01). B. Original image showing nitrotyrosine‐staining of hippocampus CA1 and CA3 regions. Arrows indicate cells positive for nitrotyrosine (cytoplasm stained in red).
Discussion
In general, during ischemia and reperfusion, the following cascade of neuronal damage might occur: initially, ischemic conditions lead to ATP depletion and might promote expression of HIF‐1α an indicator of low oxygen; secondly, during reperfusion phase, oxygen radicals react with NO and form reactive nitrogen species as peroxynitrite, which in turn lead to DNA damage and PARP activation with the consequence of PAR formation. This latter process, which is highly ATP consuming, will then lead to a further reduction in energy rich phosphates. Another factor––involved in AIF‐independent apoptosis––is caspase‐3. This effector caspase is activated during hypoxic conditions. cC3 inhibits PARP activity, thereby inhibiting the repair of DNA strand breaks on one hand, but on the other hand via PARP cleavage leads to less ATP consumption, which might be beneficial for the cell 8, 18.
In summary, in our study, we could demonstrate that CPB had a significant negative influence on the neural cell band of the hippocampus. We saw a significant and severe reduction of ATP concentration and a significant elevation of HIF‐1α translocation, indicating an insufficient oxygen supply. Factors of apoptosis such as AIF and cC3 were also significantly elevated during CPB. Additionally, nitrotyrosine and PAR formation was stimulated, which may point towards tyrosine‐nitrosylation and DNA strand breaks by peroxynitrite. Interestingly, cellular damages in CA1 and CA3 regions were not evenly, but rather inhomogeneously distributed. Both drugs applied during CPB––the antioxidant EGCG and the anti‐apoptotic antibiotic agent minocycline––were able to reduce or even completely inhibit cellular damage, despite the fact that ATP was reduced.
Surprisingly, also during control conditions a small number of cC3‐positive cells could be detected, which were reduced by EGCG by about one‐third. This slight elevation might be due to an impairment of hippocampal cells by the volatile anesthetic isoflurane, from which it is known that it might induce cellular apoptosis 35. On the other hand, a certain neutroprotective effect has been ascribed to isoflurane during mild to moderate ischemia, as outlined the comprehensive review of Kitano et al 27. However, as all piglets received the same amounts of isoflurane the differences among the groups are unlikely attributable to isoflurane effects.
The histologic damages seen in our experimental model resemble a hypoperfusion/reperfusion injury. It should be noted that despite a normal perfusion flow, an impairment of hippocampal cells were seen. We found cellular injury, which occurred in consequence of CPB, as we did not see severe cellular changes in our controls.
Histopathologic changes in the hippocampus following CPB have also been described by others 44, 48, who demonstrated ischemic damage and neuronal apoptosis in the hippocampus of piglets undergoing CPB. However, in contrast to our study, these authors used prolonged low flow conditions, whereas in our experimental setting a perfusion flow of 100 mL/kg/min was administered, which is commonly used during CPB in man. Nevertheless, although the piglets of our study had a normal blood pressure and oxygen saturation with no differences between the experimental groups injuring of hippocampal structures could be demonstrated. This is remarkable as it seems imaginable to assume that similar damages in the CA regions following CPB might also be detectable in paediatric patients undergoing repair of severe inborn heart diseases. Besides, congenital heart diseases are often corrected in hypothermic circulatory arrest, which makes damages even more likely and more severe.
In contrast to our study Han et al 19 did not see any PARP activation within the hippocampus of their CPB control group. However, these authors used different experimental conditions: they used adult rats and a high CPB flow of 180 mL/kg b.w., which makes a direct comparison difficult, although––in line with our study––they also found enhanced PARP and nitration in the group of 75‐minute cardiac arrests.
To counteract the negative effects of CPB two different drugs were applied during our experimental setting: EGCG and minocycline. From the first one it is known that this ingredient of green tea has positive effects on memory and learning and furthermore has antioxidant and caspase‐3‐blocking properties, which can––as EGCG passes the blood–brain barrier––exert influence on neuronal cells 20, 26. Regarding the latter drug, besides its antibiotic properties, beneficial side effects have been described. Minocycline has a protective cerebral potential during ischemic conditions and in addition has also anti‐apoptotic qualities by inhibiting caspase‐3‐dependent and ‐independent cell death pathways 5, 21. Moreover, it has––at least in rats––positive effects on cognitive impairments during general anesthesia 29. Furthermore, according to other studies both drugs also seem to have anti‐inflammatory activities, which lower PARP activation and thereby PAR formation 2, 25. Thus, apoptosis induction, which might occur on several routs could be deactivated by EGCG or minocycline.
Cerebral edema formation after ischemia and/or inflammation is not uncommon and might result in an increase in intracerebral pressure resulting in a fatal and irreversible damage of hippocampal pyramidal cells. Both drugs investigated in our study were able to reduce edema formation whereas minocycline was more effective probably because of its anti‐inflammatory activity 24, 31. The mechanism of edema prevention is not known––maybe a general membrane damage by ROS––but interestingly for EGCG not restricted to the hippocampus as the same positive effect was seen in kidneys 42.
Surprisingly, although as mentioned earlier, we used a normal clinically relevant perfusion flow of 100 mL/kg/min together with hypothermia alterations of hippocampal neural cells, which were most likely due to ischemic conditions during CPB were seen. The transcription factor HIF‐1α and its downstream target vascular endothelial growth factor has its function in mediating cellular oxygen supply by promoting the formation of new blood vessels. Moreover, several genes that allow survival under hypoxic conditions like erythropoietin or glycolysis enzymes are up‐regulated by HIF‐1α to stabilize energy balance of the cell 16. In this context, it seems remarkable that despite low ATP levels during CPB, both drugs were able to attenuate HIF‐1α translocation. To explain this phenomenon, it is important to mention that HIF‐1α is activated not only by hypoxia, but also by reactive oxygen species (ROS), which promote HIF‐1α translocation 47. As EGCG is able to scavenge ROS this might be the mechanism by which HIF‐1α translocation was lowered in our study 3. Moreover, a decrease of ROS has also been ascribed to minocycline which has, like EGCG, a polycyclic aromatic ring system 37.
Another issue to discuss is the positive effects of both drugs on markers of cellular apoptosis. ROS and peroxynitrite, which are part of ischemia and reperfusion injury at the end lead to nitrosylated proteins, DNA damage, AIF and caspase‐3 activation. In a very recent study it was demonstrated in a mouse model of reduced AIF expression that cardiac ischemic/reperfusion injury was significantly attenuated if AIF translocation was shut down even if ROS levels were not altered 10. Thus, AIF relocation seems to be important in the development of tissue damage.
The anti‐apoptotic action of EGCG has been related to its radical scavenging properties. In a model of cultured neuronal cells it was demonstrated that EGCG preferentially protects against apoptosis resulting from mitochondrial oxidative stress probably by an accumulation in the mitochondria 38. Furthermore, NO measurements in rat hippocampus revealed that EGCG scavenges NO during ischemia (about 70%); however, without lowering hippocampal blood flow 32. In addition, minocycline also reduced nitrotyrosine amount and restored blood–brain barrier in a model of Parkinson's disease 41. Thus, these findings link well with our data of reduced nitrosative stress (i.e., less nitrotyrosine) in the pharmaceutical groups. On the other hand, in a recent study, the protective effects of nitrite therapy on heart and brain after cardiac arrest and resuscitation have been pointed out 12. The authors showed that nitrite therapy increased S‐nitrosothiol levels (a radical scavenger) and reduced mitochondrial ROS formation. However, nitrite and NO must not be confounded. While “normal” doses of NO are surely necessary for regular cellular function excessive NO can cause nitration of proteins leading to pathologic consequences. Thus, this chapter of NO and neuroprotection has not been closed.
Taken together our results indicate that pharmacologic brain protection against CPB‐associated injury is in principle possible. Both drugs seem to interfere with the post‐ischemic signaling. The present results obtained in a large animal model in a clinical setting may stimulate further research on the brain‐protective activity of EGCG and minocycline.
Limitations
First of all, the study was carried out in piglets and not in humans for obvious ethical reasons. However, we used a setup and operation protocol as typically used in the clinic.
In order to see whether the histologic changes may finally result in functional deficits, a 4–12‐week follow‐up with neuropsychological tests would be highly interesting. However, as only ∼10% of children undergoing CPB exhibit neuropsychological impairment, very large groups of piglets would be required to assess drug effects on this parameter. Thus, at present, the effects of the different treatments, which showed acute positive neuroprotective effects here in our study, on long‐term neuropsychological outcome remain to be investigated.
Supporting information
Figure S1. Original immunoblots of hypoxia‐inducible factor‐1α (HIF‐1α), apoptosis‐inducing factor (AIF), cleaved caspase 3 (cC3), poly‐ADP‐ribose (PAR) and nitrotyrosine. Lanes 1–3 indicate hippocampal lysates of three different control piglets.
Appendix S1. Immunoblots of hypoxia‐inducible factor 1‐alpha, apoptosis‐inducing factor, cleaved caspase 3, poly‐ADP‐ribose and nitrotyrosine.
Acknowledgments
We thankfully acknowledge the technical support of our residential staff.
References
- 1. Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ, Antonetti DA (2013) Minocycline prevents retinal inflammation and vascular permeability following ischemia‐reperfusion injury. J Neuroinflammation 10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alano CC, Kauppinen TM, Valls AV, Swanson RA (2006) Minocycline inhibits poly(ADP‐ribose) polymerase‐1 at nanomolar concentrations. Proc Natl Acad Sci U S A 103:9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. An Z, Qi Y, Huang D, Gu X, Tian Y, Li P et al (2014) EGCG inhibits Cd(2+)‐induced apoptosis through scavenging ROS rather than chelating Cd(2+) in HL‐7702 cells. Toxicol Mech Methods 24:259–267. [DOI] [PubMed] [Google Scholar]
- 4. Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B (2004) Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 10:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM (2002) Minocycline markedly protects the neonatal brain against hypoxic‐ischemic injury. Ann Neurol 52:54–61. [DOI] [PubMed] [Google Scholar]
- 6. Bellinger DC, Newburger JW, Wypij D, Kuban KC, duPlesssis AJ, Rappaport LA (2009) Behaviour at eight years in children with surgically corrected transposition: the Boston Circulatory Arrest Trial. Cardiol Young 19:86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellinger DC, Rivkin MJ, Demaso D, Robertson RL, Stopp C, Dunbar‐Masterson C et al (2014) Adolescents with tetralogy of Fallot: neuropsychological assessment and structural brain imaging. Cardiol Young 11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke 40:e331–e339. [DOI] [PubMed] [Google Scholar]
- 9. Bruggemans EF (2013) Cognitive dysfunction after cardiac surgery: pathophysiological mechanisms and preventive strategies. Neth Heart J 21:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Q, Szczepanek K, Hu Y, Thompson J, Lesnefsky EJ (2014) A deficiency of apoptosis inducing factor (AIF) in Harlequin mouse heart mitochondria paradoxically reduces ROS generation during ischemia‐reperfusion. Front Physiol 5:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chugani HT (1998) Biological basis of emotions: brain systems and brain development. Pediatrics 102:1225–1229. [PubMed] [Google Scholar]
- 12. Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP et al (2009) Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation 120:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Eusanio M1, Pantaleo A, Petridis FD, Folesani G, Cefarelli M, Berretta P, Di Bartolomeo R (2013) Impact of different cannulation strategies on in‐hospital outcomes of aortic arch surgery: a propensity‐score analysis. Ann Thorac Surg 96:1656–1663. [DOI] [PubMed] [Google Scholar]
- 14. Drabek T, Janata A, Wilson CD, Stezoski J, Janesko‐Feldman K, Tisherman SA et al (2014) Minocycline attenuates brain tissue levels of TNF‐α produced by neurons after prolonged hypothermic cardiac arrest in rats. Resuscitation 85:284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan LW, Lin S, Pang Y, Rhodes PG, Cai Z (2006) Minocycline attenuates hypoxia‐ischemia‐induced neurological dysfunction and brain injury in the juvenile rat. Eur J Neurosci 24:341–350. [DOI] [PubMed] [Google Scholar]
- 16. Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ (1994) Oxygen‐regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc Natl Acad Sci U S A 91:6496–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forbess JM, Visconti KJ, Bellinger DC, Howe RJ, Jonas RA (2002) Neurodevelopmental outcomes after biventricular repair of congenital heart defects. J Thorac Cardiovasc Surg 123:631–639. [DOI] [PubMed] [Google Scholar]
- 18. Garnier P, Ying W, Swanson RA (2003) Ischemic preconditioning by caspase cleavage of poly(ADP‐ribose) polymerase‐1. J Neurosci 23:7967–7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han F, Drabek T, Stezoski J, Janesko‐Feldman K, Stezoski SW, Clark RS et al (2008) Protein nitration and poly‐ADP‐ribosylation in brain after rapid exsanguination cardiac arrest in a rat model of emergency preservation and resuscitation. Resuscitation 79:301–310. [DOI] [PubMed] [Google Scholar]
- 20. Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O (2006) Long‐term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr 136:1043–1047. [DOI] [PubMed] [Google Scholar]
- 21. Heo K, Cho YJ, Cho KJ, Kim HW, Kim HJ, Shin HY et al (2006) Minocycline inhibits caspase‐dependent and ‐independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci Lett 398:195–200. [DOI] [PubMed] [Google Scholar]
- 22. Hövels‐Gürich HH, Bauer SB, Schnitker R, Willmes‐von Hinckeldey K, Messmer BJ, Seghaye MC, Huber W (2008) Long‐term outcome of speech and language in children after corrective surgery for cyanotic or acyanotic cardiac defects in infancy. Eur J Paediatr Neurol 12:378–386. [DOI] [PubMed] [Google Scholar]
- 23. Hsia TY, Gruber PJ (2006) Factors influencing neurologic outcome after neonatal cardiopulmonary bypass: what we can and cannot control. Ann Thorac Surg 81:S2381–S2388. [DOI] [PubMed] [Google Scholar]
- 24. Jiang W, Desjardins P, Butterworth RF (2009) Cerebral inflammation contributes to encephalopathy and brain edema in acute liver failure: protective effect of minocycline. J Neurochem 109:485–493. [DOI] [PubMed] [Google Scholar]
- 25. Khalatbary AR, Ahmadvand H (2011) Anti‐inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran Biomed J 15:31–37. [PMC free article] [PubMed] [Google Scholar]
- 26. Kim SJ, Lee JH, Kim BS, So HS, Park R, Myung NY et al (2012) (‐)‐Epigallocatechin‐3‐gallate protects against NO‐induced ototoxicity through the regulation of caspase‐1, caspase‐3, and NF‐κB activation. PLoS ONE 7:e43967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ (2007) Gender‐specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab 27:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koivisto SP, Wistbacka JO, Rimpiläinen R, Nissinen J, Loponen P, Teittinen K, Biancari F (2010) Miniaturized versus conventional cardiopulmonary bypass in high‐risk patients undergoing coronary artery bypass surgery. Perfusion 25:65–70. [DOI] [PubMed] [Google Scholar]
- 29. Kong F, Chen S, Cheng Y, Ma L, Lu H, Zhang H, Hu W (2013) Minocycline attenuates cognitive impairment induced by isoflurane anesthesia in aged rats. PLoS ONE 8:e61385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lange M, Connelly R, Traber DL, Hamahata A, Nakano Y, Esechie A et al (2010) Time course of nitric oxide synthases, nitrosative stress, and poly(ADP ribosylation) in an ovine sepsis model. Crit Care 14:R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee H, Bae JH, Lee SR (2004) Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res 77:892–900. [DOI] [PubMed] [Google Scholar]
- 32. Nagai K, Jiang MH, Hada J, Nagata T, Yajima Y, Yamamoto S, Nishizaki T (2002) (−)‐Epigallocatechin gallate protects against NO stress‐induced neuronal damage after ischemia by acting as an anti‐oxidant. Brain Res 956:319–322. [DOI] [PubMed] [Google Scholar]
- 33. Nollert G, Möhnle P, Tassani‐Prell P, Uttner I, Borasio GD, Schmoeckel M, Reichart B (1995) Postoperative neuropsychological dysfunction and cerebral oxygenation during cardiac surgery. Thorac Cardiovasc Surg 43:260–264. [DOI] [PubMed] [Google Scholar]
- 34. Pua HL, Bissonnette B (1998) Cerebral physiology in paediatric cardiopulmonary bypass. Can J Anaesth 45:960–978. [DOI] [PubMed] [Google Scholar]
- 35. Sabir H, Bishop S, Cohen N, Maes E, Liu X, Dingley J, Thoresen M (2013) Neither xenon nor fentanyl induces neuroapoptosis in the newborn pig brain. Anesthesiology 119:345–357. [DOI] [PubMed] [Google Scholar]
- 36. Salvesen GS (2002) Caspases: opening the boxes and interpreting the arrows. Cell Death Differ 9:3–5. [DOI] [PubMed] [Google Scholar]
- 37. Schmitz T, Endesfelder S, Chew LJ, Zaak I, Bührer C (2012) Minocycline protects oligodendroglial precursor cells against injury caused by oxygen‐glucose deprivation. J Neurosci Res 90:933–944. [DOI] [PubMed] [Google Scholar]
- 38. Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks FA et al (2009) Green tea epigallocatechin 3‐gallate accumulates in mitochondria and displays a selective antiapoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal 11:469–480. [DOI] [PubMed] [Google Scholar]
- 39. Smith TG, Robbins PA, Ratcliffe PJ (2008) The human side of hypoxia‐inducible factor. Br J Haematol 141:325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Su XW, Undar A (2010) Brain protection during pediatric cardiopulmonary bypass. Artif Organs 34:E91–E102. [DOI] [PubMed] [Google Scholar]
- 41. Tomás‐Camardiel M, Rite I, Herrera AJ, de Pablos RM, Cano J, Machado A, Venero JL (2004) Minocycline reduces the lipopolysaccharide‐induced inflammatory reaction, peroxynitrite‐mediated nitration of proteins, disruption of the blood‐brain barrier, and damage in the nigral dopaminergic system. Neurobiol Dis 16:190–201. [DOI] [PubMed] [Google Scholar]
- 42. Twal M, Kiefer P, Salameh A, Schnabel J, Ossmann S, von Salisch S et al (2013) Reno‐protective effects of epigallocatechingallate in a small piglet model of extracorporeal circulation. Pharmacol Res 67:68–78. [DOI] [PubMed] [Google Scholar]
- 43. Walther T, Dhein S, Ullmann C, Schneider K, Bilz T, Rastan A et al (2013) Cerebral protection during controlled hypoperfusion in a piglet model: comparison of moderate (25°C) versus deep (18°C) hypothermia at various flow rates using intraoperative measurements and ex vivo investigation. Thorac Cardiovasc Surg 61:546–552. [DOI] [PubMed] [Google Scholar]
- 44. Wang X, Xue Q, Yan F, Li L, Liu J, Li S, Hu S (2013) Ulinastatin as a neuroprotective and anti‐inflammatory agent in infant piglets model undergoing surgery on hypothermic low‐flow cardiopulmonary bypass. Paediatr Anaesth 23:209–216. [DOI] [PubMed] [Google Scholar]
- 45. Ye H, Cande C, Stephanou NC, Jiang S, Gurbuxani S, Larochette N et al (2002) DNA binding is required for the apoptogenic action of apoptosis inducing factor. Nat Struct Biol 9:680–684. [DOI] [PubMed] [Google Scholar]
- 46. Yu SW, Andrabi SA, Wang H, Kim NS, Poirier GG, Dawson TM, Dawson VL (2006) Apoptosis‐inducing factor mediates poly(ADP‐ribose) (PAR) polymer‐induced cell death. Proc Natl Acad Sci U S A 103:18314–18319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zepeda AB, Pessoa A Jr, Castillo RL, Figueroa CA, Pulgar VM, Farías JG (2013) Cellular and molecular mechanisms in the hypoxic tissue: role of HIF‐1 and ROS. Cell Biochem Funct 31:451–459. [DOI] [PubMed] [Google Scholar]
- 48. Zhang TJ, Hang J, Wen DX, Hang YN, Sieber FE (2006) Hippocampus bcl‐2 and bax expression and neuronal apoptosis after moderate hypothermic cardiopulmonary bypass in rats. Anesth Analg 102:1018–1025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Original immunoblots of hypoxia‐inducible factor‐1α (HIF‐1α), apoptosis‐inducing factor (AIF), cleaved caspase 3 (cC3), poly‐ADP‐ribose (PAR) and nitrotyrosine. Lanes 1–3 indicate hippocampal lysates of three different control piglets.
Appendix S1. Immunoblots of hypoxia‐inducible factor 1‐alpha, apoptosis‐inducing factor, cleaved caspase 3, poly‐ADP‐ribose and nitrotyrosine.


