Abstract
Aim
To determine whether de‐escalating from advanced insulin therapy (AIT) to the combined use of metformin, an SGLT2 inhibitor, a GLP1 receptor agonist and basal insulin is the better option than multiple daily insulin injections (MDI) in obese patients with poorly controlled T2DM.
Methods
This was a 16‐week, prospective, randomized, controlled trial. Twenty‐two obese patients with T2DM on AIT were randomized to intervention (step‐down) or control (MDI) group. In the intervention group, all prandial insulin injections were discontinued, but the patient remained on basal insulin and metformin, to which an SGLT2i and a GLP1 RA were added. In the control group, the patient remained on MDI.
Results
Compared to control group (n = 8), A1c was significantly lower at week 4 (9.54% vs 8.25%; p = .0088) and week 16 (9.7% vs 7.31%; p < .001) in intervention group (n = 10). In intervention group, compared to baseline, there was a significant decrease in weight (−16.38 pounds; p = .003), BMI (−3.06; p < .001), LDL cholesterol (−15.7 mg/dl; p = .0378), total cholesterol (−18.5 mg/dl; p = .0386), total daily insulin dose (−57.3 units; p < .001) and a significant improvement in DM‐SAT patient satisfaction 0‐100 scores: total score (+45.3; p < .001) and subscale scores (Convenience + 35.28, p = .019; Lifestyle + 35.8, p = .0052; Medical control + 51.3, p < .001; Wellbeing + 47.2, p = .0091) at week 16.
Conclusion
De‐escalating from AIT to the combined use of metformin, SGLT2i, GLP1 RA and basal insulin in obese patients with poorly controlled T2DM on MDI resulted in significant improvement in glycaemic control, weight loss and significantly higher patient satisfaction. This stepping‐down approach may be the better option than continuing MDI in these patients.
Keywords: GLP‐1 analogue, glycaemic control, insulin therapy, SGLT2 inhibitor, type 2 diabetes
De‐escalating from advanced insulin therapy (AIT) to the combined use of metformin, an SGLT2 inhibitor, a GLP1 receptor agonist, and basal insulin may be the better option than multiple daily insulin injections (MDI) in obese patients with poorly controlled T2DM.

1. INTRODUCTION
In traditional step‐up approach, the patients with poorly controlled type 2 diabetes (T2DM) on noninsulin antihyperglycaemic agents are advised to step up to advanced insulin therapy (AIT) with multiple daily insulin injections (MDI). However, these patients often continue to have poor glycaemic control. The common reasons are the poor adherence with multiple insulin injections and the patient's reluctance to accept insulin‐induced weight gain. The recent guidelines in diabetes management have significantly changed to accommodate the newer generation of noninsulin antihyperglycaemic agents. The combination use of the these agents such as sodium glucose co‐transporter‐2 inhibitors (SGLT2i) and glucagon‐like peptide‐1 receptor agonists (GLP1 RA), that can induce weight loss, together with a basal insulin is now an alternative treatment option before the patient is advanced to MDI. 1 , 2 In this approach, the medication‐induced weight loss may give the patients an extra motivation to take medications regularly. Similarly, the patient does not require to take multiple insulin injections with meals throughout the day that may also improve the medication adherence and treatment satisfaction.
It has been observed in the real‐world clinical practice that the simple stepping‐down approach resulted in significant, sometimes surprising, improvement in glycaemic control in this patient population. 3 This improvement can be attributed to the better compliance with fewer injections and the lower insulin resistance through medication‐induced weight loss in addition to the potent glucose‐lowering effect of the newer medications.
There are still the obese T2DM patients with poor glycaemic control who are on MDI. Some of them were initiated on MDI before the availability of newer generations of medications and new treatment recommendations. Some were started simply because the physician was not aware of or not familiar with the new recommendations. Regardless of the reason, these patients are likely to remain on MDI despite chronic poor glycaemic control since the physicians are understandably reluctant or uncomfortable to step down the most advanced insulin therapy. In addition, there have been no data on the benefits and safety of the stepping‐down approach in which MDI is de‐escalated to the combination use of the noninsulin antihyperglycaemic agents, metformin, an oral SGLT2i and a GLP1 RA, together with a basal insulin only.
The aim of this study was to determine whether de‐escalating from advanced insulin therapy to the combined use of metformin, an SGLT2 inhibitor, a GLP1 receptor agonist and a basal insulin is the better option than multiple daily insulin injections (MDI) in obese patients with poorly controlled T2DM.
2. METHODS
2.1. Patients
The patients with T2DM who met all of the following criteria were included in the study: over 21 years of age, body mass index (BMI) ≥30 kg/m2, using insulin at least 2 times daily comprising both a basal and a prandial insulin or a premix insulin with or without other noninsulin medications for a least past 3 months, A1c over 8%, eGFR over 45% The patients with any of the following criteria were excluded: pregnancy, using an SGLT2i or a GLP1 RA or U‐500 insulin, T1DM, C‐peptide below normal range if measured in the past, a history of diabetes ketoacidosis, a history of recent and frequent (≥2 times within past 3 months) urinary tract infection or genito‐urinary candidiasis requiring antibiotic and/or antifungal therapies within past 3 months, a personal or family history of medullary thyroid carcinoma (MTC) or in patients with multiple endocrine neoplasia syndrome type 2 (MEN 2), a history of acute pancreatitis.
2.2. Design
This prospective, randomized, open‐label, controlled, parallel‐group study was conducted at the community clinics. Patients were allocated 1:1 to either intervention (ie step‐down) or control (MDI) group by using block randomization with computer‐generated random sequence from http://www.randomization.com.
2.3. Procedures
All participants in both groups made a total of 3 visits over a 16‐week period. The 2nd visit was at week 4 and 3rd visit at week 16 after initial visit. At each visit, all patients had blood test for A1c, CMP, CBC, fasting lipid and measurements of body weight, height, blood pressure and heart rate, answered adverse reaction questions and completed the Diabetes Medications Satisfaction (DM‐SAT) Questionnaire form.
At first visit, the following changes were made in intervention group:
All prandial insulin injections (Humalog, Novolog, Apidra, Novolin R or Humulin R) were discontinued.
Basal insulin (NPH, Lantus, Levemir, Toujeo or Tresiba) were continued at 80% of the home dose. The dose was gradually increased until the patient is back on the home dose (the dose that the patient has been taking at home prior to the enrolment) or fasting BG of 80–130 mg/dl was achieved by using the self‐titration regimen (Appendix 1).
If the patient was on premixed insulin 2–3 times daily, it was switched to a basal insulin alone and Glargine was given at 40% of total daily dose of premixed insulin. The dose was gradually increased until fasting BG of 80–130 mg/dl is achieved by using the self‐titration regimen (Appendix 1).
Metformin at home dose was continued, but other noninsulin diabetes medications were discontinued. If the patient was not on metformin, then metformin ER was started at 500 mg daily with a meal for 2 weeks and then 1000 mg daily as a maintenance dose if tolerated.
Both SGLT2i, empagliflozin 10 mg or 12.5 mg once daily, and GLP1 RA, dulaglutide 0.75 mg subcutaneously once weekly, were added to metformin and a basal insulin.
The patients were trained on the injection technique of the once‐weekly GLP1 RA and given information on potential side effects, risk and benefits of all new medications in detail, hypoglycaemia management and the self‐titration regimen for the basal insulin.
In the control group, the patients were advised to remain on MDI and to have the usual and standard care through the primary care provider. They were also advised to gradually increase the basal insulin until fasting BG of 80–130 mg/dl is achieved by using the self‐titration regimen as in the intervention group (Appendix 1).
The patients in both groups were advised to monitor FPGs daily at minimum.
A research co‐ordinator made a phone call to all participants in both groups at weeks 1, 2, 8 and 12 to review fasting glucose measurements, ask for possible adverse events, incidents of hypoglycaemia and any change in medication.
At each visit, the patients were questioned for adverse events (nausea, vomiting, diarrhoea, headache, acute pancreatitis, bacterial or fungal genito‐urinary tract infection, severe hypoglycaemia with blood glucose <40, mild hypoglycaemia with BG 41–69, diabetes ketoacidosis, any hospitalization for hyper or hyper‐glycaemia).
At 2nd visit, empagliflozin and GLP1 RA, dulaglutide were increased to maximum doses of 25 mg and 1.5 mg, respectively, if the patient tolerated the starting dose and if the additional glycaemic control is required.
2.4. Outcome measurements
The primary outcome was the change in A1c at the end of study period at week 16 and secondary outcomes were the changes in fasting blood glucose, weight, blood pressure, heart rate, fasting lipids, serum sodium and potassium, serum creatinine, liver enzymes, CBC and Diabetes Medications Satisfaction (DM‐SAT) scores at week 16.
Treatment satisfaction was measured using the Diabetes Medication Satisfaction Tool (DM‐SAT). 4 The DM‐SAT measures satisfaction with the patient's diabetes medications regimen. The instrument consists of 16 items which create 4 subscales (3 items for wellbeing, 3 items for medical control, 5 items for lifestyle and 5 items for convenience) and a total score. Responses are summed and converted to a score from 0 to 100 for each subscale and overall, with higher scores representing more satisfaction.
2.5. Statistical analysis
We estimated that 20 patients in each group would provide at least 80% power to detect a statistically significant difference (α = 0.05) in this continuous end‐point, two independent sample study, assuming a treatment group difference in haemoglobin A1c of 12%–15%.
The data were analysed by using the software R version 3.5.1 from the R Foundation. Significance testing was conducted at the two‐sided 5% level. Continuous variables were examined for normality, and if assumption is met, differences in mean values were tested using Student's t test or Mann–Whitney U test. If not normally distributed, nonparametric procedures will be used, including Wilcoxon rank‐sum test. Categorical data were analysed using Fisher's exact test and chi‐square analysis. Since before/after comparisons will also be performed on the same study patients, we will utilize paired t tests and McNemar's chi‐square test.
3. RESULTS
Overall, 22 patients were enrolled in this study with 10 in control group and 12 in intervention group (Figure 1). Two patients in control group were excluded from the study. One decided to self‐withdraw from the study because of poor glycaemic control on MDI alone, and one was excluded since primary care provider discontinued basal‐prandial insulin. Two patients in intervention group were excluded from the study. One withdrew from the study for nausea and headache, and one was lost to follow‐up. All patients in both groups were already on metformin at the time of enrolment.
FIGURE 1.
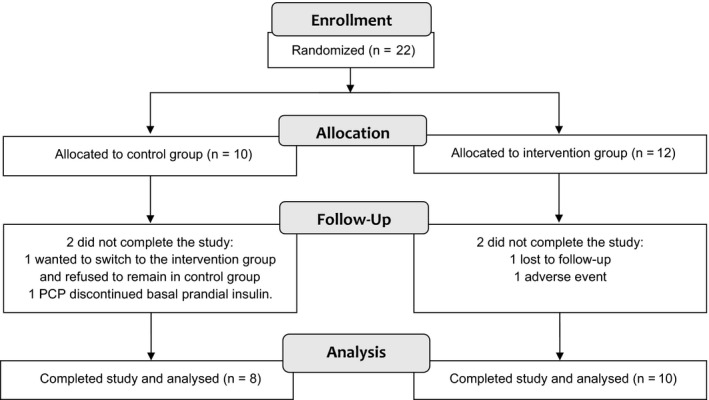
CONSORT flow diagram of patient participation and follow‐up
The demographic and baseline characteristics at randomization were similar between groups (Table 1).
TABLE 1.
Demographics and baseline characteristics
| Control (n = 8) | Intervention (n = 10) | p value | |
|---|---|---|---|
| Male | 6 (75%) | 3 (30%) | .153 |
| Age (years) | 55.62 ± 11.78 | 51.8 ± 11.14 | .494 |
| Ethnicity | |||
| Hispanics | 5 (62.5%) | 8 (80%) | .405 |
| Whites | 3 (37.5%) | 2 (20%) | .655 |
| Duration of DM (years) | 15.75 ± 6.88 | 13.4 ± 7.99 | .513 |
| Weight (pounds) | 226 ± 52.82 | 212.7 ± 39.06 | .563 |
| BMI | 36.73 ± 4.83 | 36.27 ± 4.52 | .841 |
| Systolic BP (mmHg) | 121.5 ± 16.87 | 127.2 ± 17.25 | .491 |
| Diastolic BP (mmHg) | 76 ± 10.35 | 77 ± 7.9 | .825 |
| Total daily insulin dose (units) | 97.6 ± 54.0 | 107.7 ± 64.14 | .728 |
| Metformin use at home | 8 (100%) | 10 (100%) | 1.0 |
| A1c (%) | 10.36 ± 0.877 | 9.69 ± 1.067 | .171 |
| Haemoglobin (g/dl) | 13.775 ± 2.076 | 14.21 ± 1.68 | .629 |
| Serum creatinine (mg/dl) | 0.775 ± 0.183 | 0.71 ± 0.208 | .50 |
| eGFR (mL/min/1.73m2) | 85.5 ± 6.63 | 85.4 ± 8.53 | .979 |
| LDL (mg/dl) | 84.5 ± 27.59 | 72.4 ± 24.33 | .338 |
| HDL (mg/dl) | 43.25 ± 8.15 | 40.6 ± 10.91 | .577 |
| Triglyceride (mg/dl) | 186.0 ± 83.06 | 133.4 ± 55.36 | .127 |
| Total cholesterol (mg/dl) | 165.5 ± 35.09 | 139.7 ± 30.79 | .116 |
| DM‐SAT scores | |||
| Total | 51.875 ± 16.92 | 43.70 ± 19.45 | .363 |
| Wellbeing | 53.75 ± 21.19 | 45.50 ± 19.84 | .834 |
| Medical control | 40.13 ± 24.80 | 45.6 ± 22.58 | .827 |
| Lifestyle | 48.75 ± 13.6 | 48.75 ± 13.6 | .734 |
| Convenience | 61.0 ± 20.67 | 45.7 ± 19.60 | .128 |
All values are expressed in mean ± SD.
3.1. Primary outcome
In control group, there was no significant difference in A1c between baseline and at week 4 (10.36% vs 9.7%; p = .146) and also at week 16 (10.36% vs 9.54%; p = .156) (Table 2). However, there was a significant decrease in A1c between baseline and at week 4 (9.69% vs 8.25%; p < .001) and also at week 16 (9.69% vs 7.31%; p < .001) in intervention group (Table 3).
TABLE 2.
Outcome measures in the same group: baseline vs week 4 and baseline vs week 16 in control group
| Variable | Baseline Mean (SD) | Week 4 Mean (SD) | Week 16 Mean (SD) | Difference Week 4—Baseline (CI 95%) | p‐value | Difference Week 16—Baseline (CI 95%) | p‐value |
|---|---|---|---|---|---|---|---|
| Haemoglobin A1c (%) | 10.36 (0.88) | 9.7 (0.9) | 9.54 (1.11) | −0.38 (−0.96–0.19) | .1459 | −0.83 (−2.05–0.40) | .1558 |
| Patients with A1c < 7% | 0 (0%) | 0 (0%) | 0 (0%) | 0 | 0 | ||
| Weight (pounds) | 225.98 (52.82) | 236.1 (54.77) | 225 (47.98) | 1.13 (−1.08–3.34) | .2444 | −0.98 (−9.25–7.3) | .7885 |
| Systolic blood pressure (mmHg) | 121.5 (16.87) | 117.67 (16.51) | 136.5 (20.04) | −5 (−13.81–3.81) | .2042 | 15 (2.56–27.44) | .0247 |
| Diastolic blood pressure (mmHg) | 76 (10.35) | 71.33 (10.01) | 78.25 (8.01) | −5 (−11.26–1.26) | .0953 | 2.25 | .2033 |
| eGFR (mL/min/1.73m2) | 85.5 (6.63) | 82.17 (12.86) | 84.13 (10.11) | −1.5 | .7874 | −1.38 | .4227 |
| Serum creatinine (mg/dl) | 0.78 (0.18) | 0.85 (0.26) | 0.81 (0.22) | 0.03 (−0.09–0.16) | .5301 | 0.04 (−0.04–0.11) | .2849 |
| LDL cholesterol (mg/dl) | 84.5 (27.59) | 85.67 (38.24) | 105.5 (33.88) | 0.33 (−11.1–11.77) | .9432 | 21 (−9.9–51.9) | .152 |
| HDL cholesterol (mg/dl) | 43.25 (8.15) | 43.5 (10.15) | 43.25 (7.74) | −1.83 (−6.5–2.83) | .3588 | 0 (−7.11–7.11) | 1 |
| Triglyceride (mg/dl) | 186 (83.06) | 175.17 (92.19) | 176.13 (35.93) | 10.17 (−33.43–53.77) | .575 | −9.88 (−67.57–47.82) | .6977 |
| Total cholesterol (mg/dl) | 165.5 (35.09) | 163.83 (42.23) | 183.88 (39.38) | −0.67 (−14.02–12.68) | .9029 | 18.38 (−22.38–59.13) | .3217 |
| Haemoglobin (g/dl) | 13.78 (2.08) | 13.92 (1.42) | 14.25 (1.03) | 0.45 | .7874 | 0.48 (−0.79–1.74) | .4025 |
| Serum sodium (mmol/L) | 137.13 (2.36) | 136.67 (2.25) | 136.88 (3.14) | −0.67 (−2.38–1.05) | .3632 | −0.25 (−2.43–1.93) | .7939 |
| Serum potassium (mmol/L) | 4.25 (0.52) | 4.43 (0.69) | 4.36 (0.39) | 0.08 (−0.56–0.73) | .7545 | 0.11 (−0.15–0.37) | .3442 |
| Serum bicarbonate (mmol/L) | 37.75 (27.11) | 41.83 (31.8) | 37.13 (26.36) | 0.17 | 1 | −0.63 | .4403 |
| Total daily insulin dose (units) | 97.63 (54.04) | 111.67 (33.97) | 107.88 (45.82) | −1.83 (−45.55–41.88) | .9183 | 10.25 (−18.93–39.43) | .4336 |
| Metformin dose (mg) | 1750 (462.91) | 1750 (462.91) | 1875 (353.55) | 0 | – | 125 | 1 |
| DM‐SAT Total Score | 51.88 (16.92) | 61.83 (14.57) | 56.5 (15.45) | 9.67 (3.14–16.19) | .0125 | 4.63 (−0.83–10.08) | .085 |
| DM‐SAT Subscale Score Wellbeing | 53.75 (21.19) | 62.83 (23.15) | 60.75 (14.3) | 11.17 (−13.29–35.62) | .2933 | 7 | .7518 |
| DM‐SAT Subscale Score Medical Control | 40.12 (24.8) | 55 (18.79) | 48 (17.5) | 15.5 (1.27–29.73) | .038 | 7.88 (−4.28–20.03) | .1695 |
| DM‐SAT Score Lifestyle | 48.75 (13.6) | 57 (23.42) | 55.5 (15.52) | 7 (−6.06–20.06) | .2266 | 6.75 (−1.19–14.69) | .0843 |
| DM‐SAT Subscale Score Convenience | 61 (20.67) | 70.67 (15) | 59.5 (19.53) | 8.67 (−9.23–26.56) | .2682 | −1.5 (−10.25–7.25) | .6972 |
TABLE 3.
Outcome measures in the same group: baseline vs week 4 and baseline vs week 16 in intervention group
| Variable | Baseline Mean (SD) | Week 4 Mean (SD) | Week 16 Mean (SD) | Difference Week 4—Baseline (CI 95%) | p‐value | Difference Week 16—Baseline (CI 95%) | p‐value |
|---|---|---|---|---|---|---|---|
| Haemoglobin A1c (%) | 9.69 (1.07) | 8.25 (0.78) | 7.31 (0.81) | −1.44 (−1.94–−0.94) | <.001 | −2.38 (−2.93–−1.83) | <.001 |
| Patients with A1c < 7% | 0 (0%) | 1 (10%) | 4 (40%) | 1 | 4 | ||
| Weight (pounds) | 212.68 (39.06) | 204.72 (35.61) | 196.3 (36.53) | −7.96 (−11.6–−4.32) | <.001 | −16.38 (−25.63–−7.13) | .003 |
| Systolic blood pressure (mmHg) | 127.2 (17.25) | 116.1 (20.82) | 111.2 (16.01) | −11.1 | .0248 | −16 (−33.37–1.37) | .0669 |
| Diastolic blood pressure (mmHg) | 77 (7.9) | 72.8 (7.22) | 69.8 (9.61) | −4.2 (−9.17–0.77) | .0880 | −7.2 (−18.24–3.84) | .1742 |
| eGFR (mL/min/1.73m2) | 85.4 (8.53) | 80.3 (15) | 81.1 (14.93) | −5.1 | .0579 | −4.3 | .2012 |
| Serum creatinine (mg/dl) | 0.71 (0.21) | 0.77 (0.33) | 0.75 (0.35) | 0.06 (−0.04–0.16) | .2172 | 0.04 (−0.08–0.16) | .462 |
| LDL cholesterol (mg/dl) | 72.4 (24.33) | 47.9 (22.46) | 56.7 (30.15) | −24.5 (−36.33–12.67) | .0011 | −15.7 (−30.29–−1.11) | .0378 |
| HDL cholesterol (mg/dl) | 40.6 (10.91) | 39.5 (9.72) | 39 (10.22) | −1.1 (−3.27–1.07) | .2813 | −1.6 (−5.8–2.6) | .4113 |
| Triglyceride (mg/dl) | 133.4 (55.36) | 115.4 (56.17) | 126.8 (50.13) | −18 (−41.52–5.52) | .1174 | −6.6 (−41.08–27.88) | .6752 |
| Total cholesterol (mg/dl) | 139.7 (30.79) | 110.4 (28.14) | 121.2 (33.63) | −29.3 (−44.61–−13.99) | .0019 | −18.5 (−35.79–−1.21) | .0386 |
| Haemoglobin (g/dl) | 14.21 (1.68) | 14.79 (1.53) | 14.29 (2.01) | 0.58 (0.21–0.95) | .0062 | 0.08 | .9527 |
| Serum sodium (mmol/L) | 137.6 (2.59) | 138.4 (2.41) | 137.7 (1.7) | 0.8 (−0.81–2.41) | .2901 | 0.1 (−1.85–2.05) | .9102 |
| Serum potassium (mmol/L) | 4.35 (0.29) | 4.26 (0.31) | 4.25 (0.4) | −0.09 | .4768 | −0.1 | .7211 |
| Serum bicarbonate (mmol/L) | 26.4 (1.78) | 26.2 (3.12) | 25.6 (2.59) | −0.2 (−1.74–1.34) | .7753 | −0.8 (−2.01–0.41) | .1679 |
| Total daily insulin dose (units) | 107.7 (64.14) | 55.1 (36.16) | 50.4 (38.03) | −52.6 (−77.31–−27.89) | <.001 | −57.3 (−81.18–−33.42) | <.001 |
| Metformin dose (mg) | 1500 (577.35) | 1750 (424.92) | 1750 (424.92) | 250 | .3711 | 250 | .3711 |
| DM‐SAT Total Score | 43.7 (19.45) | 80.33 (12.43) | 89 (8.59) | 34.78 (15.46–54.1) | .003 | 45.3 (28.19–62.41) | <.001 |
| DM‐SAT Subscale Score Wellbeing | 45.5 (19.84) | 83 (11.31) | 92.7 (5.7) | 36.56 | .0078 | 47.2 | .0091 |
| DM‐SAT Subscale Score Medical Control | 42.5 (20.71) | 81.33 (12.73) | 93.8 (5.05) | 36.67 (16.03–57.3) | .0034 | 51.3 (35.75–66.85) | <.001 |
| DM‐SAT Score Lifestyle | 45.6 (22.58) | 76.89 (14.94) | 81.4 (16.06) | 29.11 | .0273 | 35.8 (13.71–57.89) | .0052 |
| DM‐SAT Subscale Score Convenience | 45.7 (19.6) | 83.11 (15.69) | 80.98 (26.18) | 35 (14.37–55.63) | .0045 | 35.28 | .019 |
There was no difference in A1c between control and intervention groups at baseline (10.36% vs 9.69%; p = .171). However, A1c was significantly lower at week 4 (9.54% control vs 8.25% intervention; p = .0088) and at week 16 (9.7% control vs 7.31% intervention; p < .001) in intervention group than in control group (Table 4).
TABLE 4.
Outcome comparison between control and intervention groups: baseline vs week 4 and baseline vs week 16 between groups
| Variable | Week 4 | Week 16 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control Mean (SD) | Treatment Mean (SD) | Treatment vs Control | % | p‐value | Control Mean (SD) | Treatment Mean (SD) | Treatment vs Control | % | p‐value | |
| Haemoglobin A1c (%) | 9.7 (0.9) | 8.25 (0.78) | −1.45 | −14.95% | .0088 | 9.54 (1.11) | 7.31 (0.81) | −2.23 | −23.38% | <.001 |
| Patients with A1c < 7% | 0 (0%) | 1 (10%) | 1 | 10% | 1.0 | 0 (0%) | 4 (40%) | 4 | 40% | .0915 |
| Weight (pounds) | 236.1 (54.77) | 204.72 (35.61) | −31.38 | −13.29% | .2473 | 225 (47.98) | 196.3 (36.53) | −28.7 | −12.76% | .1857 |
| Heart rate (per min) | 85.33 (12.21) | 76.9 (11.96) | −8.43 | −9.88% | .2061 | 84.5 (10.62) | 77.8 (5.92) | −6.7 | −7.93% | .1403 |
| Systolic blood pressure (mmHg) | 117.67 (16.51) | 116.1 (20.82) | −1.57 | −1.33% | .8706 | 136.5 (20.04) | 111.2 (16.01) | −25.3 | −18.53% | .0121 |
| Diastolic blood pressure (mmHg) | 71.33 (10.01) | 72.8 (7.22) | 1.47 | 2.06% | .6189 | 78.25 (8.01) | 69.8 (9.61) | −8.45 | −10.8% | .0590 |
| eGFR | 82.17 (12.86) | 80.3 (15) | −1.87 | −2.28% | .8651 | 84.13 (10.11) | 81.1 (14.93) | −3.03 | −3.6% | .8397 |
| Serum creatinine (mg/dl) | 0.85 (0.26) | 0.77 (0.33) | −0.08 | −9.41% | .5977 | 0.81 (0.22) | 0.75 (0.35) | −0.06 | −7.41% | .6524 |
| LDL cholesterol (mg/dl) | 85.67 (38.24) | 47.9 (22.46) | −37.77 | −44.09% | .0629 | 105.5 (33.88) | 56.7 (30.15) | −48.8 | −46.26% | .0065 |
| HDL cholesterol (mg/dl) | 43.5 (10.15) | 39.5 (9.72) | −4 | −9.2% | .4557 | 43.25 (7.74) | 39 (10.22) | −4.25 | −9.83% | .3306 |
| Triglyceride (mg/dl) | 175.17 (92.19) | 115.4 (56.17) | −59.77 | −34.12% | .1925 | 176.13 (35.93) | 126.8 (50.13) | −49.33 | −38.9% | .0275 |
| Total cholesterol (mg/dl) | 163.83 (42.23) | 110.4 (28.14) | −53.43 | −32.61% | .0258 | 183.88 (39.38) | 121.2 (33.63) | −62.68 | −34.09% | .0031 |
| Haemoglobin (g/dl) | 13.92 (1.42) | 14.79 (1.53) | 0.87 | 6.25% | .2705 | 14.25 (1.03) | 14.29 (2.01) | 0.04 | 0.28% | .6334 |
| Serum sodium (mmol/L) | 136.67 (2.25) | 138.4 (2.41) | 1.73 | 1.27% | .1739 | 136.88 (3.14) | 137.7 (1.7) | 0.82 | 0.6% | .5182 |
| Serum potassium (mmol/L) | 4.43 (0.69) | 4.26 (0.31) | −0.17 | −3.84% | .5835 | 4.36 (0.39) | 4.25 (0.4) | −0.11 | −2.52% | .5517 |
| Serum bicarbonate (mmol/L) | 41.83 (31.8) | 26.2 (3.12) | −15.63 | −37.37% | .2519 | 37.13 (26.36) | 25.6 (2.59) | −11.53 | −31.05% | .0386 |
| Total daily insulin dose (units) | 111.67 (33.97) | 55.1 (36.16) | −56.57 | −50.66% | .0091 | 107.88 (45.82) | 50.4 (38.03) | −57.48 | −53.28% | .0132 |
| Metformin dose (mg) | 1750 (462.91) | 1750 (424.92) | 0 | 0% | .9548 | 1875 (353.55) | 1750 (424.92) | −125 | −6.67% | .4624 |
| DM‐SAT Total Score | 61.83 (14.57) | 80.33 (12.43) | 18.5 | 29.92% | .0296 | 56.5 (15.45) | 89 (8.59) | 32.5 | 57.52% | <.001 |
| DM‐SAT Subscale Score Wellbeing | 62.83 (23.15) | 83 (11.31) | 20.17 | 32.1% | .0903 | 60.75 (14.3) | 92.7 (5.7) | 31.95 | 52.59% | .0024 |
| DM‐SAT Subscale Score Medical Control | 55 (18.79) | 81.33 (12.73) | 26.33 | 47.87% | .0169 | 48 (17.5) | 93.8 (5.05) | 45.8 | 95.42% | <.001 |
| DM‐SAT Score Lifestyle | 57 (23.42) | 76.89 (14.94) | 19.89 | 34.89% | .0442 | 55.5 (15.52) | 81.4 (16.06) | 25.9 | 46.67% | .0034 |
| DM‐SAT Subscale Score Convenience | 70.67 (15) | 83.11 (15.69) | 12.44 | 17.6% | .15 | 59.5 (19.53) | 80.98 (26.18) | 21.48 | 36.1% | .0637 |
3.2. Secondary outcomes
3.2.1. Outcome comparison in the same group between baseline and week 16
In control group, there was no significant change at outcome variables other than higher systolic BP at week 16 (+15 mmHg; p = .0247) (Table 2).
In intervention group, there was a significant decrease in weight (−16.38 pounds; p = .003), BMI (−3.06; p < .001), LDL cholesterol (−15.7 mg/dl; p = .0378), total cholesterol (−18.5 mg/dl; p = .0386) and total daily insulin dose (−57.3 units; p < .001) at week 16 in addition to A1c. There was a significant improvement in DM‐SAT patient satisfaction 0–100 scores: total score (+45.3; p < .001) and subscale scores (Convenience + 35.28; p = .019) (Lifestyle + 35.8; p = .0052) (Medical control + 51.3; p < .001) (Wellbeing + 47.2; p = .0091) (Table 3).
3.2.2. Outcome comparison between control and intervention groups at week 16
There were statistically significant differences between two groups in the following variables, and all were in favour of intervention group : A1c (−23.38% difference; p < .01), systolic blood pressure (−18.53% difference; p = .012), LDL cholesterol (−46.26% difference; p = .007), triglyceride (−38.9% difference; p = .0275), total cholesterol (−34.09% difference; p = .003), total daily insulin dose (−53.28% difference; p = .0132), DM‐SAT total score (+57.52% difference; p < .001), DM‐SAT Subscale Score Wellbeing (+52.59% difference; p = .0024), DM‐SAT Subscale Score Medical Control (+95.42% difference; p < .001) and DM‐SAT Score Lifestyle (+46.67% difference; p = .0034) at week 16. Serum bicarbonate was lower in intervention group (37.16 ± 26.36 vs 25.6 ± 2.59 mmol/L, −31.05% difference; p = .039), but none of the patients in intervention group had bicarbonate level lower than 23 mmol/L or below normal range (22–28 mmol/L). More patients in the intervention group than in the control group (40% vs 0%) achieved A1c of less than 7% (Table 4).
3.3. Safety and tolerability
The portion of patents with any hypoglycaemia event was lower in intervention group than in control group (8.3% vs 30%) (Table 5). None of the patients in intervention group experienced severe hypoglycaemia.
TABLE 5.
Summary of adverse events
| Control (n = 10) | Intervention (n = 12) | |
|---|---|---|
| Patients with at least one AE, n (%) | 3 (30%) | 10 (83.33%) |
| Nausea | 0 | 9 (75%) |
| Vomiting | 0 | 1 (8.3%) |
| Diarrhoea | 0 | 1 (8.3%) |
| Headache | 0 | 1 (8.3%) |
| Urinary tract infection | 0 | |
| Genital yeast infection | 0 | 1 (8.3%) |
| Skin reaction at injection site | 0 | 1 (8.3%) |
| Any hypoglycaemia event (BG <70 mg/dL) | 3 (30%) | 1 (8.3%) |
| Mild hypoglycaemia event (BG 40‐69 mg/dL) | 2 (20%) | 1 (8.3%) |
| Severe hypoglycaemia event (BG <40 mg/dL) | 1 (10%) | 0 |
| Acute pancreatitis | 0 | 0 |
| Diabetes ketoacidosis | 0 | 0 |
| Withdrawal from study due to AE | 0 | 1 (8.3%) Nausea/vomiting/headache |
| Hospitalization due to AE | 0 | 0 |
At least one adverse event (AE) was observed in 83.3% of patients in intervention group. However, most of these AE were mild nausea lasting less than 2 weeks. Only one patient in intervention group withdrew from the study due to AE (Appendix 2). The symptoms, nausea, vomiting and headache resolved completely and did not require hospital admission. One patient in intervention group had vaginal yeast infection which was successfully treated with a 2‐day course of fluconazole. There was no patent in intervention group who had severe AE such as acute pancreatitis, diabetes ketoacidosis or hospitalization resulting from AE.
4. DISCUSSION
This is the first study in which the multiple daily prandial insulin injections were replaced with an SGLT2i and GLP1 RA in patients with poorly controlled T2DM on the advanced insulin therapy or MDI regimen. Our study population was typical of the patients we see in the real‐world clinical situation (middle‐aged, obese, poor glycaemic control, chronic duration of diabetes) though the number of the participants was small. In those with poor glycaemic control on a basal insulin, they are usually advanced from basal insulin alone to basal‐prandial insulin regimen or multiple daily insulin injections. In those who are advanced to MDI regimen and continue to have poor glycaemic control, they are often not given an opportunity to step down from MDI to a simpler regimen with fewer injection: basal insulin alone together with SGLT2i and/or GLP1 RA. One of the main reasons is the lack of the data on efficacy, safety and tolerability of this step‐down approach though the combination use of these noninsulin antihyperglycaemic agents has been proven safe and effective in the traditional step‐up approach. 5 , 6 , 7 , 8 , 9 , 10
In our study, there was a significant improvement in glycaemic control and weight when prandial insulin was replaced with oral SGLT2i and once‐weekly GLP1 RA. The statistically significant decreases in A1c and weight were observed as early as at week 4 and also at the end of study at week 16 in the intervention group (A1c from 9.68% to 8.25% at week 4 and 7.31% at week 16; weight from 212.68 pounds to 204.72 pounds at week 4 and 196.3 pounds at week 16). When compared between groups, the difference in decrease in A1c (−23.38%) was again significant favouring the intervention group. Nearly half (40%) of those in the intervention group achieved A1c of less than 7% at week 16 compared with none in the control group.
The weight loss achieved by the intervention group was not statistically significant (225 vs 196.3 pounds, −28.7 pounds or −12.76%, p = .1857); however, the amount of weight loss achieved may be considered clinically relevant and there may have been a statistically significant difference with higher number of study patients.
In addition to above important benefits, we also observed that, in intervention group, there was a significant decrease in systolic BP and LDL cholesterol at the end of study at week 16. Total daily insulin requirement was reduced by over 50%, and the patient satisfaction DM‐SAT scores were significantly higher. We did not observe these benefits in the control group. The patients who are currently on MDI and who are regarded as poor adherence with insulin therapy may have a significant improvement in their glycaemic control with the stepping‐down approach. In addition to weight loss and improvement in glycaemic control, SGLT2 inhibitors and GLP1 RAs in this stepping‐down approach will provide the cardiovascular and renal benefits in patients with T2DM who have or are at risk for ASCVD or diabetic nephropathy. 11 , 12 , 13 , 14 , 15
Regarding safety, none of the patients in the intervention group experienced serious adverse events. Though total daily insulin dose and frequency of insulin injections were much lower and fewer in the intervention group, none of the patients developed diabetes ketoacidosis or worsening of glycaemic control. The most common adverse event in the intervention group was mild nausea that lasted less than 2 weeks in most patients. Patients with mild or severe hypoglycaemia were also fewer in the intervention group.
We acknowledge the limitations of our study. Our study was underpowered and had a small sample size that limits the quality and precision of data. The results, therefore, need to be confirmed with larger studies with adequate statistical power. The study period was only 16‐weeks, and therefore, the long‐term risks or benefits of the treatment cannot be determined based on our study results. Despite all these limitations, our study showed encouraging results in several outcomes with a statistically significant decline in A1c, clinically relevant weight loss and significant increase in patient's treatment satisfaction scores in treatment group.
In conclusion, the stepping‐down approach from advanced insulin therapy to the combined use of metformin, an SGLT2 inhibitor, a GLP1 receptor agonist and a basal insulin in obese patients with poorly controlled T2DM resulted in the significant improvement in glycaemic control and clinically relevant weight loss in short term. The patient satisfaction was much higher with this approach than with MDI. There was no serious adverse event in the intervention group. This approach may be a better option than multiple daily insulin injections in obese patients with poorly controlled T2DM on advanced insulin therapy. These results need to be confirmed in a trial with a larger sample size.
CONFLICT OF INTEREST
All authors declared no conflict of interest for this study.
AUTHOR CONTRIBUTIONS
SN and PM conceived and designed the study and interpreted the data. SN, GR, JG, AP, SK screened, recruited and consented the patients, acquired the data and contributed to drafting the manuscript. All authors are responsible for the work described in this paper. All authors either drafted the manuscript or critically reviewed the manuscript for important intellectual content. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENT
The authors thank UC Merced Biostatistics and Data Support team for data analysis.
Appendix 1. Basal insulin self‐titration instruction to the patients
Please follow the instruction to adjust your basal insulin dose (Lantus, Basaglar, Toujeo, Tresiba, Levemir, NPH) by 1 unit every day.
Start the basal insulin at ___________ Units before bedtime
Check fasting blood glucose level before breakfast or on waking every day. Your target is between 80–130 mg/dl.
-
If your fasting glucose level is:
over 130 mg/dl, add 1 unit of insulin
less than 80 mg/dl, reduce the insulin dose by 2 to 3 units.
between 80 and 130 mg/dl, do not change the dose.
Please adjust the dose EVERY DAY.
Write down the date and new dose on the glucose log sheet every time you adjust it.
Appendix 2. Characteristics of 4 patients who were withdrawn from the study
| Assigned group | Age (years) | Gender | Weight in pounds/BMI | Baseline A1c | Reason for withdrawal | Final outcome after withdrawal |
|---|---|---|---|---|---|---|
| Control | 77 | M | 230/35 | 9.4% | He wanted to switch MDI to the intervention group and refused to remain in control group. | Taking the study medications from PCP. |
| Control | 59 | F | 208/31.6 | 8.3% | PCP discontinued basal prandial insulin. | Taking U‐500 insulin. |
| Intervention | 28 | F | 186.6/32.2 | 9.1% | Lost to follow‐up/did not return to 3rd visit | She continues to take the same study medication through PCP. |
| Intervention | 27 | F | 223.4/43.1 | 11.4% | adverse event (nausea/vomiting/headache) | Withdrew from study after 3 weeks. Complete resolution of symptoms after stopping study medications. |
PCP, Primary Care Provider.
Naing S, Ramesh G, Garcha J, Poliyedath A, Khandelwal S, Mills PK. Is the stepping‐down approach a better option than multiple daily injections in obese patients with poorly controlled Type 2 diabetes on advanced insulin therapy?. Endocrinol Diab Metab.2021;4:e00204. 10.1002/edm2.204
Funding informationThis study was funded by the Central California Faculty Medical Group (CCFMG) Research Grant. Grant number 2014434.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request from the researchers.
REFERENCES
- 1. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm – 2020 executive summary. Endoc Pract. 2020;26(1):107‐139. [DOI] [PubMed] [Google Scholar]
- 2. Pharmacologic Approaches to Glycemic Treatment . Standards of Medical Care in Diabetes‐2020. Diabetes Care. 2020;43(Supplement 1):S98‐S110. [DOI] [PubMed] [Google Scholar]
- 3. Bell DSH. The potent synergistic effects of the combination of liraglutide and canagliflozin on glycemic control and weight loss. Am J Case Rep. 2014;15:152‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson RT, Girman CJ, Pawaskar MD, et al. Diabetes medication satisfaction tool. Diabetes Care. 2009;32(1):51‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clegg LE, Penland RC, Bachina S, et al. Effects of exenatide and open‐label SGLT2 inhibitor treatment, given in parallel or sequentially, on mortality and cardiovascular and renal outcomes in type 2 diabetes: Insights from the EXSCEL trial. Cardiovasc Diabetol. 2019;18(1):1–12. 10.1186/s12933-019-0942-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION‐8): a 28 week, multicentre, double‐blind, phase 3, randomised controlled trial. Lancet Diabet Endocrinol. 2016;4(12):1004‐1016. 10.1016/S2213-8587(16)30267-4 [DOI] [PubMed] [Google Scholar]
- 7. Goncalves E, Bell DSH. Glucagon‐like peptide‐1 receptor agonists and sodium‐glucose co‐transporter‐2 inhibitors: Sequential or simultaneous start? Diabet Obes Metabol. 2017;19(6):909‐911. [DOI] [PubMed] [Google Scholar]
- 8. Goncalves E, Bell DSH. Combination treatment of SGLT2 inhibitors and GLP‐1 receptor agonists: symbiotic effects on metabolism and cardiorenal risk. Diabet Therap. 2018;9(3):919‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo M, Gu J, Teng F, et al. The efficacy and safety of combinations of SGLT2 inhibitors and GLP‐1 receptor agonists in the treatment of type 2 diabetes or obese adults: a systematic review and meta‐analysis. Endocrine. 2020;67(2):294‐304. [DOI] [PubMed] [Google Scholar]
- 10. Ludvik B, Frías JP, Tinahones FJ, et al. Dulaglutide as add‐on therapy to SGLT2 inhibitors in patients with inadequately controlled type 2 diabetes (AWARD‐10): a 24‐week, randomised, double‐blind, placebo‐controlled trial. Lancet Diabet Endocrinol. 2018;6(5):370‐381. [DOI] [PubMed] [Google Scholar]
- 11. Das SR, Everett BM, Birtcher KK, et al. 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol. 2018;72(24):3200‐3223. 10.1016/j.jacc.2018.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in Type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 13. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375(4):323‐334. [DOI] [PubMed] [Google Scholar]
- 14. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 15. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request from the researchers.


