Abstract
We aimed to investigate the association between isolated systolic hypertension (ISH) and central blood pressure (BP) in a nationally representative population, with a focus on the young and middle‐aged adults (<50 years old). A total of 2029 adults without taking antihypertensive medications, aged ≥ 19 years old, participated in the 2013–2016 National Nutrition and Health Survey in Taiwan. Central and brachial BP were simultaneously measured using a cuff‐based stand‐alone central blood pressure monitor purporting to measure invasive central BP (type II device). Central hypertension was defined by central systolic (SBP)/diastolic BP (DBP) ≥130 or 90 mm Hg, and ISH was defined by brachial SBP ≥ 140 and DBP < 90 mm Hg. Overall, the prevalence rates of ISH, isolated diastolic hypertension (IDH, brachial SBP < 140 and DBP ≥ 90 mmHg), and systolic/diastolic hypertension (SDH, brachial SBP ≥ 140 and DBP ≥ 90 mmHg) were 6.51%, 1.92%, and 4.34%, respectively. ISH subjects had significantly higher central pulse pressure (PP) (62.8 ± 9.7 mm Hg for age < 50 years and 72.4 ± 13.5 mmHg for age ≥ 50 years) than those subjects with either IDH (44.7 ± 10.7 and 44.9 ± 10.6 mmHg) or SDH (55.2 ± 14.0 and 62.6 ± 17.1 mmHg). All ISH adults had central hypertension, and a higher prevalence of central obesity than the normotensives (80.95% vs. 26.15%, for age < 50 years; and 63.96% vs. 43.37% for age ≥ 50 years). All untreated subjects with ISH, whether younger or older, had central hypertension and had significantly higher central PP than those with IDH or SDH. Central obesity was one of the major characteristics of ISH, especially in the young‐ and middle‐aged adults.
Keywords: central blood pressure, isolated systolic hypertension, national sample, prevalence
1. INTRODUCTION
Isolated systolic hypertension (ISH) is defined as systolic blood pressure (SBP) ≥140 mmHg and diastolic blood pressure (DBP)<90 mmHg. 1 The prevalence of ISH increases with age along with the decreasing DBP and the widening pulse pressure (PP). ISH is commonly viewed as the manifestation of vascular aging and is conceivably the most common subtype of hypertension in the elderly population. 2 , 3 , 4 The elderly with ISH have a significantly higher risk of cardiovascular diseases 5 , 6 and benefit substantially from antihypertensive therapy. 7 , 8 , 9
Although the prevalence of hypertension in the young adults is usually low, ISH is also the most common form of hypertension in adolescents and young adults. 10 The proportion of ISH to systolic‐diastolic hypertension (SDH) in the population aged <40 years is 2 to 1. 11 Current evidence suggests the existence of two main phenotypes of ISH in the young, one is spurious systolic hypertension, 11 , 12 , 13 , 14 and the other is ISH with increased cardiovascular risk 11 , 12 and less of a difference between central and peripheral SBP, that is, a reduced stiffness gradient between central and peripheral arteries. 15
An elevated central SBP or PP may indicate the presence of vascular aging or early vascular aging with increased arterial stiffness and/or wave reflections. 16 It has been suggested that young‐to‐middle‐age ISH individuals with low central SBP may have a lower risk of hypertension needing treatment than those with high central SBP. 17 However, the relationship between central blood pressure and ISH remains to be determined. Therefore, we aimed to investigate the association between ISH and central blood pressure in a nationally representative sample in Taiwan, with a focus on the young‐ and middle‐aged adults (<50 years old).
2. MATERIALS AND METHODS
2.1. Study population
Since 1993, the Health Promotion Administration, Ministry of Health and Welfare has conducted a series of Nutrition and Health Survey in Taiwan (NAHSIT). The protocol of the survey in 1993–1996 covered all residents in the National Household Registry in Taiwan, excluding those who lived in military institutes, medical institutes, schools, occupation/sport training centers, dormitories, and prisons. Enrolled subjects in the NAHSIT were selected using a multistage stratified sampling scheme. The detailed information of sampling methods has been described in a prior study. 18 Each enrolled resident was firstly informed of the aims and potential benefits/risks of this survey and then was asked to sign the informed consent before the interview. All subjects who had received the household interview were invited for a health check‐up, included fasting blood biochemical and urine sample testing, and central and brachial blood pressure measurements. A total of 2742 residents aged more than 19 years attended the health check‐up. Among these, a total of 2029 residents who reported not taking any antihypertensive medication were included as the study population for the present study.
2.2. Blood pressure measurement and definitions
All participants were asked to refrain from exercise, smoking, and drinking tea or coffee at least one hour before blood pressure measurement. Blood pressure measurement was carried out in the morning while subjects had been seated and relaxed for 5 min with back and arms supported, legs uncrossed, and feet flat on the floor in a quiet room at each survey site for the health check‐up. 19 All measurement sites followed the same protocol. Central and brachial blood pressures were measured simultaneously in the right arm with an appropriately sized cuff at heart level, using an validated oscillometric central blood pressure monitor (WatchBP Office Central; Microlife AG, Widnau, Switzerland). 20 The central blood pressure monitor measured brachial SBP and DBP, performed pulse volume plethysmography at cuff pressure of 60 mm Hg to provide an ensemble average brachial pressure waveform, calibrated the ensemble average waveform to the brachial SBP and DBP, analyzed the calibrated waveform, and finally calculated central SBP and PP according to separate regression equations constructed from components of the analyzed waveform. 20 , 21 The central blood pressure monitor displayed readings of brachial SBP and DBP, central SBP and PP, and heart rate, each of which was an average from 2 consecutive measurements separated by an interval of 60 s. Central DBP was calculated as the difference between central SBP and PP. 20 Measurement accuracy of the stand‐alone oscillometric central blood pressure monitor with reference to the simultaneously measured invasive central blood pressure in 85 subjects (255 measurements; age range, 30–93 years) has been previously reported. 20
In the present study, normotension (NT) was defined as brachial SBP <140 mmHg and brachial DBP <90 mmHg; ISH was defined as brachial SBP ≥140 mm Hg and DBP <90 mm Hg; isolated diastolic hypertension (IDH) was defined as brachial SBP <140 mm Hg and DBP ≥90 mm Hg; and SDH was defined as brachial SBP ≥140 mm Hg and DBP ≥90 mm Hg. Central hypertension was defined as central SBP ≥130 mm Hg or DBP ≥90 mm Hg. 22
Body mass index was calculated as weight in kilogram divided by height‐square in meter. The definitions of general obesity and central obesity in Taiwanese have been proposed by the Health Promotion Administration, Taiwan. Obesity was defined as body mass index ≥27 kg/m2, 23 and central obesity was defined as waist circumference ≥90 cm for men and ≥80 cm for women. 23 Diabetes was defined as fasting glucose ≥126 mg/dL or using anti‐diabetic medications. 24
2.3. Statistical methods
Means (±SD) and proportions were used to describe the characteristics of the sampled population. Comparisons between subjects with ISH and subjects with NT, IDH, or SDH were performed separately with t test for interval variables or chi‐squared test for categorical variables. The analysis was separately conducted in younger adults (<50 years old) and older adults (≥50 years old).
Logistic regression was used to investigate the determinants of ISH among young adults and older adults. The logistic regression with stepwise selection was conducted with ISH (vs. NT) as the dependent variable and with the candidate variables, including age (years), sex (men vs. women), heart rate (beats/min), central PP (mmHg), obesity (≥27 kg/m2), high waist circumference (≥90 cm for men and ≥80 cm for women), high triglycerides (≥150 mg/dL), low high‐density lipoprotein cholesterol (<50 mg/dL for men and <40 mg/dL for women), high low‐density lipoprotein (LDL) cholesterol (≥130 mg/dL or using lipids lowering drugs) and diabetes.
We described the associations of age (5‐year increment) with prevalence of ISH and with levels of central SBP and central PP using a histogram (prevalence of ISH) and line charts (group means of central SBP and central PP; Figure 2A, B, C). We found a J‐shaped association between age and prevalence of ISH, and between age and central PP. Therefore, we further separated the younger adults at age 30 and then to estimate and compare the ISH prevalence rates among the three age groups (<30 years, 30–49 years, and ≥50 year) by CHISQ test.
FIGURE 2.
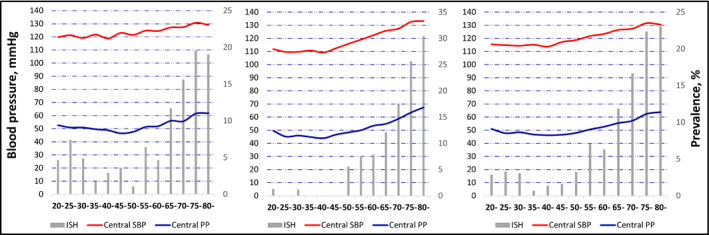
Prevalence of ISH and levels of central SBP and PP in adults without taking antihypertensive medications, according to each 5‐year age group in men (A), women (B), and (C) total population. ISH, isolated systolic hypertension; PP, pulse pressure; SBP, systolic blood pressure
All statistical tests were set at a type I error of 5% and 2 tails. P values of <.05 were considered statistically significant.
3. RESULTS
3.1. Prevalence rates of hypertension subtypes
Among the 2029 untreated participants, the prevalence rates of ISH, IDH, and SDH were 6.51%, 1.92%, and 4.34%, respectively. The prevalence rates of ISH, IDH, and SDH were 2.15%, 1.64%, and 2.97%, respectively, for adults <50 years old (Table 1). In contrast, for adults ≥50 years old, the prevalence rates were 10.54%, 2.18%, and 5.60%, respectively (Table 2).
TABLE 1.
Characteristics of the study population aged <50 years (n = 976)
| NT (n = 910) | ISH (n = 21) | IDH (n = 16) | SDH (n = 29) | |
|---|---|---|---|---|
| Age, years | 34.2 ± 8.7 | 32.0 ± 8.7 | 40.6 ± 7.6† | 39.8 ± 8.1† |
| Male, % (n) | 42.65% (388)* | 90.48% (19)† | 68.75% (11)† | 86.21% (25)† |
| Brachial SBP, mmHg | 109.8 ± 12.0* | 143.3 ± 2.8† | 132.5 ± 2.7† | 155.5 ± 13.2† |
| Brachial DBP, mmHg | 68.7 ± 8.3* | 82.9 ± 4.9† | 92.9 ± 2.4† | 100.1 ± 7.6† |
| Brachial PP, mmHg | 41.1 ± 8.2* | 60.4 ± 6.1† | 39.6 ± 3.8* | 55.4 ± 10.7† |
| Central SBP, mmHg | 113.4 ± 10.7* | 141.0 ± 7.3† | 130.3 ± 7.2† | 147.6 ± 12.5† |
| Central DBP, mmHg | 66.2 ± 7.0* | 78.2 ± 4.5† | 85.6 ± 4.4*, † | 92.3 ± 8.0† |
| Central PP, mmHg | 47.3 ± 9.5* | 62.8 ± 9.7† | 44.7 ± 10.7* | 55.2 ± 14.0† |
| Heart rate, beats/min | 76.6 ± 10.7 | 73.9 ± 9.0 | 78.4 ± 15.1 | 78.4 ± 12.4 |
| Body mass index, kg/m2 | 23.1 ± 4.0* | 30.1 ± 5.3† | 28.5 ± 4.6† | 28.9 ± 7.0† |
| Waist circumference, cm | 79.5 ± 10.8* | 98.8 ± 12.4† | 90.7 ± 10.2† | 94.4 ± 15.7† |
| Triglycerides, mg/dL | 102.7 ± 84.2* | 151.2 ± 76.5 | 188.3 ± 170.6† | 166.6 ± 110.9† |
| HDL cholesterol, mg/dL | 55.8 ± 14.9* | 42.7 ± 8.8† | 46.5 ± 14.5 | 48.6 ± 14.2 |
| LDL cholesterol, mg/dL | 111.1 ± 32.7* | 129.0 ± 27.8 | 118.4 ± 38.8 | 119.6 ± 19.7 |
| Total cholesterol, mg/dL | 181.5 ± 35.2 | 195.4 ± 30.4 | 190.6 ± 39.1 | 191.7 ± 25.2 |
| Glucose, mg/dL | 96.2 ± 15.6* | 103.6 ± 19.8 | 113.3 ± 48.1† | 102.2 ± 13.2 |
| Obesity, % | 15.05%* | 71.43%† | 75.0%† | 58.62%† |
| Central obesity, % | 26.15%* | 80.95%† | 68.75%† | 58.62%† |
| Diabetes, % | 2.86%* | 18.75%† | 14.29%† | 10.34%† |
| Smoking, % | 16.37%* | 33.33%† | 37.50%† | 27.59% |
| Low physical activity | 34.4% | 23.81% | 12.5%† | 27.59% |
Obesity was defined as body mass index ≥27 kg/m2; central obesity was defined as waist circumference ≥90 cm for men and ≥80 cm for women.
Abbreviations: DBP, diastolic blood pressure; HDL, high‐density lipoprotein; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; LDL, low‐density lipoprotein; NT, normotension; PP, pulse pressure; SBP, systolic blood pressure; SDH, systolic/diastolic hypertension.
p‐value < .05 in comparison with ISH group.
p‐value < .05 in comparison with NT group.
TABLE 2.
Characteristics of the study population aged ≥ 50 years (n = 1053)
| NT (n = 860) | ISH (n = 111) | IDH (n = 23) | SDH (n = 59) | |
|---|---|---|---|---|
| Age, years | 63.1 ± 9.0* | 69.0 ± 9.0 | 61.1 ± 7.8† | 63.6 ± 8.2* |
| Male, % (n) | 49.53% (426) | 45.05% (50) | 73.91% (17)† | 71.19% (42)† |
| Brachial SBP, mmHg | 116.7 ± 11.9* | 148.1 ± 9.4† | 132.7 ± 7.4† | 156.2 ± 16.6† |
| Brachial DBP, mmHg | 70.6 ± 8.1* | 80.1 ± 6.9† | 93.1 ± 3.4† | 97.7 ± 9.0† |
| Brachial PP, mmHg | 46.1 ± 9.1* | 68.1 ± 11.3† | 39.6 ± 9.8† | 58.4 ± 12.7† |
| Central SBP, mmHg | 119.3 ± 11.1* | 147.8 ± 11.0† | 132.0 ± 9.6*, † | 152.3 ± 18.1† |
| Central DBP, mmHg | 68.1 ± 6.9* | 75.4 ± 6.5† | 87.1 ± 3.8† | 89.7 ± 8.5† |
| Central PP, mmHg | 51.2 ± 10.2* | 72.4 ± 13.5† | 44.9 ± 10.6* | 62.6 ± 17.1† |
| Heart rate, beats/min | 73.0 ± 10.2 | 73.9 ± 9.9 | 76.0 ± 10.1 | 75.5 ± 11.8 |
| Body Mass Index, kg/m2 | 23.7 ± 3.3* | 25.2 ± 3.8† | 24.2 ± 2.8 | 25.4 ± 3.4† |
| Waist circumference, cm | 83.8 ± 9.6* | 88.0 ± 10.0† | 87.6 ± 6.8 | 89.1 ± 9.8† |
| Triglycerides, mg/dL | 119.0 ± 67.9* | 135.5 ± 98.8 | 138.1 ± 65.7 | 124.9 ± 62.8 |
| HDL cholesterol, mg/dL | 54.9 ± 16.1 | 52.8 ± 15.5 | 49.7 ± 12.8 | 51.9 ± 13.8 |
| LDL cholesterol, mg/dL | 122.5 ± 31.6 | 122.8 ± 35.2 | 135.1 ± 28.6 | 124.9 ± 27.5 |
| Total cholesterol, mg/dL | 194.3 ± 34.3 | 196.0 ± 39.4 | 206.8 ± 34.6 | 193.9 ± 32.5 |
| Glucose, mg/dL | 107.0 ± 29.1 | 112.7 ± 29.5 | 110.8 ± 30.7 | 113.0 ± 43.7 |
| Obesity, % | 15.81%* | 27.03%† | 21.74% | 30.51%† |
| Central obesity, % | 43.37%* | 63.96%† | 52.17% | 57.63%† |
| Diabetes, % | 13.26%* | 21.62%† | 17.39% | 15.25% |
| Smoking, % | 14.42% | 10.81% | 26.09% | 16.95% |
| Low physical activity | 29.30% | 29.73% | 17.39% | 27.12% |
Obesity was defined as body mass index ≥27 kg/m2; central obesity was defined as waist circumference ≥90 cm for men and ≥80 cm for women.
Abbreviations: DBP, diastolic blood pressure; HDL, high‐density lipoprotein; IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; LDL, low‐density lipoprotein; NT, normotension; PP, pulse pressure; SBP, systolic blood pressure; SDH, systolic/diastolic hypertension.
p‐value < .05 in comparison with ISH group.
p‐value < .05 in comparison with NT group.
Both younger and older subjects with IDH and SDH were predominantly men. In contrast, there was a sex difference in the epidemiology of ISH. Adults with ISH were predominantly men before age 50 years but were predominantly women after age 50 (Tables 1 and 2, Figure 1A and B).
FIGURE 1.
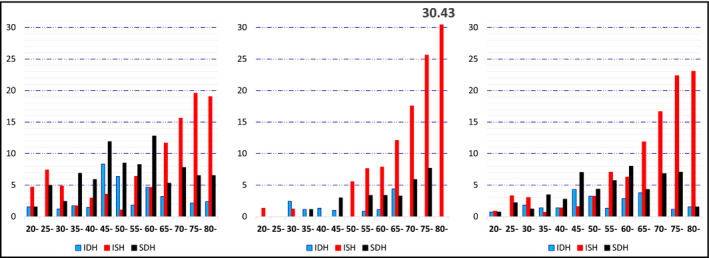
Prevalence of IDH, ISH, and SDH among adults without taking antihypertensive medications, according to each 5‐year age group in men (A), women (B), and (C) total population. IDH, isolated diastolic hypertension; ISH, isolated systolic hypertension; SDH, systolic/diastolic hypertension
ISH was the dominant hypertension subtype in men aged 20–30 years and 65–80 years (Figure 1A). In contrast, the prevalence of ISH in women was very low before age 50 years but increased dramatically after age 50, and ISH was the dominant hypertension subtype in women across the age range of 50–80 year (Figure 1B). The prevalence of IDH peaked at age 45 and declined with increasing age thereafter in men (Figure 1A), but it was consistently low in women, especially after 70 years old (Figure 1B). SDH was the dominant hypertension subtype in men aged 35–60 years (Figure 1A). In women, SDH was rare before age 40 and the rate increased gradually after age 50 (Figure 1B). Overall, ISH was the dominant hypertension subtype in the separate age ranges of 20–30 years and 65–80 years, respectively (Figure 1C).
3.2. Characteristics of ISH
Among younger adults (<50 years old), ISH was associated with male gender, greater body mass index and waist circumference, higher levels of triglycerides, total cholesterol, LDL cholesterol and fasting glucose, lower level of high‐density lipoprotein cholesterol, and higher prevalence of obesity, central obesity, diabetes, and smoking, when compared with NT (Table 1). Moreover, among hypertension subtypes, ISH patients had the youngest age and the highest measures of body mass index and waist circumference, and the highest prevalence of central obesity and diabetes (Table 1). When compared with NT, subjects with IDH or SDH had older age, more male gender, greater body mass index, waist circumference, level of triglycerides, and prevalence of obesity, central obesity, and diabetes (Table 1). Heart rate was not significantly different among NT and all subtypes of hypertension (Table 1).
Among older adults (≥50 years old), ISH was associated with age, greater body mass index and waist circumference, higher level of triglycerides, and higher prevalence of obesity, central obesity, and diabetes, when compared with NT (Table 2). Moreover, among hypertension subtypes, ISH patients had the highest age and the highest prevalence of central obesity, diabetes, and low physical activity (Table 2). When compared with NT, subjects with IDH or SDH had more male gender (Table 2). Heart rate was not significantly different among NT and all subtypes of hypertension (Table 1).
3.3. ISH and central blood pressure
Among younger adults (<50 years old), compared with NT subjects, subjects with ISH had significantly higher brachial and central SBP, DBP, and PP (Table 1). Compared with subjects with IDH, brachial and central DBP were significantly lower in ISH individuals, but brachial and central PP and central SBP were significantly higher (Table 1). Compared with subjects with SDH, brachial SBP and DBP and central DBP were significantly lower in ISH individuals (Table 1). There were no differences in brachial or central PP between ISH and SDH subjects (Table 1). Central hypertension was found in all subjects with ISH or SDH and in 62.5% of subjects with IDH (Table S1).
Among older adults (≥50 years old), compared with NT subjects, subjects with ISH had significantly higher brachial and central SBP, DBP, and PP (Table 2). Compared with subjects with IDH, brachial and central DBP were significantly lower in ISH individuals, but brachial and central SBP and PP were significantly higher (Table 2). Compared with subjects with SDH, brachial and central SBP and DBP were significantly lower in ISH individuals, but brachial and central PP were significantly higher (Table 2). Central hypertension was found in all subjects with ISH, in 98.31% of SDH, and in 69.67% of IDH (Table S1).
Overall, levels of central SBP and PP according to each 5‐year age group are shown in Figure 2. In both men and women, central SBP and PP increased with age roughly after age 50 years (Figure 2A and 2B). However, central PP was high at age 20 years and declined with age until age 50 (Figure 2A, 2B, and 2C). The J‐shaped association between age and central PP roughly simulates the association between age and ISH prevalence (Figure 2C). ISH prevalence rates were 2.95%, 1.73%, and 10.54% (p‐value of χ2 < .0001), the average levels of central PP were 49.5, 47.0, and 54.0 mmHg, and the prevalence rates of central obesity were 22.1%, 32.7%, and 46.53% for subjects aged <30, between 30 and 49, and ≥50 years, respectively.
Among younger adults (<50 years old), multivariable logistic regression showed that male gender, central PP, central obesity, and high LDL cholesterol were independently associated with ISH (Table 3). Among older adults (≥50 years old), multivariable logistic regression showed that central PP and central obesity were independently associated with ISH (Table 3). Among all adults, multivariable logistic regression showed that age, high LDL, central PP, and central obesity were independently associated with ISH (Table 3). Heart rate was not significantly associated with ISH in any multivariable models.
TABLE 3.
Determinants of isolated systolic hypertension when compared with normotensives, multivariable analysis
| Age < 50 years | Age ≥ 50 years | All | |
|---|---|---|---|
| Age, years | 1.02 (1.01–1.04) | ||
| Men vs. women | 10.2 (2.21–47.16) | ||
| Central pulse pressure, mmHg | 1.10 (1.05–1.15) | 1.17 (1.14–1.20) | 1.15 (1.12–1.17) |
| Central obesity, yes vs. no | 11.46 (3.19–41.22) | 1.75 (1.05–2.93) | 2.37 (1.50–3.76) |
| High LDL, yes vs. no | 3.84 (1.30–11.32) | 1.82 (1.16–2.85) |
Numbers are odds ratios and 95% confidence intervals. Heart rate as a co‐variate did not enter any of the models.
Abbreviation: LDL, low‐density lipoprotein cholesterol.
4. DISCUSSION
4.1. Main findings
Among the 2029 untreated adults from a nationally representative population, ISH was the most common subtype of hypertension. Younger adults with ISH were predominantly men and had a high prevalence of central obesity, whereas older adults with ISH were significantly associated with age and central obesity. All subjects with ISH had central hypertension and had a higher central PP than those with IDH or SDH. Moreover, there was a J‐shaped relationship between age and ISH prevalence and also between age and central PP. The association of central hypertension, central obesity, and high central PP with ISH may provide insights into the management for ISH, especially in the young‐ and middle‐aged adults.
4.2. Prevalence of ISH in the young‐ and middle‐aged adults
According to the analysis based on National Health and Nutrition Examination Survey (NHANES) III (1988–1994), for the younger hypertensive group (<50 years old), IDH was most common among untreated individuals, followed by SDH and ISH. 2 However, the 1999–2004 NHANES data indicated that ISH among young adults (aged 18–39 years and not on antihypertensive medications) was increasing in prevalence (1.57%) and was more common than SDH (0.93%). 25 In 3744 untreated subjects, aged <40 years from a multi‐ethnic prospective cohort study in Amsterdam, The Netherlands (the HELIUS study), the most common hypertension subtype was SDH (3.98%), followed by ISH (2.91%) and IDH (2.27%). 12 In 432 male Japanese workers and University students aged 18–49 years, the prevalence for ISH, IDH, and SDH was 5.78%, 10.87%, and 12.04%, respectively. 26 In contrast, among 1008 subjects aged 17 to 27 years from the ENIGMA study, the prevalence of ISH was 8% and other hypertension subtypes, 4%. 11
In the present study of an untreated nationally representative sample, as expected, the prevalence rate for ISH (2.15%) was lower among the young‐ and middle‐aged adults (<50 years old) in comparison to the older adults (≥50 years old, 10.54%). The prevalence of ISH was higher than IDH (1.64%) but lower than SDH (2.97%). Our results are in agreement with that of the HELIUS study. 12
Population studies have found that PP values may be high around the age of 20 years and low in the age range between 20 and 40 years. 27 , 28 , 29 , 30 The Hypertension Ambulatory Recording Venetia Study found a J‐shaped association between PP and age for subjects aged 18–45 years old. 31 Similarly, the HELIUS study found a U‐shaped association between brachial/central SBP and age for subjects aged <40 years old. 12 The J‐shaped association between age and ISH prevalence has been reported in prior studies. 12 , 25 , 31 For example, the NHANES 1999–2004 that male aged 30–39 years old had a lower ISH prevalence rate than those male aged 18–29 years old (1.94% vs. 2.48%). 25 In the present study, we also found a J‐shaped relationship between age and ISH prevalence, and between age and central PP. Moreover, we found that central PP and central obesity were the independent determinants of ISH in both younger and older groups. Therefore, we speculate that central PP/central hypertension and central obesity may contribute to the J‐shaped association between age and ISH. Furthermore, the finding that the prevalence of ISH was high in the age range 20–30 years and low in the age range 35–45 years also supports the finding from the ENIGMA study that ISH is the dominant hypertension subtype in the young adults. 11
Although arterial stiffness and wave reflection phenomenon are major determinants of central blood pressure and both are heart rate dependent, 16 heart rate was not associated with ISH in prior studies. 11 , 12 , 14 , 26 , 32 For example, the ENIGANA study showed that ISH had almost the same levels of heart rate with NT (69 vs. 68 beats/min). 11 In the present study, heart rate was not significantly different between ISH and NT and heart rate was not an independent determinant of ISH in either group of younger or older subjects.
4.3. Clinical characteristics of ISH in the young and middle‐aged adults
The 1999–2004 NHANES reported that obesity (body mass index ≥30 kg/m2), male sex, education level less than high school, and current smoking were characteristics independently associated with higher odds of ISH among young‐ and middle‐aged adults. 25 The prevalence of obesity in the young‐ and middle‐aged Americans with ISH was about 40%. 25 In the HELIUS study, subjects with ISH had the greatest height, were younger, had a lower body mass index and cholesterol than those with SDH or IDH, while they were comparable in age, body mass index, and cholesterol with subjects having high‐normal blood pressure. 12 In the male Japanese workers and University students, the ISH participants had greater body mass index and waist circumference than participants with optimal blood pressure. 26 In the ENIGMA study ISH subjects were taller, heavier, had a greater body mass index, and were more likely to be male, when compared with normotensive subjects. 11
In the present study, high prevalence rates of central obesity and general obesity were found in both the younger (80.95%, 71.43%, respectively) and older (63.96%, 27.03%, respectively) adults with ISH. Previous studies have indicated that central obesity may be more important than general obesity in causing ISH. In 1031 adult individuals without manifest cardiovascular disease randomly selected from population, central obesity parameters were more closely associated with aortic stiffness than body mass index. 33 Furthermore, the Whitehall II cohort with 16‐year follow‐up concluded that central obesity was a strong predictor of aortic pulse wave velocity 16 years later in both sexes, even after adjustment for body mass index. 34 Even among normal weight individuals without manifest cardiovascular disease (n = 136), only central fat distribution was significantly associated with higher carotid‐femoral pulse wave velocity after adjustment for age and mean blood pressure. 35 Our results strongly support the role of central obesity in the pathogenesis of ISH in the young‐ and middle‐aged adults. Our study also reconfirms that male sex is a determinant of ISH in the young‐ and middle‐aged adults. 11 , 12 , 25 , 26
4.4. ISH and central blood pressure
Current evidence suggests two main phenotypes of ISH in the young, one is spurious systolic hypertension, and the other is ISH with increased cardiovascular risk. Young subjects with spurious systolic hypertension are characterized with normal arterial stiffness 14 and normal central blood pressure. 13 In contrast, young subjects with ISH and increased cardiovascular risk are characterized with increased arterial stiffness 11 , 12 and increased central blood pressure. 11 , 12 , 14
In the present study, young‐ and middle‐aged adults with ISH had central hypertension and higher central PP. In the HELIUS study, central SBP in subjects with ISH was higher when compared with subjects with high‐normal blood pressure but was lower than that of IDH and SDH. 12 In the male Japanese workers and University students, central SBP in subjects with ISH was higher when compared with subjects with high‐normal blood pressure but was lower than that of SDH, but central PP in ISH was higher than that of high‐normal blood pressure, IDH and SDH. 26 In the ENIGMA study, subjects with ISH had significantly higher peripheral and central SBP, DBP, and PP when compared with normotensive subjects. 11 Compared with subjects with IDH/SDH, peripheral and central PP were higher in ISH individuals. 11 Thus, a higher central PP but not central SBP appears to be a consistent finding in the young‐ and middle‐aged adults with ISH, irrespective of ethnicity. The ENIGMA study suggested that ISH in the youth appears to result from an increased stroke volume and/or aortic stiffness, whereas the major hemodynamic abnormality underlying IDH/SDH is an increased peripheral vascular resistance. 11 Therefore, the consistent finding of a high central PP is likely due to an increased stroke volume and/or aortic stiffness and does not support the role of exaggerated pulse pressure amplification in the young ISH. 13 , 36
Prevalence of spurious hypertension was 0% in the younger adults in the present study. In contrast, the Atherosclerosis Risk in Young Adults study reported that prevalence rates of spurious hypertension were 16.1% for men and 8% for women. 14 The Atherosclerosis Risk in Young Adults study used the SphygmoCor (type I central blood pressure device) to measure central blood pressure and spurious hypertension was defined as ISH with low central SBP (median 124 mmHg for men and 120 mmHg for women). 14 Our study used Microlife central blood pressure monitor (type II central blood pressure device) to measure central blood pressure. 37 Type I central blood pressure devices could underestimate the true central SBP. 37 The use of different central blood pressure measurement devices apparently may affect the estimation of the prevalence of spurious hypertension.
Recent studies have found that ISH in the young‐ and middle‐aged adults had significantly higher future cardiovascular risk. 5 , 38 , 39 The Chicago Heart Association Detection Project in Industry study revealed that younger‐ and middle‐aged adults (aged 18–49 years, during 31‐year average follow‐up period) with ISH had higher relative risk for cardiovascular disease and coronary heart disease mortality than those with optimal‐normal blood pressure. 5 Recently, from a nationwide health screening database of 6 424 090 participants (aged 20–39 years, with a median follow‐up of 13.2 years) who were not taking antihypertensive medications, stage 1 ISH, IDH, and SDH were all associated with higher CVD risks than normal blood pressure. 38 Furthermore, a sample with 4776 adults aged between 30 and 49 years from the NIPPON DATA80 (1980–2009) with 29‐year follow‐up revealed that ISH was independently associated with higher risk of cardiovascular disease mortality later in life. 39 We have shown that central hypertension is associated with increased cardiovascular risks. 22 Since central hypertension was detected in all ISH subjects in the present study, irrespective of age, our results may also support that ISH in the young‐ and middle‐aged adults is not benign.
4.5. Strengths and limitations
There are several limitations in the present study. First, some cardiovascular hemodynamic parameters, such as stroke volume and carotid‐formal pulse wave velocity, were not available in the National Nutrition and Health Survey. Second, we measured the central SBP and central PP using a type II central blood pressure monitor. 37 Therefore, our central blood pressure results may not be comparable to those measured using a type I device. 14 , 26 , 32
The major strength of the present study is that the study population was derived from a nationally representative sample of ethnic Chinese.
5. CONCLUSIONS
In a nationally representative population without taking antihypertensive medications, ISH was the dominant hypertension subtype. Subjects with ISH were characterized by the presence of central hypertension, a higher central PP, and central obesity, irrespective of age. Our findings support that ISH in the young‐ and middle‐aged adults is not benign and future studies are required to develop effective management strategies.
CONFLICT OF INTEREST
Microlife Co., Ltd., and National Yang‐Ming University have signed a contract for transfer of the noninvasive central blood pressure technique. The contract of technology transfer includes research funding for conducting studies validating this technique.
Supporting information
Table S1
Chuang S‐Y, Chang H‐Y, Tsai T‐Y, Cheng H‐M, Pan W‐H, Chen C‐H. Isolated systolic hypertension and central blood pressure: Implications from the national nutrition and health survey in Taiwan. J Clin Hypertens. 2021;23:656–664. 10.1111/jch.14105
Funding information
This work was funded by the Health Promotion Administration, Ministry of Health and Welfare. The content of this research may not represent the opinion of the Health Promotion Administration, Ministry of Health and Welfare. MOHW109‐HPA‐H‐114‐144702.
Contributor Information
Shao‐Yuan Chuang, Email: chuangsy@nhri.org.tw.
Chen‐Huan Chen, Email: chench@vghtpe.gov.tw.
DATA AVAILABILITY STATEMENT
Data from the National Nutrition and Health survey in Taiwan cannot be shared publicly because of the Law of Personal Information Protection in Taiwan. Data are available from the Center for Welfare and Health Data Sciences (contact via the Center for Welfare and Health Data Sciences, https://dep.mohw.gov.tw/dos/np‐2497‐113.html) for researchers who meet the criteria for access to confidential data.
REFERENCES
- 1. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 2. Franklin SS, Jacobs MJ, Wong ND, L'Italien GJ, Lapuerta P. Predominance of Isolated Systolic Hypertension Among Middle‐Aged and Elderly US Hypertensives : Analysis Based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37(3):869‐874. [DOI] [PubMed] [Google Scholar]
- 3. Chuang SY, Cheng HM, Chou P, Chen CH. Prevalance of isolatged systolic hypertension and the awareness, treatment, and control rate of hypertension in Kinmen. ACS. 2006;22:83‐90. [Google Scholar]
- 4. Chou P, Chen CH, Chen HH, Chang MS. Epidemiology of isolated systolic hypertension in Pu‐Li, Taiwan. Int J Cardiol. 1992;35(2):219‐226. [DOI] [PubMed] [Google Scholar]
- 5. Yano Y, Stamler J, Garside DB, et al. Isolated systolic hypertension in young and middle‐aged adults and 31‐year risk for cardiovascular mortality: The Chicago Heart Association Detection Project in Industry Study. J Am Coll Cardiol. 2015;65(4):327‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355(9207):865‐872. [DOI] [PubMed] [Google Scholar]
- 7. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265(24):3255‐3264. [PubMed] [Google Scholar]
- 8. Staessen JA, Fagard R, Thijs L, et al. Randomised double‐blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Lancet. 1997;350(9080):757‐764. [DOI] [PubMed] [Google Scholar]
- 9. Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst‐China) Collaborative Group. J Hypertens. 1998;16(12 Pt 1):1823‐1829. [DOI] [PubMed] [Google Scholar]
- 10. McEniery CM, Franklin SS, Cockcroft JR, Wilkinson IB. Isolated Systolic Hypertension in Young People Is Not Spurious and Should Be Treated: Pro Side of the Argument. Hypertension. 2016;68(2):269‐275. [DOI] [PubMed] [Google Scholar]
- 11. McEniery CM, Yasmin WS, et al. Increased stroke volume and aortic stiffness contribute to isolated systolic hypertension in young adults. Hypertension. 2005;46(1):221‐226. [DOI] [PubMed] [Google Scholar]
- 12. Eeftinck Schattenkerk DW, van Gorp J, Vogt L, Peters RJ. van den Born BH. Isolated systolic hypertension of the young and its association with central blood pressure in a large multi‐ethnic population, The HELIUS study. Eur J Prevent Cardiol. 2018;25(13):1351‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med. 2000;5(3):141‐145. [DOI] [PubMed] [Google Scholar]
- 14. Hulsen HT, Nijdam ME, Bos WJ, et al. Spurious systolic hypertension in young adults; prevalence of high brachial systolic blood pressure and low central pressure and its determinants. J Hypertens. 2006;24(6):1027‐1032. [DOI] [PubMed] [Google Scholar]
- 15. Palatini P, Rosei EA, Avolio A, et al. Isolated systolic hypertension in the young: a position paper endorsed by the European Society of Hypertension. J Hypertens. 2018;36(6):1222‐1236. [DOI] [PubMed] [Google Scholar]
- 16. Cheng HM, Park S, Huang Q, et al. Vascular aging and hypertension: Implications for the clinical application of central blood pressure. Int J Cardiol. 2017;230:209‐213. [DOI] [PubMed] [Google Scholar]
- 17. Saladini F, Santonastaso M, Mos L, et al. Isolated systolic hypertension of young‐to‐middle‐age individuals implies a relatively low risk of developing hypertension needing treatment when central blood pressure is low. J Hypertens. 2011;29(7):1311‐1319. [DOI] [PubMed] [Google Scholar]
- 18. Chuang SY, Chang HY, Cheng HM, Pan WH, Chen CH. Prevalence of hypertension defined by central blood pressure measured using a Type II device in a nationally representative cohort. Am J Hypertens. 2018;31(3):346‐354. [DOI] [PubMed] [Google Scholar]
- 19. Chiang CE, Wang TD, Ueng KC, et al. 2015 guidelines of the taiwan society of cardiology and the taiwan hypertension society for the management of hypertension. J Chinese Med Assoc. 2015;78(1):1‐47. [DOI] [PubMed] [Google Scholar]
- 20. Cheng HM, Sung SH, Shih YT, Chuang SY, Yu WC, Chen CH. Measurement accuracy of a stand‐alone oscillometric central blood pressure monitor: a validation report for Microlife WatchBP Office Central. Am J Hypertens. 2013;26(1):42‐50. [DOI] [PubMed] [Google Scholar]
- 21. Sung SH, Cheng HM, Chuang SY, et al. Measurement of central systolic blood pressure by pulse volume plethysmography with a noninvasive blood pressure monitor. AmJ Hypertens. 2012;25(5):542‐548. [DOI] [PubMed] [Google Scholar]
- 22. Cheng HM, Chuang SY, Sung SH, et al. Derivation and validation of diagnostic thresholds for central blood pressure measurements based on long‐term cardiovascular risks. J Am Coll Cardiol. 2013;62(19):1780‐1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pan WH, Lee MS, Chuang SY, Lin YC, Fu ML. Obesity pandemic, correlated factors and guidelines to define, screen and manage obesity in Taiwan. Obes Rev. 2008;9(Suppl 1):22‐31. [DOI] [PubMed] [Google Scholar]
- 24. American DA. Diagnosis and classification of diabetes mellitus. Diabet Care. 2010;33(Suppl 1):S62‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999–2004 US National Health And Nutrition Examination Survey. J Hypertens. 2010;28(1):15‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakagomi A, Imazeki F, Nishimura M, et al. Central blood pressure and pulse wave velocity in young and middle‐aged Japanese adults with isolated systolic hypertension. Hypertens Res. 2020;43(3):207‐212. [DOI] [PubMed] [Google Scholar]
- 27. Shen W, Zhang T, Li S, et al. Race and sex differences of long‐term blood pressure profiles from childhood and adult hypertension: The Bogalusa Heart Study. Hypertension. 2017;70(1):66‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Franklin SS, Wt G, Wong ND, et al. Hemodynamic patterns of age‐related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96(1):308‐315. [DOI] [PubMed] [Google Scholar]
- 29. Ayer JG, Harmer JA, Marks GB, Avolio A, Celermajer DS. Central arterial pulse wave augmentation is greater in girls than boys, independent of height. J Hypertens. 2010;28(2):306‐313. [DOI] [PubMed] [Google Scholar]
- 30. McEniery CM, Yasmin MB, et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo‐Cardiff Collaborative Trial II. Hypertension. 2008;51(6):1476‐1482. [DOI] [PubMed] [Google Scholar]
- 31. Saladini F, Fania C, Mos L, Mazzer A, Casiglia E, Palatini P. Office pulse pressure is a predictor of favorable outcome in young‐ to middle‐aged subjects with stage 1 hypertension. Hypertension. 2017;70:537‐542. [DOI] [PubMed] [Google Scholar]
- 32. Radchenko GD, Torbas OO, Sirenko YM. Predictors of high central blood pressure in young with isolated systolic hypertension. Vasc Health Risk Manag. 2016;12:321‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wohlfahrt P, Somers VK, Cifkova R, et al. Relationship between measures of central and general adiposity with aortic stiffness in the general population. Atherosclerosis. 2014;235(2):625‐631. [DOI] [PubMed] [Google Scholar]
- 34. Johansen NB, Vistisen D, Brunner EJ, et al. Determinants of aortic stiffness: 16‐year follow‐up of the Whitehall II study. PLoS One. 2012;7(5):e37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wohlfahrt P, Somers VK, Sochor O, Kullo I, Jean N, Lopez‐Jimenez F. Influence of body fatness distribution and total lean mass on aortic stiffness in nonobese individuals. Am J Hypertens. 2015;28(3):401‐408. [DOI] [PubMed] [Google Scholar]
- 36. Mahmud A, Feely J. Spurious systolic hypertension of youth: fit young men with elastic arteries. Am J Hypertens. 2003;16(3):229‐232. [DOI] [PubMed] [Google Scholar]
- 37. Sharman JE, Avolio AP, Baulmann J, et al. Validation of non‐invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38(37):2805‐2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee H, Yano Y, Cho SMJ, et al. Cardiovascular Risk of Isolated Systolic or Diastolic Hypertension in Young Adults. Circulation. 2020;141(22):1778‐1786. [DOI] [PubMed] [Google Scholar]
- 39. Hisamatsu T, Miura K, Ohkubo T, et al. Isolated systolic hypertension and 29‐year cardiovascular mortality risk in Japanese adults aged 30–49 years. J Hypertens. 2020;38(11):2230‐2236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
Data from the National Nutrition and Health survey in Taiwan cannot be shared publicly because of the Law of Personal Information Protection in Taiwan. Data are available from the Center for Welfare and Health Data Sciences (contact via the Center for Welfare and Health Data Sciences, https://dep.mohw.gov.tw/dos/np‐2497‐113.html) for researchers who meet the criteria for access to confidential data.


