Abstract
Obstructive sleep apnea (OSA) is a risk of hypertension and is associated with cardiovascular disease (CVD) incidence. In Asian countries, the prevalence of OSA is high, as in Western countries. When blood pressure (BP) is evaluated in OSA individuals using ambulatory BP monitoring (ABPM), the BP phenotype often indicates abnormal BP variability, such as increased nighttime BP or abnormal diurnal BP variation, that is, non‐dipper pattern, riser pattern, and morning BP surge, and all these conditions have been associated with increased CVD events. Asians have a higher prevalence of increased nighttime BP or morning BP surge than Westerners. Therefore, this review paper focused on OSA and hypertension from an Asian perspective to investigate the importance of the association between OSA and hypertension in the Asian population. Such abnormal BP variability has been shown to be associated with progression of arterial stiffness, and this association could provoke a vicious cycle between abnormal BP phenotypes and arterial stiffness, a phenomenon recognized as systemic hemodynamic atherothrombotic syndrome (SHATS). OSA may be one of the background factors that augment SHATS. An oxygen‐triggered nocturnal oscillometric BP measurement device combined with a pulse oximeter for continuous SpO2 monitoring could detect BP variability caused by OSA. In addition to treating the OSA, accurate and reliable detection and treatment of any residual BP elevation and BP variability caused by OSA would be necessary to prevent CVD events. However, more detailed detection of BP variability, such as beat‐by‐beat BP monitoring, would further help to reduce CV events.
Keywords: blood pressure variability, hypertension, obstructive sleep apnea
1. INTRODUCTION
Sleep conditions, such as obstructive sleep apnea (OSA), sleep duration, and nocturia, are strongly associated with the risk of cardiovascular disease (CVD) events. 1 , 2 , 3 , 4 , 5 The repetitive episodes of breathing cessation due to upper airway collapse during sleep in individuals with OSA confer a risk of hypertension and are also associated with organ damage and CVD events. 6 , 7 , 8 As in Western countries, the prevalence of OSA is high in Asia. 9 , 10 The termination of an episode of apnea or hypopnea in OSA causes an acute and transient rise in BP. Conventionally, ambulatory blood pressure monitoring (ABPM) is the gold standard for measuring nighttime BP. However, ABPM by itself is not sufficient for detecting this transient BP elevation during sleep in OSA. To obtain BP readings induced by an OSA episode in a clinical setting, we developed an oxygen‐triggered nocturnal BP (TNP) monitoring method that initiates measurements when oxygen desaturation occurs. 11 , 12
Continuous positive airway pressure (CPAP) is the primary therapy for patients with severe OSA. 4 , 5 , 8 However, hypertensive patients with severe OSA are sometimes asymptomatic. Since non‐symptomatic OSA patients often have poor compliance for continuing CPAP therapy, we need to manage and select alternative treatments. This review provides current information on the pathophysiology, BP assessment, and treatment of hypertension with OSA (Table 1).
TABLE 1.
Main points of this review
|
The prevalence of obstructive sleep apnea (OSA) is high in Asia as in Western countries. |
|
OSA may be one of the predisposing factors for systemic hemodynamic atherothrombotic syndrome. |
|
OSA induces transient blood pressure (BP) elevation during sleep. The oxygen‐triggered nocturnal BP monitoring method that we developed may detect this BP variability. |
|
Continuous positive airway pressure is an established treatment for the symptoms of OSA regardless of the presence or absence of hypertension. Catheter‐based renal sympathetic denervation may be an alternative treatment to achieve BP reduction in patients with OSA. |
2. CHARACTERISTICS OF OSA IN ASIA
Obesity is one of the most important risk factors for OSA. Although the degree of obesity is lower in Asians than Caucasians, OSA is a common disease in both populations. Recent data showed that the country with the highest prevalence of OSA is China, followed in order by the United States, India, and Brazil. The numbers of individuals with an apnea‐hypopnea index (AHI) ≥15/hr were 66 million, 54 million, 29 million, and 20 million, respectively. 13 However, in other Asian countries, the prevalence of OSA is still high in the population aged 30‐69 years (Figure 1). 13 Abnormalities in craniofacial structure are also an important factor in OSA. When comparing Asians with Caucasians, Asians have a shorter cranial base, and shorter maxilla and mandible length after matching body mass index (BMI) or severity of OSA. 14 Because of the high prevalence of OSA and the association of OSA with CV risk in Asia, the diagnosis and treatment of OSA are important.
FIGURE 1.
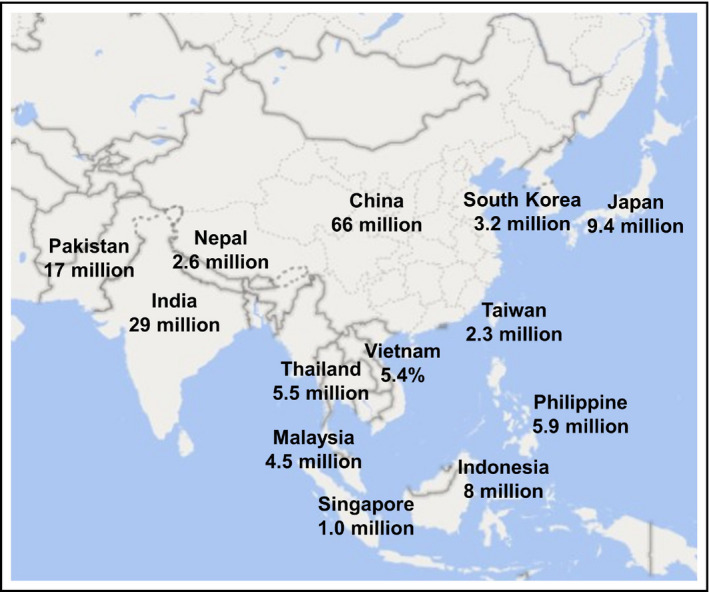
Prevalence of obstructive sleep apnea with AHI ≥ 15 events per hr in the population aged 30‐69 years by country or region
3. OSA AND HYPERTENSION
Obstructive sleep apnea syndrome (OSAS) is one of the causes of secondary and resistant hypertension. There are several pathological mechanisms causing hypertension in individuals with OSA. Intermittent arousal or hypoxia, especially in the presence of hypercapnia, as well as the initiation of elevation of sympathetic nervous system activity and decreased parasympathetic nervous activity, all can result in vasoconstriction and increased BP and heart rate (HR). Moreover, large intrathoracic pressure swings may increase cardiac preload and afterload, which also result in BP elevation. Chronically, prolonged hypoxia induces activation of oxidative stress, systematic inflammation, and endothelial dysfunction, which are also linked to elevation of BP and thereby to hypertension. Peter‐Derex L et al 15 outlined the comprehensive mechanism underlying the association between OSA and wake‐up stroke, which is defined as the situation where a patient awakens with stroke symptoms that were not present prior to falling asleep. That paper demonstrated that the main mechanisms responsible for the occurrence of wake‐up stroke are hypertension, arterial fibrillation, a hypercoagulable state, decreased cerebral vasoreactivity, and right‐to‐left shunting with subsequent paradoxical embolism in the case of the patients with patent foramen ovale caused by OSA. Although their review was intriguing, there may be racial differences in some of these conditions, such as a hypercoagulable state 16 and the prevalence of patent foramen ovale. 17 Further studies will be needed to determine whether a comprehensive mechanism underlying the association between OSA and wake‐up stroke exists in Asian populations.
When BP is evaluated in individuals with OSAS using ambulatory BP monitoring (ABPM), the resulting BP phenotype often indicates abnormal BP variability, increased nighttime BP, or abnormal circadian BP variation, that is, a non‐dipper or riser pattern (Figure 2). 18 , 19 These abnormal BP variabilities have been associated with the progression of arterial stiffness, and this association could provoke a vicious cycle between abnormal BP phenotypes and arterial stiffness, a phenomenon that is now recognized as systematic hemodynamic atherothrombotic syndrome (SHATS). 20 OSA may be one of the predisposing factors for SHATS. As an explanation for the association between OSA and the risk of cardiovascular events, it is plausible that BP elevation and abnormal BP variability lead to cardiovascular events. However, further studies will be needed to clarify whether it is the BP elevation and abnormal BP variability caused by OSA that contribute to cardiovascular events or whether it is merely the presence of OSA itself that causes CV events.
FIGURE 2.
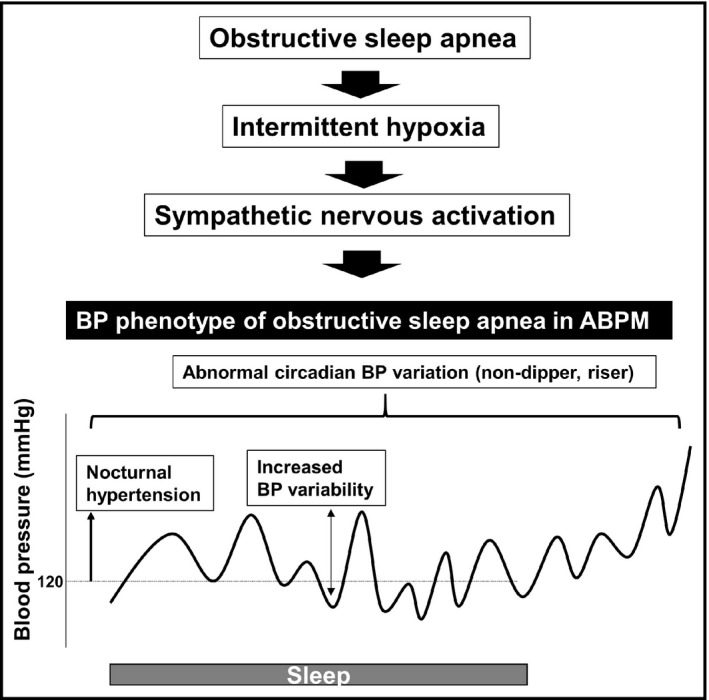
Obstructive sleep apnea and blood pressure phenotype observed in ABPM. ABPM indicates ambulatory blood pressure monitoring; BP, blood pressure
Over the last two decades, several studies have reported on the association between OSA and an abnormal dipping BP pattern as assessed by ABPM in the Japanese population. Akashiba et al examined whether a dipping BP pattern could be modified by CPAP treatment in 38 Japanese patients with severe OSA (AHI ≥ 30 events/hr and severe arterial oxygen desaturation [SaO2 < 75%]). They found that, after CPAP treatment for 3 days, both daytime BP and nighttime BP as assessed by ABPM were significantly reduced, and 15 of 22 participants who were non‐dippers before CPAP treatment became dippers. 21 In regard to the association between dipping BP patterns and OSA in Western populations, the Wisconsin Sleep Cohort Study examined 323 employees who performed the overnight sleep study protocol at baseline, and ABPM was performed twice or more during follow‐up (average 7.2 years). The results showed that the individuals with AHI of 5 to 15 events/hr had a threefold increased risk of developing a non‐dipper pattern. 22 In an open‐label, randomized, multicenter clinical trial of patients with OSA on the Spanish Resistant Hypertension Registry who did or did not receive 12 weeks of CPAP treatment, the patients who received the CPAP treatment had significantly reduced 24‐hr BP levels and significantly increased prevalence of the dipper pattern compared to those without CPAP treatment. 23 Recently, in 541 Japanese patients with severe OSA (AHI ≥ 30 events/hr) diagnosed using polysomnography and ABPM, the cumulative percentage of time at a saturation below 90% was found to be an independent risk factor for the non‐dipper and riser pattern in the younger group (<60 years of age). 24 Cuspidi et al 25 reported that in a meta‐analysis including 1562 patients with OSA and 957 non‐OSA controls from 14 studies, including the paper mentioned above, OSA significantly increased the risk of non‐dipper status by approximately 1.5‐fold. However, to our knowledge there has been no study investigating whether the improvement of abnormal diurnal BP variation contributes to a reduction in the progression of organ damage or cardiovascular events.
In addition, primary aldosteronism (PA) is known to be a cause of secondary and resistant hypertension. Li et al 26 reported that snoring patients with PA had a higher prevalence of moderate‐to‐severe OSAS. Therefore, we need to take care not to overlook the presence of OSA in patients with PA.
4. OSA AND BP SURGE
Currently, to detect transient BP elevation at the time of an apneic episode, invasive or complicated BP measurements such as peripheral arterial tonometry are needed. A new TNP system that combines an oscillometric BP measurement device (HEM‐780 system; Omron Healthcare Co., Ltd., Kyoto, Japan) with a pulse oximeter for continuous SpO2 monitoring is now available for use. 11 , 12 During sleep, the oxygen‐triggered function initiates 3 BP measurements at 15‐second intervals when the SpO2 level falls below a variable threshold. Previously, we reported the case of a 36‐year‐old male patient with severe OSA (AHI 75.5/hr) who experienced the recurrence of stroke while using this system. Under antihypertensive medication (telmisartan 40 mg, amlodipine 5 mg, and bisoprolol 5 mg), an office BP level of less than 140/90 mmHg was achieved in this patient. However, the maximum SBP triggered by hypoxia using this TNP system was 209 mmHg. 27
In a related study, we used the TNP system to investigate the association between the BP level triggered by hypoxia during an apneic episode and the characteristics of the preceding apneic episode in 42 patients with OSA (average age 62 years; averaged AHI 32.6/hr). We found that rapid eye movement (REM) sleep and severe oxygen desaturation were associated with the extent of BP surge triggered by hypoxia during the apneic episode. 12 These results indicate that BP surge from midnight to early morning may confer a risk of CVD events associated with OSA, because REM sleep is mostly concentrated in the second half of the sleep period. Elevated nighttime BP, morning hypertension, and exaggerated morning BP surge are the characteristics of hypertension in OSA, and all three have been associated with target organ damage and CVD incidence. 28 , 29 , 30 Asian individuals have higher nighttime BP and morning BP surge than their Western counterparts. 31 , 32 When morning hypertension or morning BP surge is detected, we need to consider the presence of OSA, and this may be of particular importance in the Asian population.
As mentioned above, ABPM would be a more suitable approach to gain detailed information on BP during the nighttime, when OSA episodes occur, compared to home BP measurement, because there is not enough evidence about nighttime BP assessed by home BP monitoring. However, we recently reported that nighttime BP assessed by home BP monitoring was associated with a risk of CVD incidence in a Japanese clinical population. 29 Another noninvasive technique is based on the method of pulse transit time (PTT) for the assessment of BP variability, although this method awaits appropriate validation according to international protocols. Most recently, Misaka et al reported on the association between BP variability measured using PTT and subclinical organ damage in 242 Japanese patients with suspected sleep‐disordered breathing. Their results demonstrated that the PTT index was significantly associated with AHI, ODI, and minimal SpO2. In addition, very short‐time BP variability defined as a standard deviation of the diastolic BP component of the PTT index was shown to be associated with the presence of chronic kidney disease and cardiac hypertrophy. 33
5. TREATMENT
As the primary treatment for OSA, exercise, especially aerobic exercise, is recommended, and exercise would also contribute to weight loss, which would result in positive effects on multiple cardiovascular and metabolic diseases. CPAP is an established treatment for the symptoms of OSA regardless of the presence or absence of hypertension. However, the use of CPAP for the purpose of reducing BP in hypertensive patients with OSA has been debated. The 2017 AHA/ACC BP guideline mentions that the effectiveness of CPAP for reducing BP is not well established in adults with hypertension and OSA (class IIb). 34 Non‐symptomatic OSA patients also have poor compliance for continuing CPAP therapy. Hypertensive patients with OSA are sometimes asymptomatic for OSA, and poor compliance with CPAP is associated with resistant hypertension. 35 However, as described in the clinical case of a patient with hypertension and symptoms of OSA, CPAP can achieve a dramatic improvement of symptoms and reduction in BP. Adding lifestyle modification to CPAP therapy is also important for BP reduction in OSA patients. For increased CPAP adherence, appropriate education, monitoring of CPAP use, and behavioral intervention are needed. Chirinos et al compared CPAP therapy, weight‐loss therapy, and CPAP plus weight‐loss therapy in terms of their ability to reduce office BP in patients with severe OSA and obesity. Although all three groups showed office BP reduction, the per‐protocol analysis, which also took into consideration the compliance with CPAP use, showed that only the group with CPAP treatment alone showed a reduction in office BP at the 24‐week follow‐up. Thus, an individualized approach would be needed for the treatment of hypertension with OSA.
The mandibular advancement device (MAD) is an alternative option for OSA treatment in patients who are unable to tolerate CPAP. In a recent network meta‐analysis comparing the effect of CPAP, MAD, or an inactive control on office BP, CPAP, and MAD were associated with a reduction in office SBP of 2.5 mmHg (95% CI, 1.5‐3.5 mmHg, p < .001) and 2.1 mmHg (95%CI, 0.8‐3.4 mmHg, p = .002), respectively, compared with the inactive control in those with OSAS (AHI ≥ 5/hr). However, there was no significant difference between CPAP and MAD in terms of reduction in office SBP. 36
Catheter‐based renal sympathetic denervation (RDN) may be an alternative treatment for BP reduction in those with OSA. As described above, increased sympathetic nervous activity plays a key role in BP elevation in individuals with OSA. Therefore, RDN may be useful for BP reduction in OSA patients with hypertension. This concept was partly confirmed in the post hoc analysis of the Symplicity HTN‐3 study, which failed to demonstrate superiority of RDN over a sham catheter control in terms of lowering office or ambulatory BP. Recently, Warchol‐Celnska et al reported the results of a randomized trial on the effect of RDN on office and ambulatory BP in patients with resistant hypertension coexisting with moderate‐to‐severe OSA (AHI ≥ 15/hr). At 3 months of follow‐up, RDN reduced office SBP, 24‐hr SBP, daytime SBP, and nighttime SBP by −22 mmHg, −12 mmHg, −14 mmHg, and −9 mmHg, respectively, while no reduction was seen in the control (non–sham catheter) group. 37 In our 2 cases of resistant hypertension with mild OSA, RDN reduced the BP surge triggered by hypoxia during sleep using this TNP system. 38 Recently, Chen et al 39 reported that CPAP treatment may be effective in lowering daytime BP in patients with a faster pulse rate, nocturnal hypertension, and moderate‐to‐severe OSA. That study also suggested that the presence of increased sympathetic nervous activity may be important for the BP‐reducing effect of OSA treatment.
There are several studies about the association between different antihypertensive drugs and BP reduction in patients with OSA. When considering the mechanism underlying the BP elevation in OSA patients, sympatholytic antihypertensive drugs may be the most useful. Kraiczi et al reported a comparison of atenolol (50 mg), amlodipine (5 mg), enalapril (20 mg), hydrochlorothiazide (25 mg), and losartan (50 mg) on ambulatory BP in patients with hypertension and OSA (AHI ≥ 10/hr). Their results showed that atenolol achieved the greatest reduction, lowering nighttime SBP by −13.3 mmHg and DBP by −10 mmHg, followed by hydrochlorothiazide (SBP, −8.5 mmHg; DBP, −5.8 mmHg). 40
6. CONCLUSIONS
In both Asian and Western populations, OSA is highly prevalent and constitutes a major problem because of its association with hypertension and CVD events. OSA can lead to BP surges during sleep, which are associated with target organ damage and may trigger CVD events. However, the BP variability associated with OSA may be overlooked by the traditional methods, such as ABPM or TNP systems. More recently developed wearable devices that allow noninvasive, cuff‐less, beat‐by‐beat BP measurements may help to solve this problem. Accurate and reliable detection and treatment of BP elevation and BP variability caused by OSA are important goals in the prevention of CVD events. While the present work showed that BP readings induced by an OSA episode can be obtained using the proposed method, future research should be done to further evaluate the effectiveness of this approach for detecting BP elevation during sleep, as well as to discover its limitations.
ACKNOWLEDGEMENTS
We gratefully acknowledge Ms. Ayako Okura for editorial assistance.
CONFLICT OF INTEREST
SH has received research grants from Sanofi Co., Astellas Pharma Inc, and Novartis Pharma KK KK has received independent principal investigator–initiated research grants from Omron Healthcare Inc, Fukuda Denshi Inc, A&D Inc, Taisho Pharmaceutical Co. Inc, and Sanwa Kagaku Kenkyusho Co. Inc YC Chia has received an honorarium and sponsorships to attend conferences and seminars from Boehringer Ingelheim, Pfizer, Omron, Servier, and Xepa‐Soul and an investigator‐initiated research grant from Pfizer. CH Chen reports personal fees from Novartis, Sanofi, Daiichi Sankyo, Servier, Bayer, and Boehringer Ingelheim Pharmaceuticals Inc HM Cheng received speakers’ honoraria and sponsorships to attend conferences and CME seminars from Eli Lilly, AstraZeneca, Pfizer Inc, Bayer AG, Boehringer Ingelheim Pharmaceuticals Inc, Daiichi Sankyo, Novartis Pharmaceuticals Inc, Servier, Pharmaceuticals Corporation, Sanofi, and Takeda Pharmaceuticals International and has served as an advisor and consultant to ApoDx Technology Inc S Park reports a research grant from Sankyo and lecture fees from Sankyo, Servier, Daewoong, Donga, Takeda, Boryung, Hanmi, Pfizer, and Servier. All other authors report no potential conflicts of interest in relation to this review paper.
AUTHORS CONTRIBUTIONS
Hoshide S takes primary responsibility for this paper. Hoshide S wrote the manuscript. Hoshide S, Kario K, Chia YC, Siddique S, Buranakitjaroen P, Tsoi K, Tay JC, Turana Y, Chen CH, Cheng HM, Minh HV, Park S, Soenarta AA, Sogunuru GP, Wang TD, and Wang JG reviewed/edited the manuscript.
Hoshide S, Kario K, Chia Y‐C, et al. Characteristics of hypertension in obstructive sleep apnea: An Asian experience. J Clin Hypertens. 2021;23:489–495. 10.1111/jch.14184
REFERENCES
- 1. Hu H, Li H, Huang X, et al. Association of self‐reported sleep duration and quality with baPWV levels in hypertensive patients. Hypertens Res. 2020;43(12):1392‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hirata T, Nakamura T, Kogure M, et al. Reduced sleep efficiency, measured using an objective device, was related to an increased prevalence of home hypertension in Japanese adults. Hypertens Res. 2020;43:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tabara Y, Matsumoto T, Murase K, et al. Frequent nocturnal urination in older men is associated with arterial stiffness: the Nagahama study. Hypertens Res. 2019;42:1996‐2001. [DOI] [PubMed] [Google Scholar]
- 4. Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: Joint recommendations by the european society of hypertension, by the European Respiratory Society and by the members of European Cost (cooperation in scientific and technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633‐646. [DOI] [PubMed] [Google Scholar]
- 5. Parati G, Lombardi C, Hedner J, et al. Recommendations for the management of patients with obstructive sleep apnoea and hypertension. Eur Respir J. 2013;41:523‐538. [DOI] [PubMed] [Google Scholar]
- 6. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology foundation scientific statement from the American Heart Association council for high blood pressure research professional education committee, council on clinical cardiology, stroke council, and council on cardiovascular nursing. J Am Coll Cardiol. 2008;52:686‐717. [DOI] [PubMed] [Google Scholar]
- 7. Medeiros MA, Lins‐Filho OL, Patriota T, et al. Abnormal atrial function in hypertensive patients with obstructive sleep apnea assessed by speckle tracking echocardiography. Hypertens Res. 2020;43:841‐844. [DOI] [PubMed] [Google Scholar]
- 8. Tokunou T, Ando SI. Recent advances in the management of secondary hypertension‐obstructive sleep apnea. Hypertens Res. 2020;43(12):1338‐1343. [DOI] [PubMed] [Google Scholar]
- 9. Neo WL, Ng ACW, Rangabashyam M, et al. Prevalence of cardiac arrhythmias in asian patients with obstructive sleep apnea: A Singapore sleep center experience. J Clin Sleep Med. 2017;13:1265‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao A, Tey BH, Ramalingam G, Poh AG. Obstructive sleep apnoea (OSA) patterns in bariatric surgical practice and response of OSA to weight loss after laparoscopic adjustable gastric banding (LAGB). Ann Acad Med Singap. 2009;38:587‐587. [PubMed] [Google Scholar]
- 11. Kuwabara M, Hamasaki H, Tomitani N, Shiga T, Kario K. Novel triggered nocturnal blood pressure monitoring for sleep apnea syndrome: Distribution and reproducibility of hypoxia‐triggered nocturnal blood pressure measurements. J Clin Hypertens. 2017;19:30‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sasaki N, Nagai M, Mizuno H, Kuwabara M, Hoshide S, Kario K. Associations between characteristics of obstructive sleep apnea and nocturnal blood pressure surge. Hypertension. 2018;72:1133‐1140. [DOI] [PubMed] [Google Scholar]
- 13. Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature‐based analysis. Lancet Respir Med. 2019;7:687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33:1075‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peter‐Derex L, Derex L. Wake‐up stroke: from pathophysiology to management. Sleep Med Rev. 2019;48:e101212. [DOI] [PubMed] [Google Scholar]
- 16. Chan MY, Andreotti F, Becker RC. Hypercoagulable states in cardiovascular disease. Circulation. 2008;118:2286‐2297. [DOI] [PubMed] [Google Scholar]
- 17. Kuramoto J, Kawamura A, Dembo T, Kimura T, Fukuda K, Okada Y. Prevalence of patent foramen ovale in the Japanese population‐ autopsy study. Circ J. 2015;79:2038‐2042. [DOI] [PubMed] [Google Scholar]
- 18. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 19. Somuncu MU, Karakurt ST, Karakurt H, Serbest NG, Cetin MS, Bulut U. The additive effects of OSA and nondipping status on early markers of subclinical atherosclerosis in normotensive patients: a cross‐sectional study. Hypertens Res. 2019;42:195‐203. [DOI] [PubMed] [Google Scholar]
- 20. Kario K. Systemic hemodynamic atherothrombotic syndrome (SHATS): diagnosis and severity assessment score. J Clin Hypertens. 2019;21:1011‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akashiba T, Minemura H, Yamamoto H, Kosaka N, Saito O, Horie T. Nasal continuous positive airway pressure changes blood pressure "non‐dippers" to "dippers" in patients with obstructive sleep apnea. Sleep. 1999;22:849‐853. [DOI] [PubMed] [Google Scholar]
- 22. Hla KM, Young T, Finn L, Peppard PE, Szklo‐Coxe M, Stubbs M. Longitudinal association of sleep‐disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin sleep cohort study. Sleep. 2008;31:795‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martínez‐García MA, Capote F, Campos‐Rodríguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension. JAMA. 2013;310(22):2407‐2415. [DOI] [PubMed] [Google Scholar]
- 24. Sekizuka H, Osada N, Akashi YJ. The factors affecting the non‐dipper pattern in Japanese patients with severe obstructive sleep apnea. Intern Med. 2018;57:1553‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cuspidi C, Tadic M, Sala C, Gherbesi E, Grassi G, Mancia G. Blood pressure non‐dipping and obstructive sleep apnea syndrome: a meta‐analysis. J Clin Med. 2019;8(9):1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li M, Ge Q, Sheng CS, et al. Clinical characteristics of snoring patients with primary aldosteronism and obstructive sleep apnea‐hypopnea syndrome. J Hum Hypertens. 2019;33:693‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida T, Kuwabara M, Hoshide S, Kario K. Recurrence of stroke caused by nocturnal hypoxia‐induced blood pressure surge in a young adult male with severe obstructive sleep apnea syndrome. J Am Soc Hypertens. 2016;10:201‐204. [DOI] [PubMed] [Google Scholar]
- 28. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401‐1406. [DOI] [PubMed] [Google Scholar]
- 29. Kario K, Kanegae H, Tomitani N, et al. Nighttime blood pressure measured by home blood pressure monitoring as an independent predictor of cardiovascular events in general practice. Hypertension. 2019;73:1240‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Raimondo D, Musiari G, Pinto A. Nocturnal blood pressure patterns and cardiac damage: there is still much to learn. Hypertens Res. 2020;43:246‐248. [DOI] [PubMed] [Google Scholar]
- 31. Hoshide S, Kario K, de la Sierra A, et al. Ethnic differences in the degree of morning blood pressure surge and in its determinants between Japanese and European hypertensive subjects: data from the ARTEMIS study. Hypertension. 2015;66:750‐756. [DOI] [PubMed] [Google Scholar]
- 32. Li Y, Wang JG, Gao P, et al. Are published characteristics of the ambulatory blood pressure generalizable to rural Chinese? The Jingning population study. Blood Press Monit. 2005;10:125‐134. [DOI] [PubMed] [Google Scholar]
- 33. Misaka T, Niimura Y, Yoshihisa A, et al. Clinical impact of sleep‐disordered breathing on very short‐term blood pressure variability determined by pulse transit time. J Hypertens. 2020;38:1703‐1711. [DOI] [PubMed] [Google Scholar]
- 34. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 35. Navarro‐Soriano C, Martínez‐García MA, Torres G, et al. Factors associated with the changes from a resistant to a refractory phenotype in hypertensive patients: a pragmatic longitudinal study. Hypertens Res. 2019;42:1708‐1715. [DOI] [PubMed] [Google Scholar]
- 36. Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta‐analysis. JAMA. 2015;314:2280‐2293. [DOI] [PubMed] [Google Scholar]
- 37. Warchol‐Celinska E, Prejbisz A, Kadziela J, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof‐of‐concept phase II trial. Hypertension. 2018;72:381‐390. [DOI] [PubMed] [Google Scholar]
- 38. Kario K, Ikemoto T, Kuwabara M, Ishiyama H, Saito K, Hoshide S. Catheter‐based renal denervation reduces hypoxia‐triggered nocturnal blood pressure peak in obstructive sleep apnea syndrome. J Clin Hypertens. 2016;18:707‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Q, Cheng YB, Shen M, et al. A randomized controlled trial on ambulatory blood pressure lowering effect of CPAP in patients with obstructive sleep apnea and nocturnal hypertension. Blood Press. 2020;29:21‐30. [DOI] [PubMed] [Google Scholar]
- 40. Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1423‐1428. [DOI] [PubMed] [Google Scholar]


