Abstract
Objectives
Diabetic retinopathy (DR) is the most common microvascular complications seen in children and adolescents with type 1 diabetes. The aim of this study was to evaluate the prevalence of retinopathy and its association with other risk factors in young people with type 1 diabetes.
Methods
This study was a cross‐sectional study, which was done as part of the ongoing complication assessment in the paediatric diabetes clinic in BIRDEM (Bangladesh Institute of Research and Rehabilitation of Diabetes Endocrine and Metabolic Disorders), a tertiary care hospital. Children, adolescents and young adults with type 1 diabetes who were having diabetes duration >2 years were included in this study. Retinopathy was detected using fundal photography, and grading was done by National Screening Committee of UK by trained ophthalmologists.
Results
Diabetic retinopathy was observed in 44 (6.6%) patients. Majority (95.4%) of them had early diabetic retinopathy in the form of mild NPDR (nonproliferative diabetic retinopathy) (R1). Patients with retinopathy had higher HbA1c 9.6[8.4‐12.3] vs 9.1 [7.9‐10.8] (P = .013), longer duration of diabetes 7.6 [5.5‐10.7] vs 6.0 [4.5‐8.2] years (P = .001) and were older 21.5 [18.0‐23.0] vs 18 [16.0‐21.0] years (P = .0001) compared with those without retinopathy. On multivariate regression analysis, higher age and median HbA1c were significantly associated with DR.
Conclusions
Higher HbA1c was the only modifiable risk factor for development of DR in our study population. Early detection of DR with improvement of glycaemic control may reduce the risk of progression of severe stages of the disease.
Keywords: Bangladesh, retinopathy, risk factors, type 1 diabetes
The aim of this study was to investigate the prevalence and risk factors associated with, retinopathy in Youth with type 1 diabetes in Bangladesh. Diabetic retinopathy was observed in 44 (6.6%) patients. Majority 42 (95.4%) of them had early diabetic retinopathy mild NPDR (R1).

1. INTRODUCTION
Diabetic Retinopathy (DR) is the most common microvascular disease seen in children and adolescents with type 1 diabetes which is often asymptomatic in early stages but may progress to severe disease. 1 , 2 , 3 , 4 , 5 The rising number of type 1 and type 2 diabetes in children has led to an increase number of young people at risk of visual problem. 6 , 7 , 8 Almost all type 1 diabetes patients have developed some degree of retinopathy after 20 years of diabetes duration found in a study done by DCCT (Diabetes Control and Complications Trial) Research Group. 9 The pathogenesis is still not clear; various factors—metabolic, environmental, hormonal and genetic factors, may play role in development of diabetic microangiopathy. The prevalence and risk factors of DR vary widely in different studies ranged from 0% to 28%. 10 , 11 , 12 , 13 , 14 Although the prevalence of diabetic retinopathy in adolescents was quite high, approximately 41%‐42% in Australia and the United States and even higher in some European centres (46%) 15 , 16 , 17 but recently lower prevalence rate (3.9%) has also been reported. 18 Many studies reported that duration of 8‐10 years is required for the development of DR whereas few studies reported that mild DR occurred with short duration of DM (1‐2 years). 19 , 20 , 21 , 22 The risk factors for the development of DR include long duration of diabetes, poor glycaemic control, hypertension, hyperlipidaemia and genetic predisposition. 23 , 24 , 25 The importance of strict glycaemic control and blood pressure control has been shown to reduce the progression of DR in the Diabetes Control and Complication Trial. 9 ISPAD (International Society for Paediatric and Adolescent Diabetes) recommends screening for diabetic retinopathy should start from age 11 years with 2‐5 years diabetes duration. 26 Whereas ADA (American Diabetes Association) and AAO (American Academy of Ophthalmology) recommend eye examination once youth have had type 1 diabetes for 3‐5 years, provided they are of age ≥10 years or puberty has started, whichever is earlier. 27
There has been only one study done on development of DR, and associated risk factors in young patients with T1DM in Bangladesh. Hence, the purpose of this study was to assess the prevalence of retinopathy in children, adolescents and young adults with type 1 diabetes and further explore the risk factors for the development of DR.
We evaluated the DR among young people in type 1 diabetes enrolled in a largely managed comprehensive diabetes care in CDiC (Changing Diabetes in Children) and LFAC (Life for a Child) Paediatric Diabetes Center in BIRDEM (Bangladesh Institute of Research and Rehabilitation of Diabetes Endocrine and Metabolic Disorders) hospital in Bangladesh. We specifically focused on the association between retinopathy and recognized risk factors—age, diabetes duration, insulin dose and HbA1c.
2. RESEARCH DESIGN AND METHODS
This study was a cross‐sectional study, undertaken as part of the ongoing complication assessment in the CDiC and LFAC Paediatric Diabetes Center in BIRDEM 2, a tertiary care hospital. We analysed comprehensive data from patients with type 1 diabetes over 1 year period from October 2016 to October 2017 who were screened for DR. Children, adolescents and young adults with type 1 diabetes who were having diabetes duration >2 years were included in this study. Our centre is the only multidisciplinary centre where patients are referred from all over Bangladesh. Determination of the type 1 diabetes was made by the local and ISPAD criteria according to available clinical features and history: T1D was diagnosed upon abrupt onset of typical symptoms of diabetes with insulin required from diagnosis and no sign of insulin resistance—acanthosis nigricans and usually nonobese. 28 , 29 Clinical assessment was done by paediatric endocrinologist and the team during complications assessment.
The protocol was approved by the ethics committee of Diabetic Association of Bangladesh.
2.1. Assessment of Diabetic retinopathy
Screening for retinopathy has been started since 2016 in our clinic. All patients who came for follow‐up were screened (while they were ≥10 years old).
Colour fundus photography (CFP) was done in both eyes by using canon CR‐2 digital retinal camera by optometrist. Screening and grading for the presence or absence of diabetic retinopathy were performed by trained ophthalmologists. Grading was done by Disease Grading Protocol in National Guidelines on Screening for Diabetic Retinopathy Grading in England and Wales screening programmes. 30
Data were collected in predesigned data collection sheet. HbA1c (haemoglobin A1c) done during the screening was taken for analysis. The informed consent from patients and their caregivers was taken during complication assessment.
2.2. Statistical analysis
Summary statistics are reported as mean ± SD for normally distributed data and median (interquartile range, IQR) for skewed data. While comparing the data, parametric test—ANOVA test was used for continuous data and nonparametric test—Kruskal‐Wallis test was used for skewed data. A probability ‘P’ of equal or less than .05 was considered significant and value more than 0.05 was not significant.
Risk factors for the development of retinopathy were assessed using logistic regression. Potential variables used in the regression model were age during the examination, HbA1c and diabetes duration.
3. RESULTS
Overall 622 patients were assessed who met the inclusion criteria. The median age of onset of diabetes was 12.0[9.0‐14.0] years in our study population. The median age during assessment was 19.0 [IQR: 16.0‐ 21.0] years and median duration of diabetes 6.1 [IQR: 4.5‐8.3] years. A total of 295 (44.6%) were male and 367(55.4%) were female. Diabetic retinopathy was observed in 44 (6.6%) patients. Majority (95.4%) of them had early diabetic retinopathy mild NPDR (R1), one patient had severe NPDR (R2), and one had proliferative retinopathy (R3) (Figure 1). The median diabetes duration was 7.6 [5.6‐10.7] years in patients with retinopathy. The earliest retinopathy developed in 4 years 5 months in one patient. While comparing the residence, the patients from rural had more retinopathy than urban area (P = .040) (Table 1). Patients with retinopathy had higher HbA1c 9.6[8.4‐12.3] vs 9.1 [7.9‐10.8] (P = ..013), longer duration of diabetes 7.6[5.6‐10.7] vs 6.0 [4.5‐8.2] years (P = .001) and were older 21.5 [18.0‐23.0] vs 18 [16.0‐21.0] years (P = .0001) compared to patients without retinopathy (Table 1).
FIGURE 1.
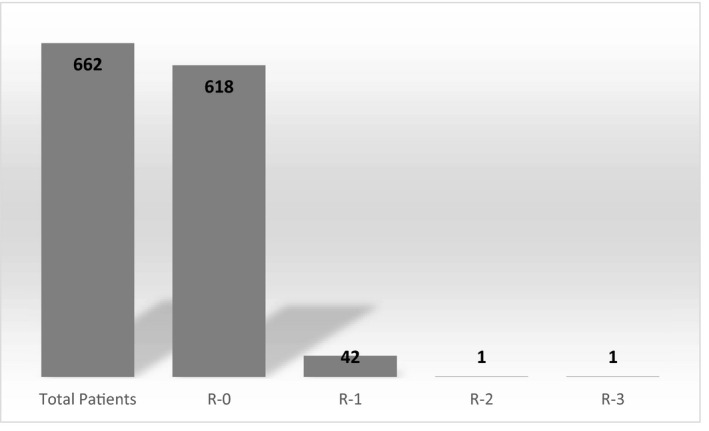
Grading of Retinopathy among the type 1 diabetes patients
TABLE 1.
Characteristics of Patients with and without retinopathy
| Parameters | Without retinopathy (n = 578) | With retinopathy (n = 44) | P value |
|---|---|---|---|
| Age at assessment | 18.0 [16.0‐21.0] | 21.5 [18.0‐23.0] | .0001 |
| Age at diagnosis | 12.0 [9.0‐14.0] | 13.0 [10.0‐15.0] | .165 |
| Sex | |||
| Male | 276 (93.6%) | 19 (6.4%) | .849 |
| Female | 342 (93.2%) | 25 (6.8%) | |
| Area of residence | |||
| Urban | 180 (97.3%) | 5(2.7%) | .040 |
| Semi‐urban | 39(92.9%) | 3 (7.1%) | |
| Rural | 330 (91.7%) | 30 (8.3%) | |
| Family history | |||
| Yes | 13 (5.7%) | 13 (5.3%) | .954 |
| No | 214 (94.3%) | 231 (94.7%) | |
| Diabetes duration (y) | 6.0 [4.5‐8.2] | 7.6 [5.6‐10.7] | .001 |
| Insulin dose (U/d) | 34 [26‐46] | 36 [26‐46] | .447 |
| HbA1c | |||
| (%) | 9.1 [7.9‐10.8] | 9.6 [8.4‐12.3] | .013 |
| mmol/mol | 76.0 [62.8‐94.5] | 81.4 [68.3‐110.9] | |
3.1. Regression analysis of complication outcome
Retinopathy was associated with higher age, longer diabetes duration and higher HbA1c. Independent predictors of retinopathy were higher age, longer duration of diabetes and high HbA1c on univariate analysis. Higher age and HbA1c remained significantly associated with DR on multivariate analysis (Table 2).
TABLE 2.
Factors associated with retinopathy in adolescents with diabetes
| Factors | Univariate model | Multivariate model | ||
|---|---|---|---|---|
| OR [95% CI] | P value | OR [95% CI] | P value | |
| Age at assessment | 1.2 [1.09‐1.31] | .0001 | 1.16 [1.06‐1.27] | .001 |
| Diabetes duration | 1.15 [1.06‐1.25] | .001 | 1.08 [1.00‐1.19] | .082 |
| HbA1c | 1.18 [1.04‐1.34] | .008 | 1.15 [1.01‐1.30] | .031 |
Age at diagnosis, sex and insulin dose were adjusted for the analysis.
4. DISCUSSION
The prevalence of retinopathy was found 6.6% in this cohort of 662 patients. In many studies, the prevalence of DR ranged from 0% to 28%. 10 , 11 , 12 , 13 , 14 A large cohort study of 1604 patients in Australia, retinopathy was found in approximately 50% of adolescents with type 1 diabetes in the early years (1990s) which reduced to 12% (i2000s), which might be due to intensive management in recent years. 31 In our study population, majority had early retinopathy R1 44 (95.4%). Similar finding (early retinopathy) was found in our previous study done in young patients with diabetes. 32 Majority had early retinopathy in a study done in 119 children with type 1 diabetes in UK. 33
Patients who are residing in rural area had more retinopathy than urban people in our study. It may be explained that the patients who are from rural area cannot visit the centre regularly and miss follow‐up often as they have to travel a long distance to come to the centre, which might also cause poorer metabolic control compared to urban people. In our study population, age of onset of diabetes was mostly at adolescence and higher age during assessment was conferred as a risk of DR. In a cohort of children with T1D in Australia, it was found that children who were diagnosed before the age of 5 years had a longer retinopathy free period than those diagnosed between 5 and 15 years. 34 The increase incidence of DR during puberty probably due to hormonal influences which may affect the microvascular cellular integrity. 34
Our patients who developed DR had longer median duration of diabetes (7.6 years) compared to non‐DR. In US SEARCH study of 265 young diabetics <20 years of age, 17% T1DM developed DR on fundus photography with median duration of 6.8 years. 35 Conversely, a large cohort of 2240 youth, 20% of had developed diabetic retinopathy at a shorter duration (median duration of 3.2 years). 36
Though there are several factors that may influence in development of DR but till now HbA1c has been found to be a modifiable risk factor. Higher HbA1c has been identified as a risk factor for not only in the development but also in progression of retinopathy in adolescents and adults. 37 , 38
Similar to other studies, we found the only modifiable risk factor was higher HbA1c for development of DR in our study population. 32 , 39 , 40 , 41 , 42 , 43 , 44 Glycaemia showed a continuous relationship with retinopathy; therefore, emphasis should be given on strict glycaemic control in type 1 diabetes patient to reduce the risk of development and progression of DR. Screening for identification of early retinopathy with optimal glycaemic control should be part of management of type 1 diabetes.
On multivariate analysis, the risks of retinopathy were higher age and median HbA1c. Improved glycaemic control certainly reduces the risk of retinopathy which can be thought of as preventative strategies. Adolescents with poor glycaemic control have a higher risk of rapid progression to severe stages of retinopathy (severe nonproliferative retinopathy) as of adults. 45 , 46 Therefore, screening from adolescence time should be started for detection of early stage of diabetic retinopathy and emphasis on reduction of HbA1c should be given as improved glycaemic control may reduce or reverse the retinopathy. 47 , 48
The strength of this study included the sample size, with 662 young people with type 1 diabetes, representative of the different age groups from adolescents to young adults. This was an observational study and patient characteristics were comparable. It was a well‐described representative population, as patients were referred from almost all parts of the country to this centre. The data were collected during the complication assessment visit. All patients were assessed at single centre, and same protocol was used in the complications assessment methods throughout the study period, and the same trained ophthalmologists did the grading of the retinal images.
The limitations of the study were‐ this was a cross‐sectional study, thus was limited to observed associations and also not including all the modifiable risk factors which could reveal the other risk factors for prediction of retinopathy in addition to glycaemic control.
In conclusion, we found there is prevalence (6.6%) of retinopathy which was associated with higher age and HbA1c in young people with T1 D. Our results provide strong support for prior literature in emphasizing the early screening of DR and optimal glycaemic control to reduce the risk and progression of retinopathy. Longitudinal follow‐up of this cohort may provide the information about the predictive value of retinopathy and its further progression.
CONFLICTS OF INTEREST
The authors declare that they have no potential conflicts of interest relevant to this article.
AUTHOR'S CONTRIBUTION
BZ, ZK, NC and KA conceptualized and designed the study. BZ and ZK prepared the first draft of the manuscript. L H, K H, AA and Y K contributed to data preservation and analysis tools. All authors have contributed to manuscript revisions and read the manuscript. BZ, ZK and KA approved the final manuscript.
ACKNOWLEDGEMENTS
We would like to acknowledge Life for a Child Programme and Orbis International for their contribution to start screening of DR in our CDiC and LFAC Paediatric Diabetes Center.
Zabeen B, Khaled MZ, Husain L, et al. Risk factors associated with retinopathy in young people with type 1 diabetes in Bangladesh. Endocrinol Diab Metab.2021;4:e00197. 10.1002/edm2.197
REFERENCES
- 1. Fong DS, Aiello LP, Ferris FL. Diabetic retinopathy. Diabetes Care. 2004;27(10):2540‐2553. [DOI] [PubMed] [Google Scholar]
- 2. Broe R. Early risk stratification in pediatric type 1 diabetes. Acta Ophthalmol. 2015;93:1‐19. 10.1111/aos.12702 [DOI] [PubMed] [Google Scholar]
- 3. Porta M, Allione A. Diabetic retinopathy and its relevance to paediatric age. An update. Pediatr Endocrinol Rev. 2004;1:404‐411. [PubMed] [Google Scholar]
- 4. Thomas RL, Dunstan FD, Luzio SD, et al. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol. 2015;99:64‐68. [DOI] [PubMed] [Google Scholar]
- 5. Lian JX, Gangwani RA, McGhee SM, et al. Systematic screening for diabetic retinopathy (DR) in Hong Kong: prevalence of DR and visual impairment among diabetic population. Br J Ophthalmol. 2016;100:151‐155. 10.1136/bjophthalmol-2015-307382 [DOI] [PubMed] [Google Scholar]
- 6. Haines L, Wan KC, Lynn R, et al. Rising incidence of type 2 diabetes in children in the U.K. Diabetes Care. 2007;30(5):1097‐1101. 10.2337/dc06-1813 [DOI] [PubMed] [Google Scholar]
- 7. Patterson CC, Dahlquist GG, Gyürüs E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027‐2033. 10.1016/S0140-6736(09)60568-7 [DOI] [PubMed] [Google Scholar]
- 8. Dabelea D, Mayer‐Davis EJ, Saydah S, et al. SEARCH for Diabetes in Youth Study. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:1778‐1786. 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long‐term complications in adolescents with insulin‐dependent diabetes mellitus: diabetes control and complications trial. J Pediatr. 1994;125:177‐188. 10.1016/s0022-3476(94)70190-3 [DOI] [PubMed] [Google Scholar]
- 10. Kristinsson JK, Gudmundsson JR, Stefansson E, Jonasson F, Gislason I, Thorsson AV. Screening for diabetic retinopathy: initiation and frequency. Acta Ophthalmol Scand. 1995;73:525‐528. [DOI] [PubMed] [Google Scholar]
- 11. Gallego PH, Wiltshire E, Donaghue KC. Identifying children at particular risk of long‐term diabetes complications. Pediatr Diabetes. 2007;8(Suppl 6):40‐48. 10.1111/j.1399-5448.2007.00298.x [DOI] [PubMed] [Google Scholar]
- 12. Massin P, Erginay A, Mercat‐Caudal I, et al. Prevalence of diabetic retinopathy in children and adolescents with type 1 diabetes attending summer camps in France. Diabetes Metab. 2007;33:284‐289. 10.1016/j.diabet.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 13. Majaliwa ES, Munubhi E, Ramaiya K, et al. Survey on acute and chronic complications in children and adolescents with type 1 diabetes at Muhimbili National Hospital in Daressalaam Tanzania. Diabetes Care. 2007;30(9):2187‐2192. 10.2337/dc07-0594 [DOI] [PubMed] [Google Scholar]
- 14. Donaghue KC, Craig ME, Chan AK, et al. Prevalence of diabetes complications 6 years after diagnosis in an incident cohort of childhood diabetes. Diabet Med. 2005;22:711‐718. 10.1111/j.1464-5491.2005.01527.x [DOI] [PubMed] [Google Scholar]
- 15. Klein R, Klein BE, Moss SE. The Wisconsin epidemiologic study of diabetic retinopathy: an update. Aust N Z J Ophthalmol. 1990;18:19‐22. 10.1111/j.1442-9071.1990.tb00579.x [DOI] [PubMed] [Google Scholar]
- 16. Bonney M, Hing SJ, Fung AT, et al. Development and progression of diabetic retinopathy: adolescents at risk. Diabet Med. 1995;12:967‐973. 10.1111/j.1464-5491.1995.tb00407.x [DOI] [PubMed] [Google Scholar]
- 17. Stephenson J, Fuller JH. Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia. 1994;37:278‐285. 10.1007/BF00398055 [DOI] [PubMed] [Google Scholar]
- 18. Tapley JL, McGwin G, Ashraf AP, et al. Feasibility and efficacy of diabetic retinopathy screening among youth with diabetes in a pediatric endocrinology clinic: a cross‐sectional study. Diabetol Metab Syndr. 2015;24:7‐56. 10.1186/s13098-015-0054-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holl RW, Lang GE, Grabert M, Heinze E, Lang GK, Debatin KM. Diabetic retinopathy in pediatric patients with type‐1 diabetes: effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J Pediatr. 1998;132(5):790‐794. 10.1016/s0022-3476(98)70305-1 [DOI] [PubMed] [Google Scholar]
- 20. Donaghue KC, Fairchild JM, Chan A, et al. Diabetes micro vascular complications in prepubertal children. J Pediatr Endocrinol Metab. 1997;10:579‐585. [DOI] [PubMed] [Google Scholar]
- 21. Verougstraete C, Toussaint D, De Schepper J, Haentjens M, Dorchy H. First microangiographic abnormalities in childhood diabetes − types of lesions. Graefes Arch Clin Exp Ophthalmol. 1991;229:24‐32. [DOI] [PubMed] [Google Scholar]
- 22. Malone JI, Grizzard S, Espinoza LR, Achenbach KE, van Cader TC. Risk factors for diabetic retinopathy in youth. Pediatrics. 1984;73:756‐761. [PubMed] [Google Scholar]
- 23. Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520‐526. 10.1001/archopht.1984.01040030398010 [DOI] [PubMed] [Google Scholar]
- 24. Sultan MB, Starita C, Huang K. Epidemiology, risk factors and management of pediatrics diabetic retinopathy. Br J Ophthalmol. 2012;96:312‐317. 10.1136/bjophthalmol-2011-300169 [DOI] [PubMed] [Google Scholar]
- 25. Ogle G, Middlehuurst A, Silink M, Hanas R, International society for pediatric and adolescent diabetes (ISPAD) . Global IDF/ISPAD guideline for diabetes in childhood and adolescence. 2011. Available at: https://www.idf.org/sites/default/files/Diabetes‐in‐Childhood‐and‐Adolescence‐Guidelines.pdf. Accessed April 9, 2017.
- 26. Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2018;19(Suppl 27):262‐274. 10.1111/pedi.12742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Children and Adolescents: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019, 42(Supplement 1): S148‐S164. 10.2337/dc19-S013 [DOI] [PubMed] [Google Scholar]
- 28. Mayer‐Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Suppl. 27):7‐19. 10.1111/pedi.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zabeen B, Govender D, Hassan Z, et al. Clinical features, biochemistry and HLA‐DRB1 status in children and adolescents with diabetes in Dhaka, Bangladesh. Diabetes Res ClinPract. 2019;158:107894. 10.1016/j.diabres.2019.107894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harding S, Greenwood R, Aldington S, et al. Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med. 2003;20(12):965‐971. 10.1111/j.1464-5491.2003.01077.x [DOI] [PubMed] [Google Scholar]
- 31. Downie E, Craig ME, Hing S, Cusumano J, Chan AKF, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 Diabetes. Diabetes Care. 2011;34(11):2368‐2373. 10.2337/dc11-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zabeen B, Huda K, Nessa M, Ahmed F, Akhter S, Azad K. Retinopathy in Bangladeshi Youth cohort with diabetes‐ a multicenter study. Pediatr Diab. 2018;19(Suppl. 26):104. [Google Scholar]
- 33. Dhillon N, Karthikeyan A, Castle A, et al. Natural history of retinopathy in children and young people with type 1 diabetes. Eye (Lond). 2016;30(7):987‐991. 10.1038/eye.2016.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donaghue KC, Fairchild JM, Craig ME, et al. Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care. 2003;26(4):1224‐1229. 10.2337/diacare.26.4.1224 [DOI] [PubMed] [Google Scholar]
- 35. Mayer‐Davis EJ, Davis C, Saadine J, et al. Diabetic retinopathy in the SEARCH for Diabetes in Youth Cohort: a pilot study. Diabet Med. 2012;29:1148‐1152. 10.1111/j.1464-5491.2012.03591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang SY, Andrews CA, Herman WH, Gardner TW, Stein JD. Incidence and risk factors for developing diabetic retinopathy among youths with type 1 or type 2 diabetes throughout the United States. Ophthalmology. 2017;124(4):424‐430. 10.1016/j.ophtha.2016.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olsen BS, Sjølie A, Hougaard P, et al. A 6‐year nationwide cohort study of glycaemic control in young people with type 1 diabetes. Risk markers for the development of retinopathy, nephropathy and neuropathy. Danish Study Group of Diabetes in Childhood. J Diabetes Complications. 2000;14(6):295‐300. 10.1016/s1056-8727(00)00078-7 [DOI] [PubMed] [Google Scholar]
- 38. Danne T, Weber B, Hartmann R, Enders I, Burger W, Hovener G. Long‐term glycemic control has a nonlinear association to the frequency of background retinopathy in adolescents with diabetes. Follow‐up of the Berlin Retinopathy Study. Diabetes Care. 1994;17(12):1390‐1396. 10.2337/diacare.17.12.1390 [DOI] [PubMed] [Google Scholar]
- 39. Henricsson M, Nystrom L, Blohme G, et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: Results from the nationwide population‐based Diabetes Incidence Study in Sweden (DISS). Diabetes Care. 2003;26(2):349‐354. 10.2337/diacare.26.2.349 [DOI] [PubMed] [Google Scholar]
- 40. Rajalakshmi R, Amutha A, Ranjani H, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 2014;28:291‐297. 10.1016/j.jdiacomp.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 41. TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care. 2013;36:1772‐1774. 10.2337/dc12-2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156‐163. 10.1007/s001250051594 [DOI] [PubMed] [Google Scholar]
- 43. Zhang L, Krzentowski G, Albert A, Lefebvre PJ. Risk of developing retinopathy in Diabetes Control and Complications Trial type 1 diabetic patients with good or poor metabolic control. Diabetes Care. 2001;24(7):1275‐1279. 10.2337/diacare.24.7.1275 [DOI] [PubMed] [Google Scholar]
- 44. Hainsworth DP, Bebu I, Aiello LP, et al. Risk factors for retinopathy in type 1 diabetes: The DCCT/EDIC study. Diabetes Care. 2019;42(5):875‐882. 10.2337/dc18-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hietala K, Harjutsalo V, Forsblom C, Summanen P, Groop PH, FinnDia ne Study Group . Age at onset and the risk of proliferative retinopathy in type 1 diabetes. Diabetes Care. 2010;33(6):1315‐1319. 10.2337/dc11-er04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stefansson E. The case for biennial retinopathy screening in children and adolescents: response to Maguire et al. Diabetes Care. 2006;29(1):178. 10.2337/diacare.29.01.06.dc05-1736 [DOI] [PubMed] [Google Scholar]
- 47. Maguire A, Chan A, Cusumano J, et al. The case for biennial retinopathy screening in children and adolescents. Diabetes Care. 2005;28(3):509‐513. 10.2337/diacare.28.3.509 [DOI] [PubMed] [Google Scholar]
- 48. Klein R, Klein BEK, Cruickshanks KJ. Assessing progress in retinopathy outcomes in type 1 diabetes: comparing findings from the Wisconsin Diabetes Registry Study and the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 2013;36(3):631‐637. 10.1016/S0161-6420(98)91020-X [DOI] [PMC free article] [PubMed] [Google Scholar]


