Abstract
Tissue fibrosis, or scar formation, is a common response to damage in most organs of the body. The central nervous system (CNS) is special in that fibrogenic cells are restricted to vascular and meningeal niches. However, disruption of the blood–brain barrier and inflammation can unleash stromal cells and trigger scar formation. Astroglia segregate from the inflammatory lesion core, and the so‐called “glial scar” composed of hypertrophic astrocytes seals off the intact neural tissue from damage. In the lesion core, a second type of “fibrotic scar” develops, which is sensitive to inflammatory mediators. Genetic fate mapping studies suggest that pericytes and perivascular fibroblasts are activated, but other precursor cells may also be involved in generating a transient fibrous extracellular matrix in the CNS. The stromal cells sense inflammation and attract immune cells, which in turn drive myofibroblast transdifferentiation. We believe that the fibrotic scar represents a major barrier to CNS regeneration. Targeting of fibrosis may therefore prove to be a valuable therapeutic strategy for neurological disorders such as stroke, spinal cord injury and multiple sclerosis.
Keywords: extracellular matrix, glia, (myo)fibroblasts, neuroinflammation, pericytes, platelet‐derived growth factor receptor
Introduction
A scar can be defined as a mark left (as on the skin) by the healing of damaged tissue. Scars are the consequence of failed tissue regeneration and represent a repair mechanism that replaces the normal tissue with extracellular matrix (ECM) consisting predominantly of fibronectin and type I collagen 24. The key effector cells in tissue remodeling and fibrosis are myofibroblasts that express α‐smooth muscle actin (SMA) and produce pathological ECM proteins 19. Recent genetic fate mapping studies revealed that resident fibroblasts and pericytes as well as circulating fibrocytes are the predominant sources of myofibroblasts in peripheral organs 32, 47, 74. In a mouse model of kidney fibrosis, 50% of myofibroblasts arise from local resident fibroblasts through proliferation, and the remaining nonproliferating myofibroblasts derive through differentiation from bone marrow (35%), endothelial‐to‐mesenchymal transition (10%) or epithelial‐to‐mesenchymal transition (5%) 46.
The central nervous system (CNS) is different in that resident astroglia have been regarded as the key effector cells in forming a dense scar in response to injury. The astroglial scar localizes at the lesion penumbra (Figure 1), confines inflammation to the lesion core and protects the intact neural tissue 22, 57. At the same time, the glial scar serves as a major barrier to regenerating axons by secreting growth‐inhibitory chondroitin sulfate proteoglycans 51. Interestingly, the glial scar is compartmentalized with resident astrocytes forming the periphery of the scar, and ependymal neural stem cell‐derived astrocytes forming the central part that provides an important source of neurotrophic factors 72. In addition, glial cells expressing chondroitin sulfate proteoglycan 4 (NG2) migrate toward the lesion and stabilize the regenerating front of dystrophic axons in the inhibitory environment of the glial scar 10.
Figure 1.
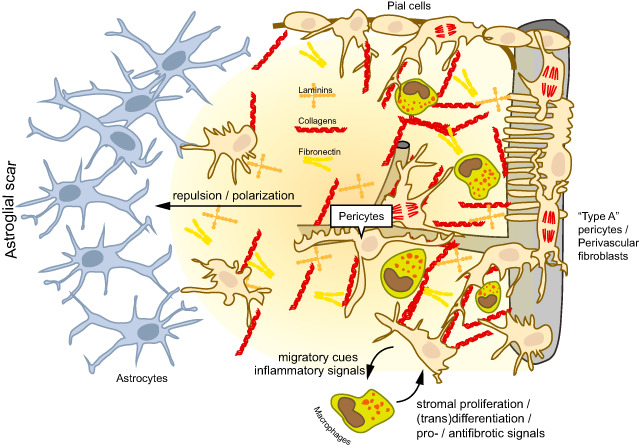
Cellular and molecular interactions in central nervous system (CNS) scar formation. The fibrotic scar is characterized by the deposition of extracellular matrix molecules that are otherwise scarcely expressed in the neural parenchyma such as collagens, laminins and fibronectin. These molecules are generated by stromal cells (myofibroblasts), which are normally absent from the CNS parenchyma. The stromal cells may originate from meningeal precursors (eg, pial cells of the leptomeningeal lining), perivascular fibroblasts or pericytes (“type A pericytes”). Blood‐borne macrophages and microglia contribute to the proliferation and differentiation of stromal cells by producing profibrotic mediators, and they are also involved in the resolution of the fibrous scar. Conversely, stromal cells modulate neuroinflammation by producing cytokines, chemokines and adhesion molecules. The astroglial scar is neatly separated from the fibrotic scar. Cellular and molecular constituents of the fibrotic scar induce the repulsion and polarization of astrocytes, and a new glia limitans is generated at the interphase of both cell populations.
In the lesion core, inflammatory cells interact with fibroblasts that form a dense fibrotic scar (Figure 1) composed of fibronectin, collagen and laminin 75. The fibroblasts segregate from astrocytes via bidirectional signaling between ephrin‐B2 on reactive astrocytes and EphB2 on meningeal fibroblasts 9. The fibrotic response is perhaps more detrimental in the CNS compared to other tissues because the brain's ECM normally contains relatively small amounts of fibrous proteins and adhesive glycoproteins 45. Instead, the neural interstitial matrix comprises a network of proteoglycans, hyaluronan, tenascins and link proteins. In addition, dense ECM aggregates form so‐called “perineuronal nets” during development and periods of synaptic maturation that are important regulators of CNS plasticity 44. Rather than purely providing mechanical support to the tissue, the ECM of the highly specialized CNS tissue serves as a substrate for the compartmentalization of the extracellular space, concentrating soluble and membrane‐bound molecules at synaptic sites, and functioning as a scaffold during development or adult neurogenesis 16. In this regard, a stiff fibrous ECM might impede the anatomical plasticity of the neural network that is required for learning and memory throughout life 102. Indeed, neural cells sense the stiffness of the microenvironment and adjust their differentiation accordingly, for example, “softer” microenvironments promote neuronal differentiation 36. There are more indications to suggest that the CNS parenchyma might represent a “fibro‐privileged” site. For instance, overexpression of transforming growth factor (TGF)‐β1 by astrocytes does not lead to parenchymal deposition of ECM molecules such as collagens, laminin or fibronectin, but the meninges and vascular vessel walls are thickened 100. In contrast, overexpression of TGF‐β1 or platelet‐derived growth factor (PDGF)‐B by hepatic cells causes increased deposition of fibrillar ECM molecules around hepatocytes as well as fibrosis in extrahepatic organs 13, 73. These results suggest that fibroblasts are absent from the normal neural parenchyma and are restricted to vascular and meningeal niches. Importantly, the basement membranes lining the cerebral microvasculature are composed of collagen IV, laminin, fibronectin and heparan sulfate proteoglycans. Three recent studies have underscored the importance of perivascular fibroblasts and pericytes, the mural cells that are embedded within the vascular basement membrane, in generating the fibrotic scar in the CNS 25, 27, 85.
Pericytes
First identified by Eberth and Rouget in the 19th century, pericytes are today considered to be a heterogenous population of mural cells. Because of the lack of specific markers and their plasticity in response to pathological conditions, the identification and fate mapping of pericytes have been challenging. We like to refer the reader to two excellent reviews on this topic 3, 42. Pericytes populate all segments of the brain vasculature with a continuum of phenotypes (Figure 2). Notably, the CNS has the highest density of pericytes in the body with a 3‐1:1 ratio between pericytes and endothelial cells 81. Pericytes in precapillary arterioles and capillaries have a round protruding cell body (∼10 to 15 μm) and extend longitudinal processes that trace the capillary endothelium. The longitudinal processes are not randomly placed but follow the endothelial cleft containing tight junctions, which underscores the important role of pericytes in the regulation of endothelial permeability 4, 76. In larger vessels, the vascular basement membrane and the basement membrane of the astrocytic glia limitans delimitate the perivascular (Virchow–Robin) space. These basement membranes are fused into a single structure in capillaries and fully enclose the pericyte 42. Besides pericytes, vascular smooth muscle cells (vSMCs) are part of the pre‐ and postcapillary vascular wall. Although there is no single marker to distinguish vSMCs from pericytes, there are important functional differences among vSMCs in arteries, the capillary pericytes, and pericytes/SMCs in venules. Obviously, different segments of the vasculature exert different functions, and the mural cells adapt to these particularities. For instance, venular pericytes lack expression of NG2 86, a proteoglycan involved in regulating endothelial permeability and adhesion of pericytes to endothelial cells 104. Currently, antibodies against platelet‐derived growth factor receptor (PDGFR)‐β, alanyl aminopeptidase (CD13), NG2, desmin and α‐SMA are used to stain pericytes in histological CNS samples 3. However, none of these markers are specific for pericytes, and the issue is complicated after CNS lesions when marker expression is dynamically regulated or shifts to other cell populations (eg, PDGFR‐β in fibroblasts, CD13 in macrophages, NG2 in glia and macrophages). Notably, ectoderm‐derived neural crest normally gives rise to pericytes and vSMCs in the CNS and thymus 21, 55, whereas mural cells in peripheral organs originate from mesoderm‐derived mesothelium 5, 71, 98. After cerebral ischemia, bone marrow‐derived cells have been reported to generate desmin‐positive pericytes in the brain by cell fusion 38, 66, but these findings remain controversial 25.
Figure 2.
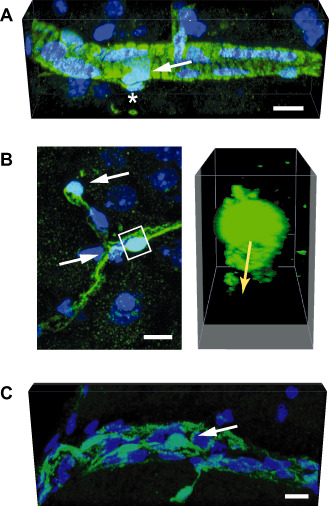
Mural cells of the central nervous system (CNS) vasculature. This figure demonstrates the different phenotypes of mural cells along different segments of the CNS vasculature. The mural cells can be identified based on their expression of the green fluorescent protein (GFP) in a mouse line where the pericyte‐specific rgs5 gene has been replaced with a GFP reporter 56. The images represent maximal projections or three‐dimensional (3D) renderings of confocal stacks obtained from GFP‐expressing cells in the brains of rgs5 GFP/ GFP mutant mice. Nuclei are counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI). Scale bars: 10 μm. A. In a precapillary arteriole, mural cells (arrow) exhibit robust and densely packed circumferential processes, suggesting a contractile phenotype. An abluminal GFP‐expressing cell is marked by an asterisk. B. Pericytes investing capillaries (white arrows in the left panel) show a typical protruding fusiform cell body with long primary processes running in the length of the capillary. The primary processes give rise to perpendicular secondary processes. The 3D reconstruction (right panel) of the volume marked by the white box demonstrates circumferential processes underneath the pericyte cell body that surround the capillary lumen (indicated by the yellow arrow). C. Mural cells (arrow) in a postcapillary venule show a flattened, stellate morphology and loosely cover the length of the vessel.
Other perivascular cells
In addition to these established cell types, other stromal cell populations have been identified in the perivascular spaces of the CNS. Using a mouse genetically engineered to express Cre recombinase under the control of the Glast promoter and originally generated to target astrocytes 84, Göritz et al discovered a perivascular cell type in the spinal cord that resides abluminal to other mural cells and gives rise to scar‐forming fibrotic cells 27. They termed these cells “type A pericytes” in order to distinguish them from the more abundant desmin‐expressing “type B pericytes” in capillaries that were not targeted in their animal model. Recently, another transgenic mouse line that expresses the green fluorescent protein (GFP) under the control of the collagen 1α1 promoter 103 was used to reveal a perivascular cell population that is associated with larger CNS blood vessels and also participates in the fibrotic response to spinal cord injury 85. It is unclear to what extent this population overlaps with “type A pericytes.” The perivascular cells described in these two publications share some markers of pericytes (PDGFR‐β, CD13) but lack others such as NG2, desmin or α‐SMA 27, 85. Pericytes in many organs, including the brain, have also been identified as multipotent mesenchymal stromal cells (MSCs) that can differentiate into various tissues such as muscle, bone, cartilage and adipose tissue 12. Moreover, MSCs isolated from the adult human brain express pericyte markers such as PDGFR‐β, CD13, NG2 or RGS5 62. Because the identification of MSCs relies on the in vitro assessment of their differentiation potential, it is not clear whether all pericytes or just a subset are multipotent. Hence, the mural and perivascular cell pool in the brain comprises heterogeneous cell populations, both in terms of marker expression and function, for which the broad term “pericyte” may be an oversimplification. Future work using genetic and imaging approaches to label and fate map distinct pericyte and perivascular cell populations will shed light on the diversity.
The fibrotic scar in the CNS
Scarring occurs after injury to any tissue in the body when regeneration fails. The evolutionary forces have likely shaped wound‐healing mechanisms to prevent infection, wall off foreign bodies and rapidly restitute missing tissue at the expense of optimal functioning 24. This can be exemplified in a number of neuropathological conditions where ectopic fibrous or laminar ECM is deposited.
Infection and parasite infestations
Bacteria, parasites and fungal infections of the CNS induce a robust and conserved fibrotic response that encapsulates affected tissue and may function to limit the spread of the pathogen. Bacterial abscesses, caused mainly by streptococcal strains and Staphylococcus aureus, are associated with rapid fibrotic wall formation within days after their seed, organized as a capsule containing myofibroblasts that secrete collagen I and fibronectin. These cells originate from bone marrow‐derived fibrocyte‐like cells and local precursors 2. Interestingly, the size of the capsule is reduced over time, which suggests tissue remodeling by myofibroblasts. A similar fibrotic wall formation occurs during infestation with parasites. For instance, larval stages of the Taenia solium tapeworm are rapidly encapsulated, generate little inflammatory reaction in the CNS and may therefore remain asymptomatic for years 26.
Spinal cord injury
Following the trauma, a large population of fibroblasts migrates to the lesion core, and forms a fibrotic scar with accessory glia limitans 78. It has long been assumed that fibroblasts would only enter the neural parenchyma after penetrating CNS injuries with disruption of the meningeal lining. However, this view has been challenged recently with the identification of collagen 1α1‐expressing PDGFR‐β+ perivascular fibroblasts that are distinct from pericytes and fibrocytes (negative for NG2 and CD11b), and give rise to the fibrotic scar after contusive, non‐penetrating spinal cord injury 85. Moreover, “type A pericytes” were identified in the CNS vasculature that proliferate locally, transform into myofibroblasts, and generate the fibrotic scar in response to dorsal funiculus incision or dorsal hemisection in mice 27. Notably, blocking the generation of progeny by this cell type results in failure to seal the injured CNS tissue, underscoring the importance of scar formation in rapidly replacing missing tissue.
Stroke
Recent findings from our laboratory suggest that PDGFR‐β+ stromal cells infiltrate the ischemic brain, and form a fibrotic scar surrounded by reactive astrocytes (Figures 3 and 4). Importantly, no fibrosis is detected in Alzheimer's disease brains despite substantial neurodegeneration and vascular pathology (Figure 4). Pericytes are rapidly lost after cerebral ischemia in both experimental and human stroke 25. Coincident with the pericyte loss is a massive proliferation of resident RGS5‐expressing PDGFR‐β+ CD13+ stromal cells that originate from the neurovascular unit, transform into α‐SMA+ CD105+ myofibroblasts and deposit fibrous ECM in the ischemic brain. No contribution of bone marrow‐derived cells is detected 25. In analogy to the constricting fibrotic capsules of brain abscesses or the healing of spinal cord lesions, the stroke‐induced fibrotic scar shrinks over time, resulting in the generation of cysts and the enlargement of the subarachnoid space.
Figure 3.
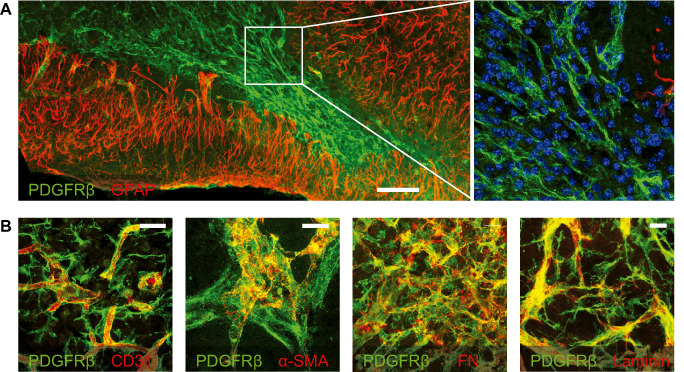
Fibrotic and glial scar after experimental stroke. Laser confocal microscopic images of immunostained mouse brain sections at 7 days after transient middle cerebral artery occlusion 25. Nuclei are counterstained with 4′,6‐diamidino‐2‐phenylindole (DAPI). Scale bars: (a) 100 μm, (b) 20 μm. A. The infarct core exhibits an abundant infiltrate of platelet‐derived growth factor receptor (PDGFR)‐β‐immunoreactive cells (green), which is surrounded by the glial fibrillary acidic protein (GFAP)‐immunoreactive astroglial scar (red). A high‐power magnification of the area marked by the white square is shown on the right and shows the inflammatory cell infiltrate. B. PDGFR‐β‐immunoreactive cells (green) detach from CD31‐immunoreactive endothelial cells, express α‐smooth muscle actin (α‐SMA) and fibronectin (FN), and juxtapose laminin‐immunoreactive extracellular matrix (ECM) deposits (all in red).
Figure 4.
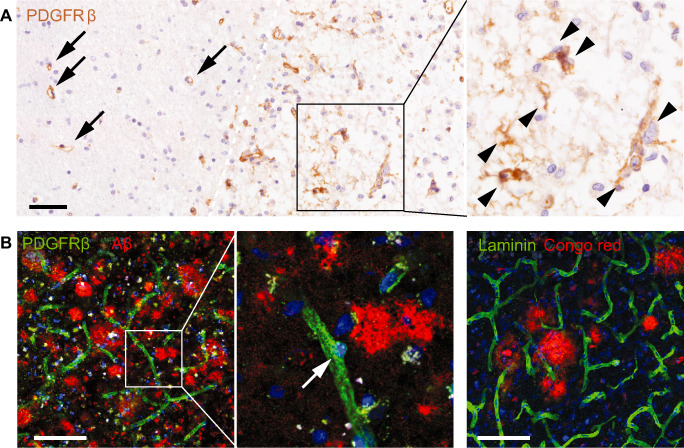
Fibrosis in human neurological disorders. Conventional and laser confocal microscopic images of immunostained human post‐mortem brain sections. Nuclei are counterstained with hematoxylin or 4′,6‐diamidino‐2‐phenylindole (DAPI). Scale bars: 100 μm. A. Platelet‐derived growth factor receptor (PDGFR)‐β‐immunoreactive cells (brown) in a subacute stroke lesion. PDGFR‐β‐immunoreactive cells are associated with vessels in the non‐ischemic tissue (arrows), but detach from the vasculature and spread into the parenchyma (arrowheads) in the ischemic tissue (right of the white dashed line). B. PDGFR‐β‐immunoreactive cells in Alzheimer's disease (left and middle panels). PDGFR‐β‐immunoreactive cells (green) remain at perivascular sites. There is no apparent proliferation of PDGFR‐β‐immunoreactive cells around amyloid plaques (red). In addition, no ectopic deposition of laminin (green) can be detected around plaques (red) in the Alzheimer's disease brain (right panel).
Scar‐free healing in the developing CNS
In contrast to the fibrotic scarring described previously, embryonic tissue is able to regenerate without scar formation 24. Only few adult vertebrates like primitive amphibians have retained this capacity, and even in these animals some tissues heal with a scar. It is not clear which factors determine the conversion to the scarring phenotype, but embryonic skin wounds that heal without a scar have very low levels of TGFβ1 and TGFβ2, whereas adult wounds contain predominantly TGFβ1 and PDGF, which are released by platelets and inflammatory cells 70, 97. In the rodent CNS, the change to mature scarring occurs around postnatal days 8–12 and is characterized by the deposition of collagen I–IV, astrocyte end‐feet alignment over a glia limitans, and the permanent presence of fibroblasts and macrophages in the core of the lesion 7, 50. Interestingly, fibrous scarring can be inhibited in the adult CNS by the transplantation of fetal tissue 43. The scarless bridging of traumatic tissue boundaries in the developing CNS is reminiscent of organisms like fish that do not develop any scarring in the CNS into adulthood 15.
Stromal cell/astrocyte interactions
When a fibrotic scar is generated in the CNS, a sharp boundary between the stromal tissue and the astroglial scar develops. Two compartments are generated: a fibrotic/inflammatory lesion core devoid of astrocytes, and an astroglial scar at the lesion penumbra 23, 35, 37, 83. This compartmentalization can also be reproduced in vitro 79. The astrocytes in the glial scar, particularly those bordering the fibrous scar, display a marked upregulation of the glial fibrillary acidic protein (GFAP) and form a mesh of highly overlapping hypertrophic cellular processes 95. Most of the astrocytes in the glial scar appear to proliferate locally and first adopt a radial or bipolar morphology oriented perpendicularly toward lesion 95. Selective deletion of STAT3 from astrocytes leads to fuzzy lesion borders, enhanced infiltration of inflammatory cells and increased neuronal loss 95. Astroglial scarring thus limits the spread of inflammation and marks the boundary of savaged neural tissue. The astroglial response appears to reproduce the development of a polarized neuroepithelial lining which culminates in the generation of the glia limitans. Notably, a glia limitans forms wherever astrocytes and meningeal cells come into contact 1. Along these lines, coculture of astrocytes with either inflammatory or fibromeningeal cells induces corralling of the meningeal cells into segregated clusters in a STAT3‐dependent manner 95. It is not clear how stromal and inflammatory cells contribute to the segregation of the fibrotic and glial scars in vivo. Interestingly, loss of the dystroglycan molecule on astrocyte foot processes at the glia limitans disturbs the binding of laminin and leads to basal lamina abnormalities as well as aberrant neuronal migration 53. Thus, ECM proteins in the fibrotic scar might contribute to restricting the expansion of neural cells into the lesion. Among the molecules involved in the confinement of astrocytes and the generation of a glia limitans, integrins play a major role as the most abundant membrane‐bound ECM receptors. Thus, deficiency of the integrin β1 subunit in astrocytes results in diffuse borders of the glial scar in vitro 64. Moreover, the ECM molecules fibronectin and laminin enhance the interleukin (IL)‐1β‐induced activation of astrocytes in an integrin‐dependent manner 88. Osteopontin is another ECM‐associated protein that is expressed by microglia and macrophages after ischemic or traumatic brain injury, and contributes to the formation of the gliotic barrier via binding to α(v)β3 integrin on astrocytes 20, 28. Last, cell contact‐mediated bidirectional signaling between ephrin‐B2 on reactive astrocytes and EphB2 on meningeal fibroblasts is an early event in the development of the glial scar and the exclusion of meningeal fibroblasts from the neural tissue (9).
Overall, it seems that fibroblasts in the lesion core together with inflammatory cells cooperate to shape the formation of the astroglial scar, partly by co‐opting molecular mechanisms of the developing CNS 48. It is important to note that pericytes in the normal CNS also seem to influence the structure of the glia limitans as their absence distorts the polarization of astrocytes in the CNS parenchyma 4. Hence, the assembly of the glia limitans might be a conserved function among CNS mural cells and their fibrogenic progeny in the injured brain.
Implications of fibrotic scarring in the CNS
In general, scar formation is considered to be a detrimental event after CNS injury because it poses a barrier for axon regrowth 23, 83. Indeed, stromal cells in the fibrotic core of brain and spinal cord lesions secrete the chemorepellent semaphorin III that exerts long‐lasting inhibitory effects on the outgrowth of injured CNS neurites 60, 61. Interestingly, vigorous regrowth of injured axons is observed in the absence of semaphorin III following early neonatal lesions in rats 61. After experimental stroke, Sema3A is induced in the ischemic core, but not in the glial scar, and impairs stroke recovery 63.
Some attempts have been made at inhibiting tissue fibrosis in the CNS. Thus, pharmacological inhibition of collagen IV production after lesion of the postcommissural fornix or spinal cord significantly improves axonal regeneration 37, 87. Moreover, inhibition of “type A pericyte” proliferation after spinal cord hemisection results in reduced ECM deposition, increased axonal regrowth across the scar tissue and improved functional recovery 27. However, this comes at the cost of failing to seal the injured tissue resulting in a large dehiscent wound 27.
In conclusion, more studies are needed to evaluate the functions of fibrotic scarring in the CNS, which are likely to be time and context dependent.
Factors mediating fibrosis in the CNS
In most organs, fibrosis is considered to be part of the inflammatory response. Protracted inflammation due to ongoing infectious or toxic tissue destruction causes excessive amounts of ECM deposition. Similarly, chronic inflammation in autoimmune diseases such as systemic sclerosis results in fibrosis. Hence, neuroinflammation needs to be considered as a potential driving force of fibrotic scarring in the CNS. Indeed, cytokines such as IL‐1β, IL‐6 and tumor necrosis factor (TNF)‐α that aggravate fibrosis in peripheral tissues 19 are also expressed by microglia or infiltrating inflammatory cells in the CNS. Macrophages in nonneural tissues adapt their function in response to pathogens, tissue damage and lymphocyte interactions. This process, termed polarization, enables the adaptive responses of innate immunity to take place 8. The appearance of alternatively activated macrophages that secrete anti‐inflammatory and profibrotic factors such as TGF‐β1 and PDGF has been associated with the accumulation of (myo)fibroblasts and the deposition of fibrillar ECM 54. At the same time, macrophages are also critical for the resolution of fibrosis 18, which is associated with the expression of IL‐13 receptor α2, IL‐10 and arginase 65, 99. Hence, different functional subsets of macrophages orchestrate different phases of fibrosis. The relative contribution of resident microglia and peripherally derived myeloid cells in this process remains to be explored 68.
Among the cytokines that act directly on fibroblasts or their precursors to induce a pro‐fibrotic phenotype are IL‐1β 34 and TGF‐β1. We have already mentioned the importance of members of the TGF‐β family for the conversion of embryonic scar‐free healing to fibrosis in the adult. TGF‐β1 is normally present at low levels in the adult CNS, with a constitutive expression in meningeal cells and some neuronal populations 89, 91. Chronic overexpression of TGF‐β1 by astrocytes only affects the meningeal lining and the microvascular compartment in transgenic mice, resulting in increased basement membrane deposition as well as degeneration of endothelial cells and pericytes 101. In cell culture, TGF‐β1 is produced at much higher levels by microglia than by astrocytes, and its production is regulated by vitronectin 96. Microglia, infiltrating monocytes/macrophages and reactive astrocytes are the major cellular sources of increased TGF‐β1 levels in CNS disorders such as ischemia, trauma and multiple sclerosis 17. After spinal cord injury, TGF‐β1 drives fibrotic scarring as demonstrated by the reduced deposition of fibrous scar tissue and attenuated limiting glial membrane formation after the administration of neutralizing TGF‐β1 antibodies 48. Type I and type II TGF‐β receptors are barely expressed in the intact brain, but gene expression is strongly induced in invading meningeal fibroblasts and much less in reactive astrocytes after penetrating injury to the striatum 39. The results suggest that fibroblasts, not reactive astrocytes, are a major target of TGF‐β1 that is upregulated after CNS injury. Pericytes also express type I and type II TGF‐β receptors, and TGF‐β1 induces ECM production and α‐SMA expression in cultured pericytes compatible with a myofibroblast phenotype 82, 90. Moreover, TGF‐β1 protects myofibroblasts from apoptosis 105, and acts as a chemoattractant for fibroblasts in vitro 67.
PDGF was originally recognized as a growth factor for glial cells, fibroblasts and vSMCs 29, 30. The family comprises four isoforms, namely PDGF‐A, ‐B, ‐C and ‐D, and two receptor chains, PDGFR‐α and ‐β 59. Pericytes and other mesenchymal cells in the vasculature and meninges express both PDGFR‐α and PDGFR‐β, as do proliferating stromal cells after CNS injury 25, 27. The interaction of ECM‐bound PDGF‐BB with PDGFR‐β is necessary for the proper attachment of pericytes to CNS blood vessels 4, 6, 14. During fibrosis, PDGFs act as potent proliferative, chemotactic and differentiation cues for myofibroblasts 59. Other important profibrotic growth factors such as connective tissue growth factor (CTGF) or fibroblast growth factor (FGF)‐2 are also induced in the injured CNS 31, 77. There is extensive cross talk with proinflammatory cytokines. For instance, TGFβ‐1 induces PDGFR‐α expression in fibroblasts 52. The cytokine IL‐1β also induces the expression of PDGFR‐α in fibroblasts, and IL‐13 promotes the autocrine release of PDGF‐AA 34. Importantly, PDGFR signaling is involved in pericyte activation, proliferation and differentiation into myofibroblasts during progressive kidney injury in mice 11. Pdgfb mRNA is induced in neurons and macrophages after experimental stroke 33, and Pdgfa mRNA is increased after lysolecithin‐induced spinal cord demyelination 31. Notably, Pdgfrb knockout mice exhibit larger infarct volumes, increased vascular leakage and reduced astroglial scar formation after cerebral ischemia 80.
Do stromal cells modulate neuroinflammation?
We have already stressed the importance of inflammation for driving fibrotic scarring in the CNS. Conversely, it is increasingly recognized that stromal cells play an active role during inflammation. Pericytes, arguably the most abundant stromal cell population in the quiescent CNS, might contribute to different stages of neuroinflammation.
Pericytes form a loose mesh in CNS venules, the main site for the diapedesis of inflammatory cells. In the inflamed cremaster muscle, leukocytes migrate along pericyte processes in the subendothelial space and later invade the parenchyma at specific locations dependent on pericyte‐expressed intercellular adhesion molecule (ICAM)‐1 and the leukocyte integrin ligands, Mac‐1 and LFA‐1 69. The transmigration sites coincide with regions of loose ECM in the basement membrane of venules 92, 94. The contraction of pericytes may influence the patency of these exit points for emigrating leukocytes 93. In the skin, capillary and arteriolar pericytes enhance the ability of extravasated neutrophils and macrophages to screen the interstitial space for damaged tissue and execute their effector functions by expressing ICAM‐1, toll‐like receptors and macrophage migration‐inhibitory factor (MIF) 86. The available data on CNS pericytes are more scarce, but it appears that cultured brain microvascular pericytes respond to lipopolysaccharide stimulation by producing a vast array of cytokines and chemokines 40. Moreover, pericytes activate a battery of immune response genes that initiate immune cell recruitment in the brains of conditional knock‐in mice with activating mutations at the Pdgfrb locus 58. Pericytes also give rise to (PDGFR‐β‐negative) follicular dendritic cells in ectopic tertiary lymphoid tissue in the context of chronic inflammation 41. Interestingly, chronic neuroinflammatory conditions such as some types of multiple sclerosis have been associated with the formation of tertiary lymphoid tissue at pial and perivascular sites 49. Altogether, it is clear that pericytes participate actively in inflammatory responses. Their specific impact on CNS disorders remains to be further explored.
Conclusion
The CNS shares more repair mechanisms with peripheral tissues than previously recognized. We believe that scar formation (fibrosis) is a conserved healing mechanism that has not received enough attention by neuroscientists in the past. Fibrosis is driven by neuroinflammation, and originates from resident stromal cell populations that have yet to be characterized in more detail. Our article leaves many questions unanswered: Is there a common origin of PDGFR‐β+ cells in different CNS pathologies? Is the fibrotic reaction necessary for the development of the astroglial scar? Do resident and peripherally derived inflammatory cells play different roles in fibrosis? What are the cues that provoke resolution and regression of the scar? Given the importance of fibrosis in many neurological disorders and the availability of novel antifibrotic therapies, the quest for answers to these issues appears very rewarding.
Acknowledgments
We wish to thank Josephine Radke and Werner Stenzel (Institute of Neuropathology, Charité—Universitätsmedizin Berlin) for providing histological samples of human stroke tissue, and Lasse Brandt (Laboratory of Molecular Psychiatry, Charité—Universitätsmedizin Berlin) for immunofluorescence stainings of human AD brain tissue. Our work was supported by grants from the DFG (FOR1336 and SFB/TRR43).
References
- 1. Abnet K, Fawcett JW, Dunnett SB (1991) Interactions between meningeal cells and astrocytes in vivo and in vitro . Brain Res Dev Brain Res 59:187–196. [DOI] [PubMed] [Google Scholar]
- 2. Aldrich A, Kielian T (2011) Central nervous system fibrosis is associated with fibrocyte‐like infiltrates. Am J Pathol 179:2952–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armulik A, Genové G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215. [DOI] [PubMed] [Google Scholar]
- 4. Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C et al (2010) Pericytes regulate the blood‐brain barrier. Nature 468:557–561. [DOI] [PubMed] [Google Scholar]
- 5. Asahina K, Zhou B, Pu WT, Tsukamoto H (2011) Septum transversum‐derived mesothelium gives rise to hepatic stellate cells and perivascular mesenchymal cells in developing mouse liver. Hepatology 53:983–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell RD, Winkler EA, Sagare AP, Singh I, Larue B, Deane R, Zlokovic BV (2010) Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berry M, Maxwell WL, Logan A, Mathewson A, McConnell P, Ashhurst DE, Thomas GH (1983) Deposition of scar tissue in the central nervous system. Acta Neurochir Suppl (Wien) 32:31–53. [DOI] [PubMed] [Google Scholar]
- 8. Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896. [DOI] [PubMed] [Google Scholar]
- 9. Bundesen LQ, Scheel TA, Bregman BS, Kromer LF (2003) Ephrin‐B2 and EphB2 regulation of astrocyte‐meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci 23:7789–7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Busch SA, Horn KP, Cuascut FX, Hawthorne AL, Bai L, Miller RH, Silver J (2010) Adult NG2+ cells are permissive to neurite outgrowth and stabilize sensory axons during macrophage‐induced axonal dieback after spinal cord injury. J Neurosci 30:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen YT, Chang FC, Wu CF, Chou YH, Hsu HL, Chiang WC et al (2011) Platelet‐derived growth factor receptor signaling activates pericyte‐myofibroblast transition in obstructive and post‐ischemic kidney fibrosis. Kidney Int 80:1170–1181. [DOI] [PubMed] [Google Scholar]
- 12. Crisan M, Yap S, Casteilla L, Chen C, Corselli M, Park T et al (2008) A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3:301–313. [DOI] [PubMed] [Google Scholar]
- 13. Czochra P, Klopcic B, Meyer E, Herkel J, Garcia‐Lazaro JF, Thieringer F et al (2006) Liver fibrosis induced by hepatic overexpression of PDGF‐B in transgenic mice. J Hepatol 45:419–428. [DOI] [PubMed] [Google Scholar]
- 14. Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood‐brain barrier integrity during embryogenesis. Nature 468:562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diaz Quiroz JF, Echeverri K (2013) Spinal cord regeneration: where fish, frogs and salamanders lead the way, can we follow? Biochem J 451:353–364. [DOI] [PubMed] [Google Scholar]
- 16. Dityatev A, Seidenbecher CI, Schachner M (2010) Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci 33:503–512. [DOI] [PubMed] [Google Scholar]
- 17. Dobolyi A, Vincze C, Pál G, Lovas G (2012) The neuroprotective functions of transforming growth factor Beta proteins. Int J Mol Sci 13:8219–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S et al (2005) Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest 115:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Duffield JS, Lupher M, Thannickal VJ, Wynn TA (2013) Host responses in tissue repair and fibrosis. Annu Rev Pathol 8:241–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ellison JA, Velier JJ, Spera P, Jonak ZL, Wang X, Barone FC, Feuerstein GZ (1998) Osteopontin and its integrin receptor alpha(v)beta3 are upregulated during formation of the glial scar after focal stroke. Stroke 29:1698–1707. [DOI] [PubMed] [Google Scholar]
- 21. Etchevers H, Vincent C, Le Douarin N, Couly G (2001) The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128:1059–1068. [DOI] [PubMed] [Google Scholar]
- 22. Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377–391. [DOI] [PubMed] [Google Scholar]
- 24. Ferguson MWJ, O'Kane S (2004) Scar‐free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 359:839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernández‐Klett F, Potas JR, Hilpert D, Blazej K, Radke J, Huck J et al (2013) Early loss of pericytes and perivascular stromal cell‐induced scar formation after stroke. J Cereb Blood Flow Metab 33:428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. García HH, Gonzalez AE, Evans CAW, Gilman RH, Cysticercosis Working Group in Peru (2003) Taenia solium cysticercosis. Lancet 362:547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J (2011) A pericyte origin of spinal cord scar tissue. Science 333:238–242. [DOI] [PubMed] [Google Scholar]
- 28. Hashimoto M, Koda M, Ino H, Yoshinaga K, Murata A, Yamazaki M et al (2004) Gene expression profiling of cathepsin D, metallothioneins‐1 and ‐2, osteopontin, and tenascin‐C in a mouse spinal cord injury model by cDNA microarray analysis. Acta Neuropathol 109:165–180. [DOI] [PubMed] [Google Scholar]
- 29. Heldin CH, Westermark B, Wasteson A (1979) Platelet‐derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A 76:3722–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heldin CH, Westermark B, Wasteson A (1981) Specific receptors for platelet‐derived growth factor on cells derived from connective tissue and glia. Proc Natl Acad Sci U S A 78:3664–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hinks GL, Franklin RJ (1999) Distinctive patterns of PDGF‐A, FGF‐2, IGF‐I, and TGF‐beta1 gene expression during remyelination of experimentally‐induced spinal cord demyelination. Mol Cell Neurosci 14:153–168. [DOI] [PubMed] [Google Scholar]
- 32. Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV et al (2010) Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 176:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iihara K, Sasahara M, Hashimoto N, Uemura Y, Kikuchi H, Hazama F (1994) Ischemia induces the expression of the platelet‐derived growth factor‐B chain in neurons and brain macrophages in vivo . J Cereb Blood Flow Metab 14:818–824. [DOI] [PubMed] [Google Scholar]
- 34. Ingram JL, Rice AB, Geisenhoffer K, Madtes DK, Bonner JC (2004) IL‐13 and IL‐1beta promote lung fibroblast growth through coordinated up‐regulation of PDGF‐AA and PDGF‐Ralpha. FASEB J 1810:1132–1134. [DOI] [PubMed] [Google Scholar]
- 35. Kawano H, Kimura‐Kuroda J, Komuta Y, Yoshioka N, Li HP, Kawamura K et al (2012) Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res 349:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keung AJ, Asuri P, Kumar S, Schaffer DV (2012) Soft microenvironments promote the early neurogenic differentiation but not self‐renewal of human pluripotent stem cells. Integr Biol (Camb) 4:1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klapka N, Hermanns S, Straten G, Masanneck C, Duis S, Hamers FP et al (2005) Suppression of fibrous scarring in spinal cord injury of rat promotes long‐distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci 22:3047–3058. [DOI] [PubMed] [Google Scholar]
- 38. Kokovay E, Li L, Cunningham LA (2006) Angiogenic recruitment of pericytes from bone marrow after stroke. J Cereb Blood Flow Metab 26:545–555. [DOI] [PubMed] [Google Scholar]
- 39. Komuta Y, Teng X, Yanagisawa H, Sango K, Kawamura K, Kawano H (2010) Expression of transforming growth factor‐beta receptors in meningeal fibroblasts of the injured mouse brain. Cell Mol Neurobiol 30:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kovac A, Erickson MA, Banks WA (2011) Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP‐1 expression in response to lipopolysaccharide. J Neuroinflammation 8:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D et al (2012) Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell 150:194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krueger M, Bechmann I (2010) CNS pericytes: concepts, misconceptions, and a way out. Glia 58:1–10. [DOI] [PubMed] [Google Scholar]
- 43. Krüger S, Sievers J, Hansen C, Sadler M, Berry M (1986) Three morphologically distinct types of interface develop between adult host and fetal brain transplants: implications for scar formation in the adult central nervous system. J Comp Neurol 249:103–116. [DOI] [PubMed] [Google Scholar]
- 44. Kwok JC, Dick G, Wang D, Fawcett JW (2011) Extracellular matrix and perineuronal nets in CNS repair. Dev Neurobiol 71:1073–1089. [DOI] [PubMed] [Google Scholar]
- 45. Lau LW, Cua R, Keough MB, Haylock‐Jacobs S, Yong VW (2013) Pathophysiology of the brain extracellular matrix: a new target for remyelination. Nat Rev Neurosci 14:722–729. [DOI] [PubMed] [Google Scholar]
- 46. LeBleu VS, Taduri G, O'Connell J, Teng Y, Cooke VG, Woda C et al (2013) Origin and function of myofibroblasts in kidney fibrosis. Nat Med 19:1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lin SL, Kisseleva T, Brenner DA, Duffield JS (2008) Pericytes and perivascular fibroblasts are the primary source of collagen‐producing cells in obstructive fibrosis of the kidney. Am J Pathol 173:1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A (1994) Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci 6:355–363. [DOI] [PubMed] [Google Scholar]
- 49. Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M et al (2007) Meningeal B‐cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 130:1089–1104. [DOI] [PubMed] [Google Scholar]
- 50. Maxwell WL, Duance VC, Lehto M, Ashurst DE, Berry M (1984) The distribution of types I, III, IV and V collagens in penetrant lesions of the central nervous system of the rat. Histochem J 16:1215–1229. [DOI] [PubMed] [Google Scholar]
- 51. McKeon RJ, Jurynec MJ, Buck CR (1999) The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci 19:10778–10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Messadi DV, Le A, Berg S, Huang G, Zhuang W, Bertolami CN (1998) Effect of TGF‐beta 1 on PDGF receptors expression in human scar fibroblasts. Front Biosci 3:a16–a22. [DOI] [PubMed] [Google Scholar]
- 53. Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A et al (2002) Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature 418:422–425. [DOI] [PubMed] [Google Scholar]
- 54. Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunology 11:723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Müller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N et al (2008) Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol 180:5344–5351. [DOI] [PubMed] [Google Scholar]
- 56. Nisancioglu MH, Mahoney WM, Kimmel DD, Schwartz SM, Betsholtz C, Genové G (2008) Generation and characterization of rgs5 mutant mice. Mol Cell Biol 28:2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834. [DOI] [PubMed] [Google Scholar]
- 58. Olson LE, Soriano P (2011) PDGFRβ signaling regulates mural cell plasticity and inhibits fat development. Dev Cell 20:815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ostendorf T, Eitner F, Floege J (2012) The PDGF family in renal fibrosis. Pediatr Nephrol 27:1041–1050. [DOI] [PubMed] [Google Scholar]
- 60. Pasterkamp RJ, De Winter F, Holtmaat AJ, Verhaagen J (1998) Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin‐1 in the regeneration of primary olfactory axons. J Neurosci 18:9962–9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pasterkamp RJ, Giger RJ, Ruitenberg MJ, Holtmaat AJ, De Wit J, De Winter F, Verhaagen J (1999) Expression of the gene encoding the chemorepellent semaphorin III is induced in the fibroblast component of neural scar tissue formed following injuries of adult but not neonatal CNS. Mol Cell Neurosci 13:143–166. [DOI] [PubMed] [Google Scholar]
- 62. Paul G, Özen I, Christophersen NS, Reinbothe T, Bengzon J, Visse E et al (2012) The adult human brain harbors multipotent perivascular mesenchymal stem cells. PLoS ONE 7:e35577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pekcec A, Yigitkanli K, Jung JE, Pallast S, Xing C, Antipenko A et al (2013) Following experimental stroke, the recovering brain is vulnerable to lipoxygenase‐dependent semaphorin signaling. FASEB J 27:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Peng H, Shah W, Holland P, Carbonetto S (2008) Integrins and dystroglycan regulate astrocyte wound healing: the integrin β1 subunit is necessary for process extension and orienting the microtubular network. Dev Neurobiol 68:559–574. [DOI] [PubMed] [Google Scholar]
- 65. Pesce JT, Ramalingam TR, Mentink‐Kane MM, Wilson MS, El Kasmi KC, Smith AM et al (2009) Arginase‐1‐expressing macrophages suppress Th2 cytokine‐driven inflammation and fibrosis. PLoS Pathog 5:e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Piquer‐Gil M, García‐Verdugo JM, Zipancic I, Sánchez MJ, Alvarez‐Dolado M (2009) Cell fusion contributes to pericyte formation after stroke. J Cereb Blood Flow Metab 29:480–485. [DOI] [PubMed] [Google Scholar]
- 67. Postlethwaite AE, Keski‐Oja J, Moses HL, Kang AH (1987) Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med 165:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Prinz M, Priller J (2014) Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci 15:300–312. [DOI] [PubMed] [Google Scholar]
- 69. Proebstl D, Voisin MB, Woodfin A, Whiteford J, D'Acquisto F, Jones GE et al (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo . J Exp Med 209:1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN et al (1995) Transforming growth factor‐beta 3 is required for secondary palate fusion. Nat Genet 11:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL (2008) Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci U S A 105:16626–16630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sabelström H, Stenudd M, Réu P, Dias DO, Elfineh M, Zdunek S et al (2013) Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science 342:637–640. [DOI] [PubMed] [Google Scholar]
- 73. Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L et al (1995) Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci U S A 92:2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Scholten D, Reichart D, Paik YH, Lindert J, Bhattacharya J, Glass CK et al (2011) Migration of fibrocytes in fibrogenic liver injury. Am J Pathol 179:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schreiber J, Schachner M, Schumacher U, Lorke DE (2013) Extracellular matrix alterations, accelerated leukocyte infiltration and enhanced axonal sprouting after spinal cord hemisection in tenascin‐C‐deficient mice. Acta Histochem 115:865–878. [DOI] [PubMed] [Google Scholar]
- 76. Schulze C, Firth JA (1993) Junctions between pericytes and the endothelium in rat myocardial capillaries: a morphometric and immunogold study. Cell Tissue Res 271:145–154. [DOI] [PubMed] [Google Scholar]
- 77. Schwab JM, Beschorner R, Nguyen TD, Meyermann R, Schluesener HJ (2001) Differential cellular accumulation of connective tissue growth factor defines a subset of reactive astrocytes, invading fibroblasts, and endothelial cells following central nervous system injury in rats and humans. J Neurotrauma 18:377–388. [DOI] [PubMed] [Google Scholar]
- 78. Shearer MC, Fawcett JW (2001) The astrocyte/meningeal cell interface—a barrier to successful nerve regeneration? Cell Tissue Res 305:267–273. [DOI] [PubMed] [Google Scholar]
- 79. Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM et al (2003) The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol Cell Neurosci 24:913–925. [DOI] [PubMed] [Google Scholar]
- 80. Shen J, Ishii Y, Xu G, Dang TC, Hamashima T, Matsushima T et al (2011) PDGFR‐β as a positive regulator of tissue repair in a mouse model of focal cerebral ischemia. J Cereb Blood Flow Metab 32:353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Shepro D, Morel N (1993) Pericyte physiology. FASEB J 7:1031–1038. [DOI] [PubMed] [Google Scholar]
- 82. Sieczkiewicz GJ, Herman IM (2003) TGF‐β1 signaling controls retinal pericyte contractile protein expression. Microvasc Res 66:190–196. [DOI] [PubMed] [Google Scholar]
- 83. Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156. [DOI] [PubMed] [Google Scholar]
- 84. Slezak M, Göritz C, Niemiec A, Frisén J, Chambon P, Metzger D, Pfrieger FW (2007) Transgenic mice for conditional gene manipulation in astroglial cells. Glia 55:1565–1576. [DOI] [PubMed] [Google Scholar]
- 85. Soderblom C, Luo X, Blumenthal E, Bray E, Lyapichev K, Ramos J et al (2013) Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. J Neurosci 33:13882–13887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Brühl ML et al (2013) Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and “instruct” them with pattern‐recognition and motility programs. Nat Immunol 14:41–51. [DOI] [PubMed] [Google Scholar]
- 87. Stichel CC, Hermanns S, Luhmann HJ, Lausberg F, Niermann H, D'Urso D et al (1999) Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur J Neurosci 11:632–646. [DOI] [PubMed] [Google Scholar]
- 88. Summers L, Kangwantas K, Nguyen L, Kielty C, Pinteaux E (2010) Adhesion to the extracellular matrix is required for interleukin‐1 beta actions leading to reactive phenotype in rat astrocytes. Mol Cell Neurosci 44:272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Unsicker K, Flanders KC, Cissel DS, Lafyatis R, Sporn MB (1991) Transforming growth factor beta isoforms in the adult rat central and peripheral nervous system. Neuroscience 44:613–625. [DOI] [PubMed] [Google Scholar]
- 90. Van Geest RJ, Klaassen I, Vogels IM, Van Noorden CJ, Schlingemann RO (2010) Differential TGF‐{beta} signaling in retinal vascular cells: a role in diabetic retinopathy? Invest Ophthalmol Vis Sci 51:1857–1865. [DOI] [PubMed] [Google Scholar]
- 91. Vincze C, Pál G, Wappler EA, Szabó ER, Nagy ZG, Lovas G, Dobolyi A (2010) Distribution of mRNAs encoding transforming growth factors‐beta1, ‐2, and ‐3 in the intact rat brain and after experimentally induced focal ischemia. J Comp Neurol 518:3752–3770. [DOI] [PubMed] [Google Scholar]
- 92. Voisin MB, Pröbstl D, Nourshargh S (2010) Venular basement membranes ubiquitously express matrix protein low‐expression regions: characterization in multiple tissues and remodeling during inflammation. Am J Pathol 176:482–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang S, Cao C, Chen Z, Bankaitis V, Tzima E, Sheibani N, Burridge K (2012) Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS ONE 7:e45499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang S, Voisin MB, Larbi KY, Dangerfield J, Scheiermann C, Tran M et al (2006) Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J Exp Med 203:1519–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray‐Thompson Z et al (2013) Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3‐dependent mechanisms after spinal cord injury. J Neurosci 33:12870–12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Welser‐Alves JV, Milner R (2013) Microglia are the major source of TNF‐α and TGF‐β1 in postnatal glial cultures; regulation by cytokines, lipopolysaccharide, and vitronectin. Neurochem Int 63:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Whitby DJ, Ferguson MW (1991) Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol 147:207–215. [DOI] [PubMed] [Google Scholar]
- 98. Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM (2005) The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132:5317–5328. [DOI] [PubMed] [Google Scholar]
- 99. Wilson MS, Elnekave E, Mentink‐Kane MM, Hodges MG, Pesce JT, Ramalingam TR et al (2007) IL‐13Ralpha2 and IL‐10 coordinately suppress airway inflammation, airway‐hyperreactivity, and fibrosis in mice. J Clin Invest 117:2941–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wyss‐Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM et al (1995) Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor‐beta 1. Am J Pathol 147:53–67. [PMC free article] [PubMed] [Google Scholar]
- 101. Wyss‐Coray T, Lin C, Sanan DA, Mucke L, Masliah E (2000) Chronic overproduction of transforming growth factor‐beta1 by astrocytes promotes Alzheimer's disease‐like microvascular degeneration in transgenic mice. Am J Pathol 156:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang G, Pan F, Gan WB (2010) Stably maintained dendritic spines are associated with lifelong memories. Nature 462:920–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M et al (2003) DNase I‐hypersensitive sites enhance alpha1(I) collagen gene expression in hepatic stellate cells. Hepatology 37:267–276. [DOI] [PubMed] [Google Scholar]
- 104. You WK, Yotsumoto F, Sakimura K, Adams RH, Stallcup WB (2014) NG2 proteoglycan promotes tumor vascularization via integrin‐dependent effects on pericyte function. Angiogenesis 17:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang HY, Phan SH (1999) Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol 21:658–665. [DOI] [PubMed] [Google Scholar]


