Abstract
Polypill is a fixed‐dose combination of medications with proven benefits for the prevention of cardiovascular disease (CVD). Its role in CVD prevention has been extensively debated since the inception of this concept in 2003. There are two major kinds of polypills in clinical studies. The first is polypill that combines multiple low‐dose medications for controlling only one CVD risk factor (such as high blood pressure or high serum cholesterol). These “single‐purpose” polypills were mostly developed from original producers and have higher cost. The polypill that combines 3‐4 pharmaceutical components, each with potential to reduce one major cardiovascular risk factors is “multi‐purpose” or “cardiovascular” polypill. Using data from various clinical trials and from meta‐analysis, Wald and Law claimed that this “cardiovascular” polypill when administered to every individual older than 55 years could reduce the incidence of CVD by more than 80%. Several short and intermediate to long‐term studies with different cardiovascular polypills in phase II and III trials showed that they could provide better adherence, equivalent, or better risk factor control and quality of life among users as compared to usual care. One recently published randomized controlled clinical trial demonstrated the effectiveness and safety of a four‐component polypill for both primary and secondary CVD prevention with acceptable number needed to treat (NNT) to prevent one major cardiovascular event. Considering the slow achievement of CVD prevention in many poor‐ and middle‐income Asian countries and also the need to further improve compliance of antihypertensive and lipid lowering medications in many high‐income Asian countries, the concept of “cardiovascular polypill” could be very useful. With further support from ongoing polypill cardiovascular outcome trials, polypill could be the foundation of the population‐based strategies for CVD prevention.
Keywords: Asian population, cardiovascular disease, polypill, primary prevention
1. INTRODUCTION
High blood pressure (BP) is the leading etiology of cardiovascular disease (CVD) and mortality worldwide. Approximately half of the global burden of CVD was reported to be located in Asia, 1 particularly in low‐, low‐middle‐, and upper‐middle‐income countries. 2 , 3 Socioeconomic inequalities are strong determinants of CVD risk and mortality globally. 4 , 5 Social determinants of health such as lack of health literacy, financial strain, inadequate housing conditions, lack of food scrutiny, and inadequate social support can affect treatment adherence and outcomes. 6 Unfortunately, these socioeconomic threats are common in many Asian countries.
In the year 2001, the World Health Organization and the Wellcome Trust had convened a meeting of experts to search evidence‐based and affordable interventions for non‐communicable disease. 7 A major discussion was the potential of a fixed‐dose combination polypill containing aspirin, statin, and blood pressure lowering agents, and there was a suggestion that “the use of a single pill could well encourage patients to adhere to treatment as well as seriously reduce the cost of the drugs.”
Originally, the idea of polypill focused on a large preventive effect at population level that would require intervention in almost everyone who was at increased cardiovascular risk irrespective of the levels of each risk factors. This polypill can be suitable for people at risk such as all individual over a defined age, without requiring risk factors to be properly measured or followed.
The concept of a multi‐purpose polypill to reduce CVD burden was taken up later by Wald and Law in 2003. 8 They did suggest that this polypill should contain six kinds of medicine (aspirin, statin, beta‐blocker, angiotensin‐converting enzyme inhibitor [ACEi], diuretic, and folic acid) and proposed that when widely applied to all persons above the age of 55 years could reduce CVD burden by 80%.
2. TYPE OF POLYPILLS
By definition, a polypill is a fixed‐dose combination of medications with proven benefits for the prevention of CVD. 8 The type of polypill that comprises of several pharmaceutical components, each lowering one of the major cardiovascular risk factors, is a “multi‐purpose polypill” or “cardiovascular polypill.”
Another kind of polypill that combines multiple low‐dose medication for the purpose of controlling only one risk factor (such as high BP, high blood glucose, or high cholesterol) is a “single‐purpose polypill.” The “single‐purpose” polypill or low fixed‐dose combination pill was originally designed to improve safety profile, given that side effects from these medications are mostly dose‐dependent, to improve personal medication adherence and to enhance treatment efficacy. The single‐purpose polypill that is most widely used is fixed‐dose combination of antihypertensive agents. Unfortunately, the fixed‐dose antihypertensive combinations were mostly introduced by producers of original pharmaceutical products and are higher priced than the separate use of its generic components (Table 1). The higher medication cost is one important factor of medication non‐adherence.
Table 1.
Types of polypills used in clinical trials
| Features | Single‐purpose polypill | Multi‐purpose (cardiovascular) polypill |
|---|---|---|
| Number of components | 2‐3 | 3‐4 |
| Targeted risk factor(s) | 1 | 3‐4 |
| Targeted population |
Individual Population with specific diagnosis |
General Clinically healthy population at moderate to high risk |
| Main purposes |
|
|
| Cost | High | Low |
| Quality | High | Unknown |
| Acceptability | Low‐moderate | Low |
3. FIXED‐DOSE SINGLE‐PILL COMBINATION OF ANTIHYPERTENSIVE AGENTS
In general, antihypertensive mono‐therapy is effective in achieving normal BP values in 30‐40% of patients with mild hypertension. In the past, most hypertension practice guidelines emphasized on initial use of different antihypertensive as mono‐therapy, and increasing the dose, or substituting for another mono‐therapy if necessary. However, increasing the dose of mono‐therapy produces little additional BP lowering and may increase the risk of side effects, whilst switching to another is time consuming and often ineffective.
A meta‐analysis of 42 studies concluded that combination therapy results in a greater reduction in BP compared with increasing the dose of a single drug, regardless of the class of drugs used in combination. 9 Initial combination therapy is invariably more effective at BP lowering than mono‐therapy. Low‐dose combination therapy is usually more effective than mono‐therapy used maximally, and the combination of any two medications from different antihypertensive drug classes was approximately 5 time more effective than doubling the dose of a single drug. 9
The combination of antihypertensive medications targeting multiple mechanisms, such as blocking the renin angiotensin system as well as inducing diuresis or vasodilatation, reduces the heterogeneity of the blood pressure responses to single medication. 10 Furthermore, a properly selected one component of combination therapy can reduce the occurrence of adverse events caused by the other component. In term of safety, two‐drug combinations have been shown to be safe and well tolerated, with only a small increase in the risk of hypotension, 9 even when given to patients with mild hypertension. 11
Even in patients well motivated to adhere to hypertension treatment plan, multiple medication regimens can be a significant barrier to full adherence. Single‐pill combination of antihypertensive agents can reduce pills burden and increase patients’ adherence.
An extensive list of antihypertensive combinations is available as single‐pill, fixed‐dose medications, most of which include an ACEi or an angiotensin receptor blocker (ARB) plus a diuretic or a calcium channel blocker (CCB).
Several retrospective studies have demonstrated that prescribing fixed‐dose combination pills can lead to increased adherence to antihypertensive treatment compared with the use multiple individual‐component pills. 12 , 13 , 14 , 15 , 16 , 17 Adherence or compliance rates ranged from approximately 60%‐70% for single‐pill combination drugs, which were much higher than for those prescribed the individual components.
A meta‐analysis of 15 studies, including 32 331 patients, reported a significant improvement in adherence to fixed‐dose combination of antihypertensive agents compared with free‐drug combinations and with a non‐significant beneficial trends in BP control and reduction of adverse effects. 18 Also, a second meta‐analysis of retrospective studies, published between 2000 and 2010, found that patients prescribed fixed‐dose antihypertensive combination therapy had significantly higher adherence compared with those prescribed free‐drug combinations. 19
4. FIXED‐DOSE SINGLE‐PILL COMBINATIONS FOR BP CONTROL IN ASIAN POPULATION
The clinical use of fixed‐dose single‐pill combinations for hypertension control may offer many advantages and potential to solve many challenges and barriers in the management of hypertension in many Asian countries. The advantages of increased efficacy can reduce the problem of therapeutic inertia in drug titration to optimal BP control. The benefits of decreased pill burden and potential reduction in adverse events can also help in improving medication adherence.
Single‐pill antihypertensive combinations have potential economic benefits to reduce health care costs compared with multiple‐pill therapies in long‐term consideration. 14 , 15 , 17
Indeed, this will cause a decreased expenditure to the health care system due to reduction in cardiovascular complications from better BP control. However, in many Asian countries, these single‐pill combinations, which initially entered the market as branded formulations, are still considerably more expensive than the generic forms of the individual drugs, so the initial cost of using them is a vital determinant of physician initiation decision. Their use has thus been restricted, especially in many resource‐limited countries in Asia, because the initial costs for both individual and health care system will be higher. 20 The cost factor is always a major barrier for the government in the acceptance of these formularies in their reimbursement lists for general population.
On 9 July 2019, WHO added fixed‐dose combination antihypertensive medications in the WHO Essential Medicines List. This decision is recommended by organizations with a shared goal of improving hypertension control worldwide and aligns with the recommendations for single‐pill combinations in many Asian hypertension treatment guidelines. All Asian countries, not only those of low‐income and middle‐income, must now implement policies that can enhance the access to single‐pill combinations for the patients who need them the most. They should include affordable single‐pill combinations in their essential drug lists, procure and promote sufficient supplies of quality‐assured, low‐cost single‐pill combinations, and encourage incorporation of single‐pill antihypertensive combinations into their national practice guidelines.
5. CHALLENGES IN USING FIXED‐DOSE, SINGLE‐PILL COMBINATIONS FOR BP CONTROL IN ASIA
Major reasons for the delayed acceptance of single‐pill combination for hypertension in many Asian countries are cost and availability. Other challenge is the problem of titrating the individual component within the combination pill. This problem is more significant among elderly patients, patients with comorbidities, and patients who already have to take many medications everyday. However, as drug patents begin to expire, more affordable ranges of generic combination pills with many dosing combinations will appear in Asian markets and this will help to alleviate the cost factor and help physicians in dose flexibility.
If possible, these single‐pill antihypertensive combinations should be in scored tablets, which allow one‐half or a quarter tablet dosing. This will produce a wide‐range titration possibility of just one pill, which could be started from very small dose and could be gradually up‐titrated to a higher dose by choosing from one‐quarter tablet to half and to full tablet. This practice will help in gaining more trust and confidence from the patients than changing from one type of pill to the other.
Regarding the quarter dose, quadruple fixed‐dose combination of antihypertensive drugs was designed specifically for initial treatment of hypertension, expecting fewer adverse effect anticipated by full‐dose single drug. Asian perspectives for this kind of drug were not defined yet. It could be more reassuring for reluctant patients in drug therapy for grade 1 hypertension when affordable. It also could facilitate earlier intervention of hypertension, especially in younger age group, which could be beneficial in reducing later complications such as cognitive impairment or heart failure.
One retrospective observational cohort study reported that prescriptions for single‐pill antihypertensive combinations carried a twofold increased risk of therapeutic duplication compared with the free combination ingredients prescribed separately. However, in this study the absolute risk of duplication was less than one percent and there was no apparent difference between the risk of drug‐drug interactions between single‐pill combination and free combination prescriptions. 21
6. CONCERNS ABOUT THE USE OF MULTI‐PURPOSE (CARDIOVASCULAR) POLYPILLS
The concept of multi‐purpose polypill had strong opposition from many in the scientific communities because, in addition to being unproven, there were concerns about the unknown consequences of giving medicines to the vast clinically healthy population. There were doubts about the balance of effectiveness and possible adverse reactions and the burdens of long‐term treatment in the so‐called healthy individuals. Another concern was the possibility of favoring unhealthy life habits in treated individuals, via the belief of living in a drug‐protected stated. However, in an individual participant data meta‐analysis of 3140 patients with previous CVD, polypill‐based care did not lead to neglect of lifestyle risk factors, at least among high‐risk patients. 22 There is no such study in healthy individuals.
7. CLINICAL RESEARCH ON MULTI‐PURPOSE, CARDIOVASCULAR POLYPILLS
In the last decade, there were development of many polypills, with varying constituents, and they have been tested in different clinical trials. The multi‐purpose polypills considered here for CVD prevention are mostly those with at least one antihypertensive medication in addition to aspirin and statin. These clinical trials compared multi‐purpose polypill to placebo or usual care, and some of the trials were performed in Asian population.
Participants in these clinical studies 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 were usually from low‐middle and upper‐middle‐income countries and from the underserved population in a high‐income country. 33 These studies had measured adherence rates, adverse events, and risk factors control with positive results. Most of these trials did not have enough power to test the impact of multi‐purpose fixed‐dose combination polypill on clinical outcomes except the recently published PolyIran trial. 34 These clinical trials are summarized in Table 2.
Table 2.
Studies with multi‐purpose polypills
| Study title (year) | Countries | Contents of polypills | Design of trials | Patients | Study groups | Study duration | Benefits of Polypills |
|---|---|---|---|---|---|---|---|
| TIPS‐1 23 (2009) | India | Atenolol, hydrochlorothiazide, ramipril, simvastatin, aspirin | Randomized, double‐blind | 2053 individuals without CVD with one risk factor | Polypill vs. usual care | 16 weeks | Non‐inferior to its individual components in lowering BP. LDL‐C reduction less than simvastatin alone |
| PILL 24 (2011) | Australia, Brazil, India, The Netherlands, New Zealand, UK, USA | Aspirin, hydrochlorothiazide, lisinopril, simvastatin | Randomized, double‐blind, placebo‐controlled | 378 individuals with 7.5% estimated 5‐year CVD risk | Polypill vs. placebo | 12 weeks | SBP and LDL‐C reduction Lowering of predicted cardiovascular risk |
| Wald et al 25 (2012) | UK | Amlodipine, losartan, hydrochlorothiazide, simvastatin | Randomized, double‐blind, placebo‐controlled | 86 patients ≥50 years with no CVD history | Polypill vs. placebo | 12 weeks | SBP and LDL‐C reduction |
| TIPS‐2 26 (2012) | India | Atenolol, hydrochlorothiazide, ramipril, simvastatin, aspirin | Randomized, double‐blind, 2 x 2, factorial controlled | 548 patients with previous CAD or diabetes | Single‐dose polypill plus placebo or two polypill capsules plus K+ | 8 weeks | Better reduction of BP and LDL‐C with 2 polypills |
| UMPIRE 27 (2013) | India, UK, Ireland, The Netherlands | Aspirin, lisinopril, simvastatin, and atenolol or hydrochlorothiazide | Randomized, open‐label, blinded end point | 2004 patients with CVD or at high risk | Polypill vs. usual care | 12‐24 months | Improved adherence, SBP, and LDL‐C |
| IMPACT 28 (2014) | New Zealand | Aspirin, lisinopril, simvastatin, and atenolol or hydrochlorothiazide | Randomized, open‐label | 513 high‐risk patients | Polypill vs. usual care | At least 12 months | Better adherence to four drugs |
| FOCUS Phase II 29 (2014) | Italy, Spain, Argentina, Paraguay | Aspirin, ramipril, simvastatin | Randomized, open‐label, active‐controlled | 695 post‐MI patients | Polypill vs. usual care | 9 months | Better adherence |
| TEMPUS 30 (2015) | The Netherlands | Aspirin, hydrochlorothiazide, lisinopril, simvastatin | Randomized, open, blinded end point, three‐period crossover | 78 patients with CVD | Morning polypill vs. evening polypill vs. usual care | 3‐6 weeks per period |
Eventing polypill had better LDL‐C reduction. Polypill had higher adherence and preference |
| Kanyini‐GAP 31 (2015) | Australia | Aspirin, lisinopril, simvastatin, and atenolol or hydrochlorothiazide | Randomized, open label | 623 patients with CVD or high risk | Polypill vs. usual care | 12‐34 months | Greater use of combination treatment |
| SPACE 32 (2016) | IMPACT, UMPIRE and Kanyini‐GAP | IMPACT, UMPIRE, and Kanyini‐GAP | Meta‐analysis | 3140 patients from 3 studies | Polypill vs. usual care | 12 months | Higher adherence, lower SBP, lower LDL‐C |
| Munoz D, et al 33 (2019) | USA | Aspirin, amlodipine, losartan, hydrochlorothiazide | Randomized, open | 303 individuals mean 10‐year risk 12.7% | Polypill vs. usual care | 12 months | Lower SBP and LDL‐C |
| PolyIran 34 (2019) | Iran | Aspirin, atorvastatin, hydrochlorothiazide, enalapril or valsartan | Clustered randomized | 6838 individual older than 50 years with/without CVD | Polypill vs. usual care | 60 months | Reduction of major cardiovascular event |
Abbreviations: BP, blood pressure; CVD, cardiovascular disease; FOCUS, Fixed‐Dose Combination Drug for Secondary Cardiovascular Prevention; IMPACT, IMProving Adherence using Combination Therapy; LDL‐C, low‐density lipoprotein cholesterol; PILL, Programme to Improved Life and Longevity; SBP, systolic blood pressure; SPACE, Single Pill to Avert Cardiovascular Events; TIPS, The International Polycap Study; UK, United Kingdom; UMPIRE, Use of a Multidrug Pill In Reducing Cardiovascular Events; USA, United Stated of America.
The PolyIran Study 34 aimed to assess the effectiveness and safety of a four‐component polypill, which consisted of aspirin, atorvastatin, hydrochlorothiazide, and either enalapril or valsartan, for both primary and secondary prevention of CVD. This two‐group, pragmatic, cluster‐randomized trial enrolled 6,838 individuals older than 50 years into the study. Clusters (villages) were randomly allocated (1:1) to either a package of non‐pharmacological preventive interventions alone (minimal care group) or together with a once‐daily polypill tablet (polypill group). There were 10.7% of pre‐existing CVD in both study groups, and 79.8% of these participants were using cardiovascular drugs at baseline.
Median adherence to polypill tablets was 80.5%. During the 60‐month follow‐up, 8.8% of 3417 participants in the minimal care group had major cardiovascular events (hospitalization for acute coronary syndrome, fatal myocardial infarction, sudden death, heart failure, coronary revascularization, and stroke) compared with 5.9% of 3421 participants in the polypill group (adjusted hazard ratio 0.66, 95% confidence interval 0.55‐0.80). When restricted to participants in the polypill group with high adherence, the reduction in the risk of major adverse cardiovascular events was even greater compared with the minimal care group. 34
To our knowledge, this is the first large‐scale, long‐term, randomized trial to prove the efficacy of a fixed‐dose cardiovascular polypill therapy. The significant reduction in major cardiovascular event risk demonstrated in the polypill group could translate into an acceptable number needed to treat (NNT) to prevent one major cardiovascular event particularly in population with high adherence.
8. PROS AND CONS OF MULTI‐PURPOSE, CARDIOVASCULAR POLYPILLS
The benefits of multi‐purpose fixed‐dose combination pills that have been proven include better adherence in short‐ and intermediate‐term studies, equivalent or better BP and LDL‐C control and probably better quality of life among combination pills user. Also, there is high possibility that polypill can be useful for reduction of cardiovascular events in both primary and secondary CVD prevention when added to the usual care with favorable NNT. 34
The polypill limitations may include difficulty with dose adjustment to targets, fear of unnecessary medication use, and too low BP or too low LDL‐C thus may preclude the acceptance among physicians. For the problem with dose adjustment, it is important to emphasize that, use of the polypill is not a contraindication to individualized, add‐on therapies for residual elevations in BP or LDL‐C levels, as judged by a physician. For the treatment targets, with many confirmed positive results from various more aggressive BP‐lowering and LDL‐C‐lowering clinical trials, most well‐accepted national and international treatment guidelines are now moving toward both lower BP and LDL‐C treatment targets. 35 , 36 Some have shifted to more risk‐based treatment with lower treatment thresholds for both BP and LDL‐C. 37 All these trends will reduce concerns about the harmful effects of too low BP and LDL‐C in polypill users. When considered as potential target for polypill, the moderate‐ to high‐risk individuals would have very small chance that their LDL‐C or BP would become too low, according to all these new guidelines.
9. SUGGESTED COMPONENTS IN A CARDIOVASCULAR POLYPILL FOR ASIAN POPULATION
All previous studies with multi‐purpose polypills 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 (Table 2) use 1 to 3 antihypertensive agent(s) in small‐to‐moderate dose together with a statin, some of the trials add aspirin 75‐100 mg into their polypill composition.
Important features of CVD in Asia include the high prevalence of stroke (especially hemorrhagic stroke) and non‐ischemic heart failure as complications of hypertension. There is also a stronger association between increasing BP and rates of CVD development. Another specific feature is higher salt sensitivity.
When considering the antihypertensive component(s) in a polypill for an Asian population, a small‐dose dihydropyridine calcium channel blocker (DHP‐CCB) should be first considered, follow by an ARB. The preferred antihypertensive agents in Asia are chosen based upon their ability to reduce BP effectively. DHP‐CCBs are widely prescribed for the management of hypertension in Asians. 38 Evaluation of data from Eastern Asians showed that reductions in 24‐h BP were greater with CCBs. 39 Data from Thailand showed that response rate to antihypertensive mono‐therapy was greatest for CCBs. 40 The second most frequently prescribed antihypertensive medications in Asia are the renin angiotensin aldosterone system (RAAS) inhibitors (ACEi or ARB). Since ARBs are better tolerated than ACEis, which have a higher incidence of dry cough in Asian population, 41 , 42 , 43 , 44 this preference supports ARBs as the second component of antihypertensive medication in a polypill after a DHP‐CCBs. The third component can be chosen from a beta‐blocker or hydrochlorothiazide. At the present time, beta‐blockers do not remain as one of the first‐line antihypertensive agents in many national and international guidelines, but they are still one of the most frequently prescribed agents in almost every Asian countries. 38
Hydrochlorothiazide may be a more potent BP‐lowering agent when compared with a beta‐blocker, 40 but in many hot countries in Asia the side effects from electrolytes imbalance are frequently encountered. This could explain why most physician in Asia prefer using beta‐blockers than diuretics. 38 If a diuretic is considered in a polypill, it should be a thiazide‐like diuretic, not a furosemide, and should be used only in small dose.
A generic, low‐cost, moderate‐intensity statin is also required in multi‐purpose cardiovascular polypill for Asians.
Aspirin use for primary cardiovascular disease prevention in individuals without CVD was associated with a lower risk of cardiovascular events but with an increased risk of major bleeding. 45 From the concern of major bleeding risk, the new Japanese Society of Hypertension Guidelines (JSH 2019) recommends the office BP target of <130/80 mm Hg for patients using antithrombotic drugs. 46 Consideration for using aspirin routinely for primary CVD prevention in underserved Asian population may have more harm than benefit because of very poor BP control in this specific population, and aspirin can have a significant gastrointestinal side effects even with low dose.
Data from the CSPPT (China Stroke Primary Prevention Trial) demonstrated a significant risk reduction of first stroke in hypertensive patients treated with enalapril plus folic acid compared with those with enalapril alone. This randomized, double‐blind clinical trial enrolled 20 702 hypertensive Chinese adults without history of stroke or myocardial infarction. The primary outcome which was first stroke occurrence (ischemic or hemorrhagic) was reduced by 21% after a mean follow‐up of 4.5 years favoring the enalapril‐folic acid group. 47 And based on the CSPPT data, the investigators estimated patient‐level lifetime stroke‐free survival for the enalapril‐folic therapy and projected that it will have a modest lifetime stroke‐free survival gain in overall sample. 48 Based on these data, adding folic acid as a component of cardiovascular polypill for the population that have high stroke risk such as Asians may be considered.
The suggested components for a cardiovascular polypill for Asian population are demonstrated in Figure 1. There are two types of multi‐purpose polypill that we suggest, the one with a diuretic and the one with a beta‐blocker. If the polypill with diuretic is preferred, then the dose of a thiazide‐like diuretic should be small, and if the one with a beta‐blocker is preferred, we suggest using a longer acting beta‐blocker at low‐to‐moderate dose. These polypills are designed only for the propose of primary CVD prevention.
Figure 1.
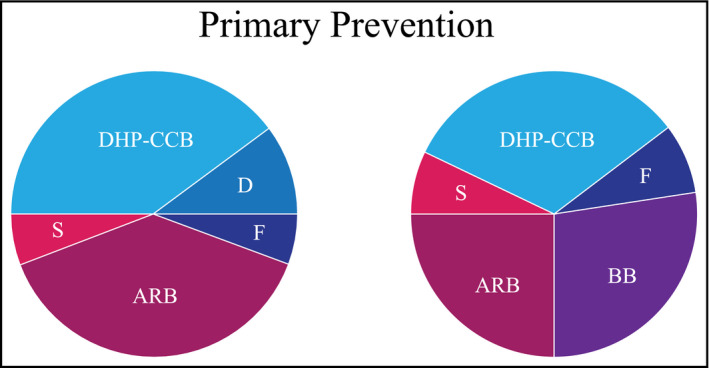
The suggested components in a cardiovascular polypill for Asian population. The areas in each segment represent the suggested dose of each medications. ARB, angiotensin receptor blocker; BB, beta‐blocker; D, thiazide‐type diuretic; DHP‐CCB, dihydropyridine calcium channel blocker; F, folic acid; S, statin
10. PHARMACOLOGICAL STANDPOINT
From a technical perspective, the inclusion of different compounds into a polypill must take in account a number of problems, which are the compatibility of chemical, stability, and pharmacokinetic properties of each component vs. the others. 49 , 50 The heat and humidity, which characterize the environments of many Asian countries, can reduce the availability as well as influence pharmacokinetic and pharmacodynamic properties of polypill contents. Even if the original pharmacological characteristics of the drugs could be retained, care should also be taken to combine drugs with complementary mechanisms. Duration of action of each polypill component should also be similar to ensure a balanced effect throughout the between‐dose interval, for example, a short‐acting beta‐blocker should not be combined with a long‐acting DHP‐CCB. These problems certainly will increase with the increasing number of drug components in a polypill.
The polypill use in the study of Munoz and coworkers 33 consisted of four low‐dose medications: atorvastatin (10 mg), amlodipine (2.5 mg), losartan (25 mg), and hydrochlorothiazide (12.5 mg). Generic versions were placed securely in sealed gelatin capsules and cost 26 US dollars per month per participant. In this trial, the participants were reminded that their physicians were free to implement any additional therapies that were deemed to be appropriate at any visit. From this example, one might argue about the duration of action of amlodipine, which was different from losartan and whether the timing of drug consumption should be in the evening (better for statin) or in the morning (as for most antihypertensive agents). Also, the cost per month of this polypill may still be unaffordable for many poor Asian communities and might even be more expensive than all generic components separately prescribed.
11. CURRENT AND FUTURE PERSPECTIVES
Implementation of pharmacologic measures to prevent CVD can be different between various levels of socioeconomic settings. The traditional strategy, which is mostly endorsed in most major guidelines, is to identify high‐risk individuals on the basis of clinical prediction algorithms. This risk‐based strategy has some drawbacks because it may be difficult in implementation, owing to the cost of laboratory tests, and requirement of several hospital or clinic visits before making treatment decision. Besides this limitation, there are still challenges about the sensitivity of this strategy in detecting these so‐called high‐risk persons. It had been shown that many persons who developed cardiovascular events were previously classified by conventional algorithms as being at low or intermediate risk before the index events. 51 , 52 In contrast, a population‐based strategy focuses on shifting the entire risk distribution in a specified population by means of a broadly applied, low‐cost intervention that has relatively few side effects such as a cardiovascular polypill.
The observed reduction in BP and LDL‐C, in most of the clinical trials using cardiovascular polypills, were statistically and clinically significant although they were still higher than optimal targets. On the basis of meta‐analysis of cardiovascular outcome trials in primary prevention, such changes, if sustained, could lead to a significant reduction in the incidence of cardiovascular events. The PolyIran study is the first clinical trial that proved the benefits of the cardiovascular polypill concept in reducing major clinical cardiovascular outcomes. 34
An ongoing secondary prevention trial using a polypill is the SECURE (secondary prevention of cardiovascular disease in the elderly) study. 53 This multicenter, randomized study is designed to evaluate the polypill (aspirin 100 mg, ramipril 2.5‐10 mg, and atorvastatin 40 mg) as a valid comprehensive strategy for secondary cardiovascular prevention. SECURE study will enroll 3206 elderly patients with recent myocardial infarction (MI), stroke or coronary revascularization from several European countries. Polypill will be compared with usual care, and the benefit will be assessed through the reduction in major cardiovascular events including revascularization over a minimum period of 2 years.
The other ongoing study in large patient population is TIPS‐3 (The International Polycap Study‐3). 54 TIPS‐3 is a purely a primary prevention trial in medium‐ to high‐risk individuals. The study employs a randomized 2 x 2 x 2 factorial, placebo‐controlled design testing whether a polypill (hydrochlorothiazide 25 mg, atenolol 100 mg, ramipril 10 mg, and simvastatin 40 mg) compared to placebo will lead to prevention of cardiovascular death, stroke and MI in male participants aged over 55 years and female participants aged over 60 years and INTERHEART risk score above 10. In addition, participants will be randomized to receive aspirin (75 mg) and vitamin D (60 000 IU monthly). This factorial design on 3 distinct treatment arms, which could also reduce fractures and cancers, could have large implications for the prevention of other important chronic disease as well as CVD.
The data supporting cardiovascular outcome for polypill as a secondary CVD prevention is small. Until we have more data from TIPS‐3, we do not support using a polypill to replace the usual treatment plan for secondary prevention, which, we believe, should be more individualized.
In the future, a cardiovascular polypill may be customized into a broader category such as for primary or secondary CVD prevention, for persons with medium or high risk. The polypill for secondary prevention among stroke patients may be slightly different from patients who survived MI.
12. CONCLUSIONS
In the past decade, the concept of polypill has emerged and progressed from a publicized concept to attaining acceptability. Most available studies favor polypill in terms of improving adherence and reducing cardiovascular risk burden of high blood pressure and high LDL‐C.
The more widely accepted polypill is the single‐purpose polypill for improving adherence and for better BP control. The drawback for this kind of polypill is the higher cost and poor accessibility in many Asian countries.
Challenging the rather slow development of cardiovascular disease prevention in many poor‐ and middle‐income Asian countries and poor medication compliance in many high‐income Asian countries, the concept of tailor‐designed cardiovascular polypill for general population with at least moderate cardiovascular risk could be a game changer. In the era of moving toward to a lower BP and LDL‐C targets, the concerns of overtreatment would become less.
With growing support from the results of cardiovascular outcome trials, polypill could be the foundation of the population‐based strategies for cardiovascular disease prevention. The availability of easily accessible low‐cost polypill in all Asian countries could be a good opportunity for these countries to achieve the UN sustainable development goal to reduce premature CVD mortality by at least a third before the year 2030.
CONFLICT OF INTERESTS
YC Chia has received honoraria and sponsorship to attend conferences and CME seminars from Abbott, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Merck Sharp & Dohme, Novartis, Orient Europharma, Pfizer, and Sanofi; and a research grant from Pfizer. JG Wang has received research grants from Bayer, Merck Sharp & Dohme, and Omron; and lecture and consulting fees from Astra‐Zeneca, Lee's Pharm, Novartis, Servier, and Takeda. J Nailes has received research grants from Pfizer. J Shin has received honoraria and sponsorship to attend seminars from Daiichi Sankyo, Takeda, Menarini, MSD, Bristol‐Myers Squibb, and Sanofi. BW Teo consulted for Servier, MSD, Astellas, AstraZeneca, and Boehringer Ingelheim. S Siddique has received honoraria from Bayer, Novartis, Pfizer, ICI, and Servier; and travel, accommodation, and conference registration support from Hilton Pharma, Atco Pharmaceutical, Highnoon Laboratories, Horizon Pharma and ICI. J Sison has received honoraria from Pfizer, AstraZeneca, AmGen, Boehringer Ingelheim, and Novartis. GP Sogunuru has received a research grant related to hypertension monitoring and treatment from Pfizer. Y Zhang has received research grants from Bayer, Novartis, and Shuanghe; and lecture fees from Bayer, Daiichi Sankyo, Novartis, Pfizer, Sanofi, Servier, and Takeda. TD Wang has received honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Medtronic, Menarini, Novartis, Omron, Pfizer, Sanofi, and Servier. S Park has received honoraria from Pfizer, Daiichi Sankyo, Takeda, Daewon pharmaceutical company, Boryung pharmaceutical company, and Servier. CH Chen has served as an advisor or consultant for Novartis Pharmaceuticals Corporation; has served as a speaker or a member of a speakers bureau for AstraZeneca; Pfizer Inc; Bayer AG; Bristol‐Myers Squibb Company; Boehringer Ingelheim Pharmaceuticals, Inc; Daiichi Sankyo, Inc; Novartis Pharmaceuticals Corporation; SERVIER; Merck & Co., Inc; Sanofi; TAKEDA Pharmaceuticals International; and has received grants for clinical research from Microlife Co., Ltd. K Kario received research grant from MSD KK, Astellas Pharma Inc, Eisai Co., Otsuka Pharmaceutical Co., Sanwa Kagaku Kenkyusho Co., Daiichi Sankyo Co., Taisho Pharmaceutical Co., Ltd, Sumitomo Dainippon Pharma Co., Takeda Pharmaceutical Co., Teijin Pharma, Boehringer Ingelheim Japan Inc, Bristol‐Myers Squibb KK, Mochida Pharmaceutical Co., and honoraria from Daiichi Sankyo Co. Ltd, Mylan EPD. All other authors report no potential conflicts of interest in relation to this article.
Sukonthasarn A, Chia Y‐C, Wang J‐G, et al. The feasibility of polypill for cardiovascular disease prevention in Asian Population. J Clin Hypertens. 2021;23:545–555. 10.1111/jch.14075
[Correction added on February 9, 2021, after first online publication: The copyright line was changed.]
REFERENCES
- 1. Ohira T, Iso H. Cardiovascular disease epidemiology in Asia: an overview. Circ J. 2013;77:1646‐1652. [DOI] [PubMed] [Google Scholar]
- 2. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371:1513‐1518. [DOI] [PubMed] [Google Scholar]
- 3. He J, Gu D, Chen J, et al. Premature deaths attributable to blood pressure in China: a prospective cohort study. Lancet. 2009;374:1765‐1772. [DOI] [PubMed] [Google Scholar]
- 4. Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137:2166‐2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khaing W, Vallibhakara SA, Attia J, et al. Effects of education and income on cardiovascular outcomes: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2017;24:1032‐1042. [DOI] [PubMed] [Google Scholar]
- 6. Malambo P, Kengne AP, De Villiers A, et al. Built Environment, selected risk factors and major cardiovascular disease outcomes: a systematic review. PLoS One. 2016;11:e0166846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Secondary prevention of non‐communicable disease in low and middle income countries through community‐based and health service interventions. World Health Organization‐Wellcome Trust meeting. Geneva: World Health Organization; 2002.
- 8. Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ. 2003;326:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wald DS, Law M, Morris JK, et al. Combination therapy versus monotherapy in reducing blood pressure: meta‐analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290‐300. [DOI] [PubMed] [Google Scholar]
- 10. MacDonald TM, Williams B, Webb DJ, et al. Combination therapy is superior to sequential monotherapy for the initial treatment of hypertension: a double‐blind randomized controlled trial. J Am Heart Assoc. 2017;6:e006986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yusuf S, Lonn E, Pais P, et al. HOPE‐3 Investigators. Blood‐pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med. 2016;374:2032‐2043. [DOI] [PubMed] [Google Scholar]
- 12. Dezii CM. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care. 2000;9:2‐6. [PubMed] [Google Scholar]
- 13. Brixner DI, Jackson KC, Sheng X, et al. Assessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide: retrospective cohorts in free‐and fixed‐dose combinations. Curr Med Res Opin. 2008;24:2597‐2607. [DOI] [PubMed] [Google Scholar]
- 14. Dickson M, Plauschinat CA. Compliance with antihypertensive therapy in the elderly: a comparison of fixed‐dose combination amlodipine/benazepril versus component‐based free‐combination therapy. Am J Cardiovasc Drugs. 2008;8:45‐50. [DOI] [PubMed] [Google Scholar]
- 15. Yang W, Chang J, Kahler KH, et al. Evaluation of compliance and health care utilization in patients treated with single pill vs. free combination antihypertensives. Curr Med Res Opin. 2010;26:2065‐2076. [DOI] [PubMed] [Google Scholar]
- 16. Zeng F, Patel BV, Andrews L, et al. Adherence and persistence of single‐pill ARB/CCB combination therapy compared to multiple‐pill ARB/CCB regimens. Curr Med Res Opin. 2010;26:2877‐2887. [DOI] [PubMed] [Google Scholar]
- 17. Panjabi S, Lacey M, Bancroft T, et al. Treatment adherence, clinical outcomes, and economics of triple‐drug therapy in hypertensive patients. J Am Soc Hypertens. 2013;7:46‐60. [DOI] [PubMed] [Google Scholar]
- 18. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension. 2010;55:399‐407. [DOI] [PubMed] [Google Scholar]
- 19. Sherrill B, Halpern M, Khan S, et al. Single‐pill vs free‐equivalent combination therapies for hypertension: a meta‐analysis of health care costs and adherence. J Clin Hypertens. 2011;13:898‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rabbani A, Alexander GC. Out‐of‐pocket and total costs of fixed‐dose combination antihypertensives and their components. Am J Hypertens. 2008;21:509‐513. [DOI] [PubMed] [Google Scholar]
- 21. Moriarty F, Bennett K, Fahey T. Fixed‐dose combination antihypertensives and risk of medication errors. Heart. 2019;105:204‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Selak V, Bullen C, Stepien S, et al. Do polypills lead to neglect of lifestyle risk factors? Findings from an individual participant data meta‐analysis among 3140 patients at high risk of cardiovascular disease. J Prev Cardiol. 2016;23:1393‐1400. [DOI] [PubMed] [Google Scholar]
- 23. Indian Polycap Study (TIPS) , Yusuf S, Pais P, et al. Effects of a polypill (Polycap) on risk factors in middle‐aged individuals without cardiovascular disease (TIPS): a phase II, double‐blind, randomized trial. Lancet. 2009;373;1341‐1351. [DOI] [PubMed] [Google Scholar]
- 24. PILL Collaborative Group , Rodgers A, Patel A, et al. An international randomized placebo‐controlled trial of a four‐component combination pill (“polypill”) in people with raised cardiovascular risk. PLoS One. 2011;6(5):e19857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7(7):e41297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yusuf S, Pais P, Sigamani A, et al. Comparison of risk factor reduction and tolerability of a full‐dose polypill (with potassium) versus low‐dose polypill (polycap) in individuals at high risk of cardiovascular diseases: the second Indian polycap study (TIPS‐2) investigators. Cir Cardiovasc Qual Outcomes. 2012;5:463‐471. [DOI] [PubMed] [Google Scholar]
- 27. Thom S, Poulter N, Field J, et al. Effects of a fixed‐dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310:918‐929. [DOI] [PubMed] [Google Scholar]
- 28. Selak V, Elley CR, Bullen C, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomized controlled trial in primary care. BMJ. 2014;348:g3318. [DOI] [PubMed] [Google Scholar]
- 29. Castellano JM, Sanz G, Peñalvo JL, et al. A polypill strategy to improve adherence: results from the FOCUS project. J Am Coll Cardiol. 2014;64:2071‐2082. [DOI] [PubMed] [Google Scholar]
- 30. Lafeber M, Grobbee DE, Schrover IM, et al. Comparison of a morning polypill, evening polypill and individual pills on LDL‐cholesterol, ambulatory blood pressure and adherence in high‐risk patients; a randomized crossover trial. Int J Cardiol. 2015;181:193‐199. [DOI] [PubMed] [Google Scholar]
- 31. Patel A, Cass A, Peiris D, et al. A pragmatic randomized trial of a polypill‐based strategy to improve use of indicated preventive treatments in people at high cardiovascular disease risk. Eur J Prev Cardiol. 2015;22:920‐930. [DOI] [PubMed] [Google Scholar]
- 32. Webster R, Patel A, Selak V, et al. Effectiveness of fixed dose combination medication ('polypills') compared with usual care in patients with cardiovascular disease or at high risk: a prospective, individual patient data meta‐analysis of 3140 patients in six countries. Int J Cardiol. 2016;205:147‐156. [DOI] [PubMed] [Google Scholar]
- 33. Muñoz D, Uzoije P, Reynolds C, et al. Polypill for cardiovascular disease prevention in an underserved population. N Engl J Med. 2019;381:1114‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roshandel G, Khoshnia M, Poustchi H, et al. Effectiveness of polypill for primary and secondary prevention of cardiovascular diseases (PolyIran): a pragmatic, cluster‐randomised trial. Lancet. 2019;394:672‐683. [DOI] [PubMed] [Google Scholar]
- 35. Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 36. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596‐e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mach F, Baigent C, Catapano AL, et al. The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). 2019 ESC/EAS guidelines for the management of dyslipidemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111‐188. [DOI] [PubMed] [Google Scholar]
- 38. Kario K, Chia YC, Sukonthasarn A, et al. Diversity of and initiatives for hypertension management in Asia‐Why we need the HOPE Asia Network. J Clin Hypertens. 2020;22:331‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang JG, Kario K, Lau T, et al. Use of dihydropyridine calcium channel blockers in the management of hypertension in Eastern Asians: a scientific statement from the Asian Pacific Heart Association. Hypertens Res. 2011;34:423‐430. [DOI] [PubMed] [Google Scholar]
- 40. Sukonthasarn A. A comparison of four primary classes of antihypertensive monotherapy in Thai patients. J Med Assoc Thai. 2000;83:1202‐1210. [PubMed] [Google Scholar]
- 41. Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38:33‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Woo KS, Nicholls MG. High prevalence of persistent cough with angiotensin converting enzyme inhibitors in Chinese. Br J Clin Pharmacol. 1995;40:141‐144. [PMC free article] [PubMed] [Google Scholar]
- 43. Ng LP, Goh PS. Incidence of discontinuation of angiotensin‐converting enzyme inhibitors due to cough, in a primary healthcare centre in Singapore. Singapore Med J. 2014;55:146‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hsu CN, Wang TD. Secular trends in prescription patterns of single‐pill combinations of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker plus a thiazide diuretic for hypertensive patients in Taiwan. Acta Cardiol Sin. 2013;29:49‐55. [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: A systematic review and meta‐analysis. JAMA. 2019;321:277‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 47. Huo Y, Li J, Qin X, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015;313:1325‐1335. [DOI] [PubMed] [Google Scholar]
- 48. Zhang T, Lin T, Wang Y, et al. Estimated stroke‐free survival of folic acid therapy for hypertensive adults: Projection based on the CSPPT. Hypertension. 2020;75:339‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kolte D, Aronow WS, Banach M. Polypills for the prevention of cardiovascular diseases. Expert Opin Investig Drugs. 2016;25:1255‐1264. [DOI] [PubMed] [Google Scholar]
- 50. Guglietta A, Guerrero M. Issues to consider in the pharmaceutical development of a cardiovascular polypill. Nat Clin Pract Cardiovasc Med. 2009;6:112‐119. [DOI] [PubMed] [Google Scholar]
- 51. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898‐904. [DOI] [PubMed] [Google Scholar]
- 52. Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631‐2639. [DOI] [PubMed] [Google Scholar]
- 53. Castellano JM, Bueno H, Fuster V. The cardiovascular polypill: clinical data and ongoing studies. Int J Cardiol. 2015;201:S8‐S14. [DOI] [PubMed] [Google Scholar]
- 54. The International Polycap Study 3 (TIPS‐3)‐Full Text View‐ Clinical Trials.gov[internet], 2017. Available from: https://clinicaltrials.gov/ctz/show/NCT01646437. Accessed 10 August 10, 2020 and October 12, 2020.


