Abstract
Hypertensive urgency (HT urgency) is an alarming sign of uncontrolled hypertension. It is aggravated by nonadherence to medication, as well as psychosocial stress. Mindfulness is beneficial for reducing stress, while deep and slow breathing is effective for lowering blood pressure (BP). In our study, we aimed to assess BP and heart rate effects in HT urgency patients practicing pursed‐lip breathing and number counting (PLB with NC)—a practice that promotes mindfulness with deep/slow breathing patterns. In a randomized controlled trial, 110 patients were equally allocated to intervention and control groups. The intervention group was trained and encouraged to do PLB with NC during their emergency room admission, while the control group received conventional medical care. The mean systolic BP (SBP), diastolic BP (DBP), and HR of the intervention group in the 3rd hour were significantly lower than the baseline values at −28.2 mm Hg (95%CI;‐23.5 to −32.4), −17.1 mm Hg (95%CI;‐14.2 to −20.0), and −4.9 beats per minute (bpm) (95%CI;‐4.0 to −5.8), respectively. In the control group, both the mean SBP and DBP were also significantly lower in the 3rd hour. However, HR reduction was inconclusive. When the two groups were compared, a greater degree of reduction was found in the intervention group for SBP (9.80 mm Hg, 95%CI; 4.10 to 15.50), DBP (7.69 mm Hg, 95%CI; 3.61 to 11.77), and HR (3.85 bpm, 95%CI; 1.99 to 5.72). In conclusion, PLB with NC was effective for lowering BP and HR. It might be used as a complementary treatment for HT urgency patients.
Keywords: emergency room, hypertensive crisis, mindfulness, slow breathing, uncontrolled hypertension
1. INTRODUCTION
The National Health and Nutrition Examination Survey (NHANES) showed that in the years 2003‐2010, nearly one‐third of American adults had hypertension, and as high as 53.5% was uncontrolled. 1 Uncontrolled blood pressure (BP) usually occurs among patients who are being treated with medication (44.8%), have unrecognized hypertension (39.4%), or who have recognized untreated hypertension (15.8%). 1 A number of studies have found that one of the important causes of uncontrolled BP is nonadherence to antihypertensive medication due to side effects. 2 , 3 , 4 , 5
Hypertensive urgency (HT urgency) is a clear and alarming sign of uncontrolled hypertension and is characterized by marked elevation of BP without evidence of target organ damage. 6 , 7 The BP cut‐off values for HT urgency are defined by a systolic BP (SBP) greater than 180 mm Hg and/or a diastolic BP (DBP) greater than 110 mm Hg. 6 , 7 Although HT urgency looks benign, many cohort studies have reported a risk of future cardiovascular complications such as atrial fibrillation (AF), heart failure (HF), myocardial infarction (MI), and/or stroke within a few months to a year. 8 Unlike HT urgency, a hypertensive emergency (HT emergency) is a fatal condition where patients usually show extremely high BP that is associated with target organ damage. 9 , 10 , 11 HT urgency can be difficult to distinguish from an HT emergency when limited to a patient's history or when doing a physical examination, especially in patients who have mild symptoms. Physicians may need to carry out further investigation, for example, chest X‐rays, electrocardiograms, or blood tests, in order to determine any possible asymptomatic organ damage. Therefore, HT patients are usually under close observation in the emergency room (ER) for a few hours. 12 , 13 While medical investigations are taking place, in addition to the usual care (bed rest and pharmacologic intervention), non‐pharmacological intervention for promoting relaxation and calmness may also be helpful for lowering BP. 14 , 15 , 16 , 17
Breathing exercises that slow respiratory rates (RR) and modify respiratory patterns help in reducing BP, as deep and slow breathing can increase baroreflex sensitivity and also affect heart rate variability. Furthermore, deep and slow breathing also increases small vessel blood flow and decreases peripheral vascular resistance. 18 A few studies have shown breathing exercises with interactive music to be a safe and feasible intervention for BP reduction. 14 , 17 It has been known for years that hypertension is associated with psychosocial stress. 19 Therefore, the National Health of Science (NHS) has proposed breathing exercises with counting techniques for stress relief and to maintain mental health and wellbeing. 20 However, there are limited data on the effectiveness of breathing interventions in an emergency setting.
The present study aimed to assess the effect of a pursed‐lip breathing technique combined with number counting versus conventional care on BP and heart rate (HR) in HT urgency patients visiting the ER.
2. MATERIALS AND METHODS
2.1. Study design, setting, and studied population
This was a single‐blinded, randomized controlled trial. We enrolled patients aged between 18‐80 years old who had been diagnosed with HT urgency after arriving at the emergency room of Srinagarind hospital (a 1000‐bed, tertiary‐care university hospital), Khon Kaen University from September 1st, 2019 to June 30th, 2020. Patients with cardiac arrhythmias, acute HF, acute coronary syndrome (ACS), acute stroke, acute respiratory failure, alteration of consciousness, or pregnancy were excluded. This study was approved by the Khon Kaen University Ethical Review Board in Human Research (HE611586). All patients gave their written informed consent before enrollment. The trial was registered retrospectively after completion to ClinicalTrials.gov (NCT04572672).
2.2. Operating definition
HT urgency was defined as SBP ≥180 mm Hg and/or DBP ≥110 mm Hg without any signs or symptoms of target organ damage including 1) ACS, 2. acute HF, 3) acute stroke, 4) intracerebral hemorrhage (ICH), 5) hypertensive encephalopathy, 6) acute aortic dissection, or 7) acute kidney injury (AKI). The target organ involvement was screened by history taking, physical examination, and investigations as needed in every patients who visited the ER with SBP ≥180 mm Hg and/or DBP ≥110 mm Hg according to the HT workflow of the hospital.
2.3. Sample size calculation
A sample size was calculated for comparing means of SBP and DBP with repeated measures. The following parameters were used: two‐tailed test, an alpha error probability of 0.05, and the power of the test = 0.8. The expected meaningful clinical difference of SBP between the intervention and control groups was 10 mm Hg. The drop‐out rate was estimated at around 15%. The total number of participants needed was 110 (55 participants for each group).
2.4. Study protocol
2.4.1. Randomization
After a written informed consent document was signed by the patient, an allocation to either the intervention or control group was then performed by a non‐team member using a computer‐generated block randomization program (block of four, 1:1). A randomization number was drawn from a sealed opaque envelope. The investigators involved in enrolling participants had no access to the randomization lists. The treatment investigators and patients knew the result of the randomization but the statistical‐analysis investigator was blinded to it. The CONSORT diagram is shown in Figure 1.
FIGURE 1.
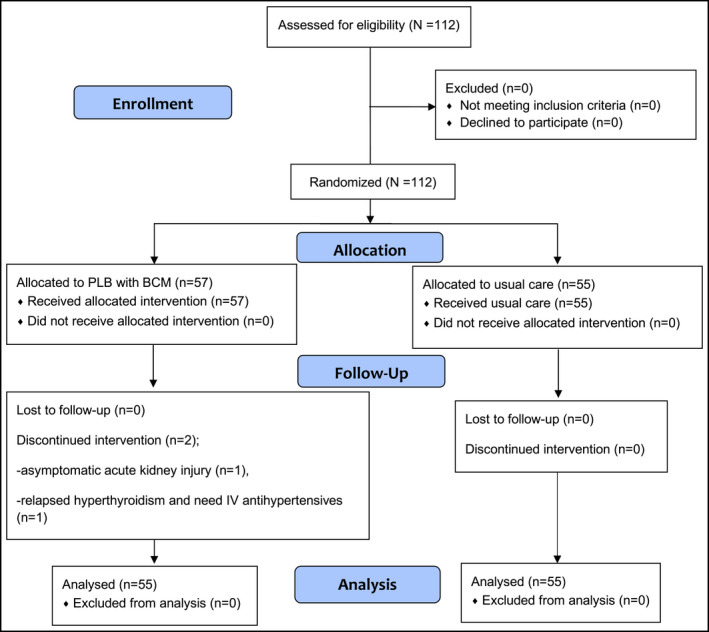
The CONSORT diagram depicts enrollment, allocation, follow up, and analysis. There were 112 patients enrolled in the present study. Fifty‐seven patients were randomized into the intervention group; two of the 57 were terminated from the study due to asymptomatic target organ damage (n = 1) and relapsed hyperthyroidism (n = 1), as both patients required intravenous antihypertensive medication. Fifty‐five patients were enrolled into the control group. In the final analysis, there were 55 patients in the intervention group and 55 patients in the control group
2.4.2. Intervention group
Using a pictorial card set, an emergency nurse trained each patient to do pursed‐lip breathing with number counting until the patient was able to do it correctly. The card set was developed and verified by five medical professional reviewers (content validity index = 0.92). The cards demonstrated: 1) Position, for which the patient lies down in a semi‐supine position with the bed adjusted to 45‐60 degrees; 2) Pursed‐lip breathing and number counting “one and two” during inhalation; and 3) Number counting “one, two, three, and four” during exhalation (Figure 2 ). The nurse advised the patient to continue pursed‐lip breathing with number counting for only the first 15 minutes (min) of each hour to prevent exhaustion. 21 The total study time for each patient was 3 hours, until the patient was discharged from the ER, or until the patient was withdrawn from the study. The nurse stayed with each patient to check whether they performed pursed‐lip breathing with number counting correctly for the first 15 minutes of each hour. In addition, every patient was advised to have a bed rest in a supine position in a quiet area (“Hypertension Corner” in the ER). Antihypertensive medication was prescribed by an emergency physician who was in charged, and usual nursing care was given to the patients according to standard practice guidelines. 22 , 23
FIGURE 2.
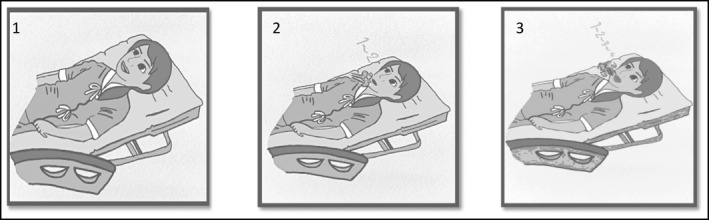
Pursed‐lip breathing and number counting pictorial cards for training. The cards demonstrated: 1) Position, for which the patient was asked to lie down in a semi‐supine position with the bed adjusted to 45‐60 degrees; 2) Pursed‐lip breathing and counting “one and two” during inhalation; and 3) Number counting “one, two, three, and four” during exhalation
2.4.3. Control group
The patients who were allocated to the control group received usual nursing care, that is, bed rest in a supine position in a quiet area (“Hypertension Corner” in the ER). Patients were advised to limit their activity. Vital sign monitoring was carried out with the same frequency for both the intervention and control groups. Pharmacologic treatment was prescribed according to the judgment of the emergency physician who was in charged. Treatment was based on the standard practice guidelines. 22 , 23
2.4.4. BP and HR measurements
Office BP and HR were measured by only one of the participating nurses for the entirety of the study. The nurse used an automatic BP device (DINAMAP V100), which was regularly calibrated by a medical equipment calibration unit at Srinagarind Hospital. BP and HR were measured and recorded as baseline data immediately after patients were enrolled in the study and at the end of each hour for 3 hours (4 times total).
2.4.5. Adverse event monitoring and withdrawal from study criteria
All patients were asked to report any possible abnormal symptoms that occurred after enrollment and during the study period. If the physician administered intravenous (IV) antihypertensive medication to a patient for immediate BP control, or if a patient needed oxygen therapy or any ventilation support, he/she was immediately withdrawn from the study for safety reasons.
2.5. Statistical analysis
Analyses were based on the intention‐to‐treat principle. Regarding baseline characteristics, data were shown as mean ± standard deviation (SD) or percentage (%). The paired t test was used to compare SBP, DBP, and HR at baseline versus after 3 hours of pursed‐lip breathing exercises with breath counting in the intervention group and usual care in the control group. The general estimating equation (GEE) was used to compare overall SBP, DBP, and HR between the two randomized groups. A probability value of <.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) software program for Windows, version 19.0.
3. RESULTS
We enrolled a total of 112 patients from which 57 and 55 patients were randomized into the intervention group and the control group, respectively. However, 2 patients were later withdrawn from the intervention group due to an urgent need for IV antihypertensive medication (study withdrawal criteria). Therefore, 110 patients were included for the final analysis (n = 55 in each group) (Figure 1). The mean age was 66.7 years in the intervention group and 60.8 years in the control group (p = .013). There were more women than men in both groups (63.6% in the intervention group and 61.8% in the control group). Excluding age, other demographic data including underlying diseases were similar in both groups (Table 1). Regarding hypertension awareness and treatment, approximately 30% of the patients in both groups had poor adherence to antihypertensive medication, while around 27% in the intervention group and 31% in the control group were untreated HT patients (p = .195; Table 1). Types of current hypertensive medication, for example, calcium channel blockers (CCB), angiotensin‐converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), and hydralazine were similar in the treated patients of both groups who had good adherence (all p > .05). In the ER, most patients in the intervention group and the control group were prescribed antihypertensive medication, 92.7% vs. 94.6% (p = .068; Table 1).
TABLE 1.
Demographic data
| Group | p | ||
|---|---|---|---|
|
Intervention n = 55 |
Control n = 55 |
||
| Age, years | 66.76 (12.64) | 60.76 (12.64) | .013 |
| Female, n (%) | 35 (63.64) | 34 (61.82) | .844 |
| Marital Status, n (%) | |||
| Married | 34 (61.82) | 30 (54.55) | .789 |
| Single | 11 (20.00) | 14 (25.45) | |
| Widowed | 7 (12.73) | 9 (19.36) | |
| Separated/Divorced | 3 (5.45) | 2 (3.64) | |
| Education, n (%) | |||
| Primary school | 6 (10.91) | 13 (23.64) | .339 |
| High school | 15 (27.27) | 11 (20.00) | |
| Bachelor's Degree or Higher | 34 (61.82) | 31 (56.38) | |
| Occupation, n (%) | |||
| Housekeeper | 3 (5.45) | 9 (16.36) | .124 |
| Farmer | 27 (49.09) | 18 (32.73) | |
| Government officer | 14 (25.45) | 19 (34.55) | |
| Personal business owner | 11 (20.00) | 9 (16.36) | |
| History of smoking, n (%) | |||
| Never smoked | 31 (56.36) | 32 (58.18) | .767 |
| Former smoker | 17 (30.91) | 15 (27.27) | |
| Actively smoking | 0 (0.00) | 8 (14.55) | |
| History of alcohol, n (%) | |||
| Never drank alcohol | 36 (65.45) | 36 (65.45) | .590 |
| Former alcohol drinker | 13 (23.64) | 14 (25.45) | |
| Actively drinks alcohol | 6 (10.91) | 5 (9.09) | |
| Underlying disease, n (%) | |||
| Hypertension | 30 (54.55) | 28 (50.91) | .849 |
| Diabetes mellitus | 5 (9.09) | 5 (9.09) | 1.000 |
| Dyslipidemia | 4 (7.27) | 4 (7.27) | 1.000 |
| Atrial fibrillation | 2 (3.64) | 2 (3.64) | 1.000 |
| Others | 14 (25.45) | 9 (16.36) | .248 |
| The duration of hypertension diagnosis, years | 2.44 (1.27) | 2.56 (1.20) | .590 |
| Creatinine, mg/dl (mean ± SD) | 0.92 ± 0.37 | 0.98 ± 0.37 | .423 |
| eGFR, ml/min/1.73m2 (mean ± SD) | 61.90 ± 26.48 | 60.67 ± 25.88 | .806 |
| Chief complaints of current visit, n (%) | |||
| Asymptomatic | 36 (65.45) | 35 (63.64) | .642 |
| Dizziness | 7 (12.73) | 5 (9.09) | |
| Headache | 4 (7.27) | 8 (14.55) | |
| Blurred vision | 4 (7.27) | 4 (7.27) | |
| Shortness of breath | 4 (7.27) | 3 (5.46) | |
| Antihypertensive medication use, n (%) | |||
| Untreated | 15 (27.27) | 17 (30.91) | .195 |
| Stopped independently | 10 (18.18) | 3 (5.45) | .041 |
| Irregularly taken | 17 (30.91) | 17 (30.91) | 1.000 |
| Regularly taken, n (%) | 13 (23.64) | 18 (32.73) | .295 |
| Calcium channel blockers | 12 (21.82) | 18 (32.73) | .284 |
| ACEI | 3 (5.45) | 3 (5.45) | 1.000 |
| ARB | 5 (9.09) | 6 (10.91) | 1.000 |
| Hydralazine | 9 (16.36) | 12 (21.82) | .628 |
| Prescribed antihypertensive medication in ER, n (%) | 51 (92.73) | 52 (94.55) | .068 |
| Amlodipine | 19 (34.55) | 34 (61.82) | .007 |
| Captopril | 0 (0) | 4 (7.27) | .118 |
| Hydralazine | 49 (89.09) | 51 (92.73) | .742 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin II receptor blockers; eGFR, estimated glomerular filtration rate; ER, emergency room.
In terms of the SBP, DBP, and HR changes from baseline values in each group, in the intervention group, the mean SBP, DBP, and HR in the 3rd hour were significantly lower compared to the baseline: −28.2 mm Hg (95% CI; −23.5 to −32.4), −17.1 mm Hg (95% CI; −14.2 to −20.0), and −4.9 beats per minute (bpm) (95% CI; −4.0 to −5.8), respectively (Table 2). In the control group, the mean SBP and DBP were also significantly lower in the 3rd hour compared to the baseline: −18.4 mm Hg (95% CI; −14.5 to −22.3), and −9.4 mm Hg (95% CI; −6.4 to −12.3), respectively. However, HR reduction by the 3rd hour was inconclusive in this group (−1.0 bpm (95% CI; −0.6 to 2.7)). The magnitude of differences in average SBP and DBP in the intervention group was greater than in the control group, as shown in Table 2.
TABLE 2.
Comparison of mean differences of SBP, DBP, and HR within and between two groups
| Measure | Intervention | Control | Mean difference between groups (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Baseline (0 hour) | 3rd hour | Mean difference (95% CI) | Baseline (0 hour) | 3rd hour | Mean difference (95% CI) | ||
| SBP ( mm Hg) | 201.38 ± 14.44 | 173.20 ± 14.62 | 28.18 (23.54, 32.42) | 199.42 ± 14.58 | 181.04 ± 15.05 | 18.38 (14.47, 22.29) | 9.80 (4.10, 15.50) |
| DBP ( mm Hg) | 105.71 ± 10.41 | 88.64 ± 8.97 | 17.07 (14.19, 19.95) | 105.09 ± 13.44 | 95.71 ± 11.54 | 9.38 (6.43, 12.33) | 7.69 (3.61, 11.77) |
| HR (bpm) | 78.02 ± 9.26 | 73.13 ± 8.47 | 4.89 (4.00, 5.78) | 77.60 ± 10.66 | 76.56 ± 10.52 | 1.04 (−0.63, 2.70) | 3.85 (1.99, 5.72) |
Abbreviations: 95% CI, 95% confidence interval; bpm, beats per minute; DBP, diastolic blood pressure; HR, heart rate; mm Hg, millimeter of mercury; SBP, systolic blood pressure.
Regarding comparisons between the two randomized groups, the mean differences of SBP (9.80 mm Hg, 95% CI; 4.10 to 15.50), DBP (7.69 mm Hg, 95% CI; 3.61 to 11.77), and HR (3.85 bpm, 95% CI; 1.99 to 5.72) were significantly different (all p < .001) (Table 2).
4. DISCUSSION
Our results showed that SBP and DBP were significantly lower in the 3rd hour compared with the baseline values in both the intervention group (pursed‐lip breathing exercise combined with breath counting) and the control group (conventional care). However, only the intervention group had HR values that decreased significantly. When comparing between groups, the degree of reduction of SBP, DBP, and HR was significantly greater in the intervention group.
Chacko et al studied the effect of slow breathing on arterial baroreflex and BP in hypertensive patients. They found that slow breathing, especially as low as 6 cycles per minute (cpm), reduced both SBP and DBP in hypertensive patients (SBP from 149.7 ± 3.7 to 141.1 ± 4 mm Hg, p < .05; and DBP from 82.7 ± 3 to 77.8 ± 3.7 mm Hg, p < .01). Their findings on arterial baroreflex showed that lowered BP during‐slow breathing practices is associated with an increase in the vagal tone. 24
A prior study on Thai hypertensive patients (BP ≥140/90 mm Hg) showed that a slow and deep respiration practice (RR around 10 cpm) over 8 weeks led to a significantly reduced office SBP (−23.59, 95% CI; −16.90 to −30.29) and office DBP (−8.57, 95% CI; −4.57 to −12.57). The magnitude of SBP reduction was similar to that found in the present study while that of DBP was greater. The population in the previous Thai study was quite similar to ours, with 60% women and a hypertension duration of no more than 10 years. They reported more than a 90% success rate for the slow and deep respiration practice. However, they did not report the effects of the intervention on HR. 21
In the present study, we did not restrict the RR. However, we trained patients to do pursed‐lip breathing combined with number counting to promote deep and slow breathing, and most importantly, to promote a state of mindfulness. Via ambulatory BP measurement (ABPM), Marquez et al demonstrated a significant reduction in SBP after mindfulness training in stage‐1 hypertensive patients. By the 8th week, the intervention group had statistically lower ABPM levels compared to the control group in terms of 24‐h and night‐time SBP (124/77 mm Hg vs 126/80 mm Hg, p < .05, and 108/65 mm Hg vs 114/69 mm Hg, p < .05, respectively). The intervention group in their study also reported that they became less judgmental, more accepting, and less depressed. 25 Therefore, BP reduction in our study might be explained by the combined mechanisms of increased parasympathetic tone resulting from the deep and slow breathing, as well as the fact that the patients had less anxiety and stress as a benefit of mindfulness.
There has also been evidence that device‐guided breathing at home can effectively reduce BP. 26 The FDA has approved use of “RESPeRATE,” an over‐the‐counter electronic device that guides slow‐paced breathing to achieve respiratory frequency <10 cpm for stress reduction and as an adjunctive treatment to lower BP. 27 From the results of our study, there is a possibility that device‐guided breathing may also help to reduce the BP of HT urgency patients in an emergency setting. However, further study is needed to prove its effectiveness.
To our knowledge, this is the first RCT that demonstrates the effect of a breathing exercise, namely, pursed‐lip breathing combined with number counting in HT urgency patients within an ER setting. The breathing exercise that we use is easy to understand, takes only a short time to teach, and requires no medical equipment. We are aware that our study had some potential limitations. First, we did not collect or compare the mean RR of each randomized group, and we did not calculate the success rate of the breathing exercise training, which may have had some effect on BP control. Secondly, the timing of each breathing cycle of the patients in the intervention group was not measured, therefore, we can not specify the dose‐effect of purse‐lip breathing combined with number counting method for the BP reduction. Third, the present study was a single‐blinded RCT, meaning a Hawthorne effect in the intervention group was inevitable. Lastly, we did not extend our study over a longer period of time. Therefore, we suggest further research in order to follow patients over a longer period and also to explore the effects of breathing exercises on more serious outcomes, such as cardiovascular events or even death.
5. CONCLUSIONS
A pursed‐lip breathing exercise combined with number counting was effective for lowering BP and HR. It is an easy and harmless complementary treatment for patients who exhibit HT urgency in the ER.
CONFLICT OF INTEREST
The authors report no conflict of interest.
AUTHOR CONTRIBUTIONS
Thapanawong Mitsungnern performed the analysis and wrote the paper. Nipa Srimookda collected the data, contributed data, and performed the analysis. Supap Imoun collected the data. Suntaraporn Wansupong collected the data. Praew Kotruchin conceived and designed the analysis, and wrote the proof manuscript.
ACKNOWLEDGMENTS
We would like to express deep gratitude to our hypertensive patients, without whom this study would have been impossible. We appreciate the assistance and collaboration from the health care team at Srinagarind Hospital, Khon Kaen. Finally, we would like to thank the HOPE ASIA Network for their wonderful and sustained hypertension activities and research opportunities.
Mitsungnern T, Srimookda N, Imoun S, Wansupong S, Kotruchin P. The effect of pursed‐lip breathing combined with number counting on blood pressure and heart rate in hypertensive urgency patients: A randomized controlled trial. J Clin Hypertens. 2021;23:672–679. 10.1111/jch.14168
Funding information
This research did not receive any grants from funding agencies in public, commercial, or non‐profit sectors.
Mitsungnern and Srimookda have contributed equally.
REFERENCES
- 1. Centers for Disease Control and Prevention (CDC) . Vital signs: awareness and treatment of uncontrolled hypertension among adults‐‐United States, 2003‐2010. MMWR Morb Mortal Wkly Rep. 2012;61:703‐709. [PubMed] [Google Scholar]
- 2. Overgaauw N, Alsma J, Brink A, et al. Drug nonadherence is a common but often overlooked cause of hypertensive urgency and emergency at the emergency department. J Hypertens. 2019;37(5):1048‐1057. [DOI] [PubMed] [Google Scholar]
- 3. Grigoryan L, Pavlik VN, Hyman DJ. Patterns of nonadherence to antihypertensive therapy in primary care. J Clin Hypertens Greenwich Conn. 2013;15(2):107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abegaz TM, Shehab A, Gebreyohannes EA, Bhagavathula AS, Elnour AA. Nonadherence to antihypertensive drugs: A systematic review and meta‐analysis. Medicine (Baltimore). 2017;96(4):e5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tedla YG, Bautista LE. Drug Side Effect Symptoms and Adherence to Antihypertensive Medication. Am J Hypertens. 2016;29(6):772‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alley WD, Copelin EL II.Hypertensive Urgency. In: StatPearls. StatPearls Publishing; 2020. http://www.ncbi.nlm.nih.gov/books/NBK513351/. Accessed August 31, 2020.
- 7. Patel KK, Young L, Howell EH, et al. Characteristics and Outcomes of Patients Presenting With Hypertensive Urgency in the Office Setting. JAMA Intern Med. 2016;176(7):981‐988. [DOI] [PubMed] [Google Scholar]
- 8. Hackett C, Garrison S, Kolber MR. What is urgent about hypertensive urgency? Can Fam Physician. 2017;63(7):543. [PMC free article] [PubMed] [Google Scholar]
- 9. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949‐1962. [DOI] [PubMed] [Google Scholar]
- 10. Feitosa‐Filho GS, Lopes RD, Poppi NT, Guimarães HP. Hypertensive emergencies. Rev Bras Ter Intensiva. 2008;20(3):305‐312. [PubMed] [Google Scholar]
- 11. Brathwaite L, Reif M. Hypertensive Emergencies: A Review of Common Presentations and Treatment Options. Cardiol Clin. 2019;37(3):275‐286. [DOI] [PubMed] [Google Scholar]
- 12. Bender SR, Fong MW, Heitz S, Bisognano JD. Characteristics and management of patients presenting to the emergency department with hypertensive urgency. J Clin Hypertens Greenwich Conn. 2006;8(1):12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maweni RM, Sunderland N, Rahim Z, et al. Clinical characteristics of Black patients with hypertensive urgency. Ir J Med Sci. 2018;187(4):1089‐1096. [DOI] [PubMed] [Google Scholar]
- 14. Grossman E, Grossman A, Schein MH, Zimlichman R, Gavish B. Breathing‐control lowers blood pressure. J Hum Hypertens. 2001;15(4):263‐269. [DOI] [PubMed] [Google Scholar]
- 15. Park SK, Lee D‐Y, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474‐1480. [DOI] [PubMed] [Google Scholar]
- 16. Campos CL, Herring CT, Ali AN, et al. Pharmacologic Treatment of Hypertensive Urgency in the Outpatient Setting: A Systematic Review. J Gen Intern Med. 2018;33(4):539‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kow FP, Adlina B, Sivasangari S, et al. The impact of music guided deep breathing exercise on blood pressure control ‐ A participant blinded randomised controlled study. Med J Malaysia. 2018;73(4):233‐238. [PubMed] [Google Scholar]
- 18. Grossman A, Grossman E. Treatment of hypertension with device‐guided breathing exercise. Harefuah. 2003;142(10):677‐679, 718. [PubMed] [Google Scholar]
- 19. Liu M‐Y, Li N, Li WA, Khan H. Association between psychosocial stress and hypertension: a systematic review and meta‐analysis. Neurol Res. 2017;39(6):573‐580. [DOI] [PubMed] [Google Scholar]
- 20. Breathing exercise for stress . nhs.uk. Published November 11, 2019. https://www.nhs.uk/conditions/stress‐anxiety‐depression/ways‐relieve‐stress/. Accessed September 1, 2020.
- 21. Ouicharoenpong M, Thongthiengdee B. Slow respiration could reduce blood pressure in hypertensive patients. Public Health J Burapha Univ. 2011;6(2):10‐47. [Google Scholar]
- 22. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertens Dallas Tex. 2018;71(6):1269‐1324. [DOI] [PubMed] [Google Scholar]
- 23. Williams B, Mancia G, Spiering W, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Blood Press. 2018;27(6):314‐340. [DOI] [PubMed] [Google Scholar]
- 24. Joseph CN, Porta C, Casucci G, et al. Slow Breathing Improves Arterial Baroreflex Sensitivity and Decreases Blood Pressure in Essential Hypertension. Hypertension. 2005;46(4):714‐718. [DOI] [PubMed] [Google Scholar]
- 25. Ponte Márquez PH, Feliu‐Soler A, Solé‐Villa MJ, et al. Benefits of mindfulness meditation in reducing blood pressure and stress in patients with arterial hypertension. J Hum Hypertens. 2019;33(3):237‐247. [DOI] [PubMed] [Google Scholar]
- 26. Gavish B. Device‐guided breathing in the home setting: technology, performance and clinical outcomes. Biol Psychol. 2010;84(1):150‐156. [DOI] [PubMed] [Google Scholar]
- 27. Sharma M, Frishman WH, Gandhi K. RESPeRATE: nonpharmacological treatment of hypertension. Cardiol Rev. 2011;19(2):47‐51. [DOI] [PubMed] [Google Scholar]


