Abstract
Hypertension is a worldwide epidemic that continues to grow, with a subset of patients responding poorly to current treatment available. This is especially relevant in Asia, which constitutes 61% of the global population. Hypertension in Asia is a unique entity that is often salt‐sensitive, nocturnal, and systolic predominant. Sacubitril/valsartan is a first‐in‐class angiotensin receptor neprilysin inhibitor that was first used in heart failure with reduced ejection fraction. Sacubitril inhibits neprilysin, a metallopeptidase that degrades natriuretic peptides (NPs). NPs exert sympatholytic, diuretic, natriuretic, vasodilatory, and insulin‐sensitizing effects mostly via cyclic guanosine monophosphate (cGMP)‐mediated pathways. As an antihypertensive agent, sacubitril/valsartan has outperformed angiotensin II receptor type 1 blockers (ARBs), with additional reductions of office systolic blood pressures ranging between 5 and 7 mmHg, in multiple studies in Asia and around the globe. The drug was well tolerated even in the elderly or those with chronic kidney disease. Its mechanisms of actions are particularly attractive for treatment of hypertension in Asia. Sacubitril/valsartan offers a novel, dual class, single‐molecule property that may be considered as first‐line antihypertensive therapy. Further investigations are needed to validate its safety for long‐term use and to explore other potentials such as in the management of insulin resistance and obesity, which often coexist with hypertension in Asia.
Keywords: angiotensin receptor neprilysin inhibitor, antihypertensive therapy, Asian patients
1. INTRODUCTION
Hypertension is a leading cause of cardiovascular mortality and morbidity worldwide. 1 According to the World Health Organization (WHO), around 1.13 billion people have hypertension globally, with adequate control in less than 20% of these patients. Worldwide burden of hypertension has been estimated to exceed 1.5 billion people by 2025. 2 In Asia, which constitutes 61% of the global population, prevalence of hypertension has reportedly ranged from 30% to 47% across various countries. 2 , 3 Although much attention and effort have been put into the control of this disease, results have remained unsatisfactory. 4 Up to 75% of patients require simultaneous use of two or more drugs. 1 “Treatment‐resistant hypertension,” which is defined as inadequate blood pressure control even with the use of three or more antihypertensive agents (including a diuretic) at their highest tolerated doses, is exhibited in approximately 10 to 20% of patients. 5
Even before the debut of sacubitril/valsartan in PARADIGM‐HF, 6 a double‐blind randomized controlled trial that examined its efficacy in heart failure with reduced ejection fraction, excitement has been aroused over the potentials of this drug. It is a first‐in‐class angiotensin receptor neprilysin inhibitor (ARNi) that is comprised of sacubitril and valsartan in equal moieties. Sacubitril inhibits the actions of neprilysin, a metallopeptidase that degrades multiple biologically active peptides. 1 , 5 , 7 Most importantly, neprilysin hydrolyzes natriuretic peptides (NPs), namely atrial natriuretic peptide (ANP), B‐type natriuretic peptide (BNP), and C‐type natriuretic peptide (CNP). NPs exert diuretic, natriuretic, vasodilatory, and sympatholytic effects, which are thus enhanced by sacubitril and, in the long run, translate into anti‐inflammatory, antifibrotic, and antihypertrophic results. 8
There has been much interest in the utilization of sacubitril/valsartan as a novel antihypertensive agent in the premarket phase. The presence of resistant hypertension suggests that there are mechanisms to hypertension that are not fully antagonized by the various classes of medication available. The synergistic actions of sacubitril and valsartan position sacubitril/valsartan as a possible solution to resistant hypertension. Its unique diuretic and natriuretic effects also lead to speculation of its use in certain patient groups, such as those with salt‐sensitive hypertension. In this review, we summarize the distinctive features of hypertension in Asians, mechanisms of sacubitril/valsartan as an antihypertensive drug, and current data on sacubitril/valsartan used in hypertension and finally conclude on the possible subtypes of hypertension that benefit most from sacubitril/valsartan.
2. HYPERTENSION IN ASIA
Hypertension in Asia is a unique entity that is distinct from that in other parts of the world in several ways. These differences arise not only from ethnicity‐specific genetic background but also from the particular demographics and dietary habits in this region. In addition, the burden of uncontrolled hypertension is great, with serious consequences. 9 Studies have reported variable rates of uncontrolled hypertension in Asia, ranging from 2.6% to 65.8%. 2 , 3 The Asia Pacific Cohort Studies Collaboration demonstrated that hypertension is more strongly associated with cardiovascular events in Asians than in Australian or New Zealand Caucasians. 10 This translated into a 4.5 times increased risk of cardiovascular events in hypertensive patients compared with their normotensive counterparts. 2 Incidence of stroke and nonischemic heart failure is observably higher in Asia compared with Western countries, 10 both of which are strongly associated with hypertension.
It is well known that Asians have higher salt intake than other parts of the world. 10 Salt sensitivity is defined by an increase in blood pressure after sodium loading. 11 Of note, the anion for sodium is poorly defined in the conventional definition of salt sensitivity. Although differential effects of various anions on blood pressure have been reported, 12 , 13 , 14 over 85% of ingested sodium is consumed as sodium chloride (NaCl) and it is thus the molecule of interest in most contexts. In this review, the term salt refers to NaCl. Blood pressure responses to sodium intake are normally distributed across human populations 11 ; thus, the concept of salt sensitivity denotes not a pathologic phenomenon, but rather a possible target for blood pressure control. This is especially relevant in Asia, where dietary sodium is high. Multiple studies have identified single nucleotide polymorphisms in Asian communities that are associated with salt sensitivity. 11 , 15 , 16 , 17 , 18 , 19 Sensitivity to sodium loading also increases with age. 10 , 20 Salt sensitivity is also related to decreased sodium excretion and impaired vasodilation that are due to excitement of the renin‐angiotensin‐aldosterone system (RAAS) and blunting of the natriuretic peptide (NP) system. 21
Aging is a global trend that is most prominent in Asia. The influence of aging on blood pressures is complex. As mentioned earlier, salt sensitivity increases with age, with concomitant decrease of glomerular filtration. 20 The resultant increase in intravascular volume causes loss of nocturnal “dipping” of blood pressure, leading to nocturnal hypertension in so‐called “nondippers” or even “risers,” 20 together with other mechanisms including sleep‐disordered breathing. As patients age, progressive arterial stiffness leads to widened pulse pressures and systolic hypertension. 20 Increased central pulse pressure, as measured in the aorta, is the combined result of both aorta stiffness, aorta geometry, and peripheral wave reflection. 10 , 20 , 22 Asians are shorter in stature compared with Westerners and have smaller aortic roots. The impact of vascular stiffness on blood pressures may thus be magnified in Asians; systolic hypertension is therefore expected to be the predominant type particularly in this population as it continues to age. 10 All in all, hypertension in Asians poses higher risk of cardiovascular events and is more likely to be salt‐sensitive, nocturnal, and systolic predominant. These are key features to consider when searching for the ideal treatment in this population.
3. MECHANISMS OF SACUBITRIL/VALSARTAN
Sacubitril/valsartan dissociates into sacubitril and valsartan after intake. Sacubitril, a prodrug also named AHU377, is metabolized in the liver and converted to its active form, LBQ657, by enzymatic cleavage. 20 , 23 LBQ657 exerts its effects by inhibiting neprilysin, which, as mentioned before, hydrolyzes several vasoactive peptides. Neprilysin is a ubiquitous, membrane‐bound metallopeptidase that is mostly expressed in the renal proximal convoluted tubules. 1 , 24 The most relevant of all substrates of neprilysin are the NPs. ANP is mainly secreted in the atria and BNP in the ventricles, both in response to increased filling pressures and cardiac chamber wall stress. 1 , 23 At baseline, serum levels of ANP are markedly higher than BNP, with the latter significantly increased only in diseased states. This raises the notion that ANP is responsible for normal homeostasis, whereas BNP acts as a compensatory response to fluid overload. 25 CNP is synthesized predominantly by vascular endothelia when triggered by inflammatory cytokines and during endothelial dysfunction; its relationship with the cardiovascular system is still under investigation. 23 , 24
The NPs act through NP receptors (NPRs), types A (NPR‐A), B (NPR‐B), and C (NPR‐C) (Figure 1). ANP and BNP are ligands of NPR‐A, whereas NPR‐B is activated by CNP. NPR‐A and NPR‐B are guanylyl cyclase‐bound receptors that exert downstream diuretic, natriuretic, and vasodilatory effects through the production of cyclic guanosine monophosphate (cGMP). 1 , 7 , 24 NPR‐C, on the other hand, is bound to inhibitory G proteins and decreases intracellular levels of cyclic adenosine monophosphate (cAMP). NPR‐C was initially thought to be responsible primarily for the degradation of NPs, 24 but has now been shown to mediate vasodilatation through regulation of potassium channels and may also inhibit atrial fibrosis. 26 , 27 , 28 , 29
Figure 1.
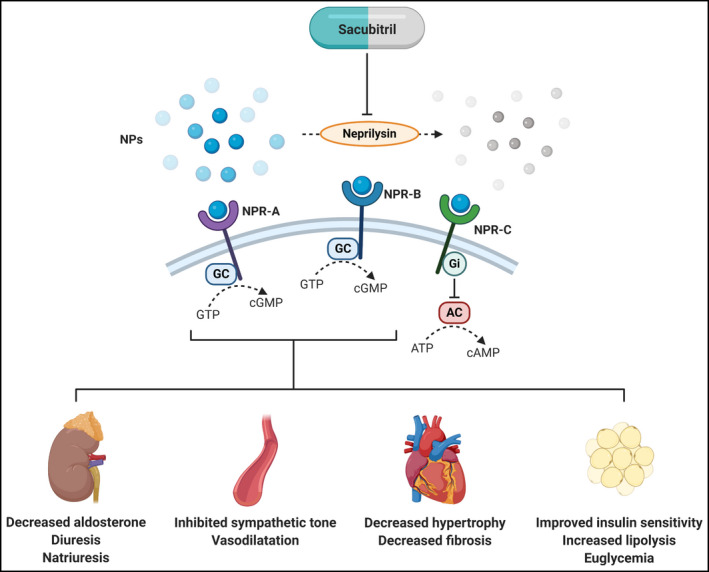
Overview of actions of sacubitril. NPs = natriuretic peptides; NPR = natriuretic peptide receptor; GC = guanylyl cyclase; GTP = guanosine‐5'‐triphosphate; cGMP = cyclic guanosine monophosphate; Gi = inhibitory G protein; AC = adenylyl cyclase; ATP = adenosine triphosphate; cAMP = cyclic adenosine monophosphate. Created with BioRender.com
Diuresis and natriuresis are the principal effects of the NPs. ANP promotes diuresis and natriuresis through cGMP‐induced suppression of sodium reabsorption in the inner medullary collecting ducts. 24 These effects are executed through inhibition of apical amiloride‐sensitive Na + channels and basolateral Na+‐K+‐adenosine triphosphatases, with the former responsible for passive reabsorption of sodium into the epithelial cells and the latter for active pumping of sodium into the blood. 24 , 30 Thus, ANP‐induced diuresis has little effect on serum potassium and uric acid levels. 5 The effects of BNP on sodium handling are similar to those of ANP. 24 , 25
Besides modulating water and sodium balance, NPs also exhibit potent vasodilatory effects via various pathways. ANP triggers the production of nitric oxide (NO) by Ca2+/calmodulin‐dependent endothelial NO synthase through the actions of cGMP‐dependent phosphokinase G (PKG), which in turn leads to arteriole vasodilatation. 30 This effect is also present in the aorta, ventricles, and kidneys. ANP also exerts similar effects via NPR‐C, with which inhibitory G proteins act to suppress adenylyl cyclase and decrease intracellular cAMP. In addition, ANP acts on the juxtaglomerular apparatus via Ca2+‐independent effects of cGMP, thereby reducing renin secretion. 30 By inhibiting the RAAS and sympathetic nervous system, ANP excites vagal afferent fibers which in turn decreases peripheral vascular resistance. 24
As sacubitril suppresses the actions of neprilysin, it indirectly augments the aforementioned processes of the NP system. Interestingly, targets of hydrolysis by neprilysin include not only NPs, but also several other vasoactive peptide, including angiotensin I (Ang I), adrenomedullin (ADM), bradykinin (BK), neurokinin A, neuropeptide Y, substance P, and endothelin (ET‐1). 7 , 24 Isolated antagonism of neprilysin may thus induce counter‐regulatory responses that negates the desirable actions of NPs. Simultaneous suppression of both the RAAS by valsartan and neprilysin by sacubitril exhibits greater results than inhibition of either alone. Target peptides of neprilysin, their effects on blood pressure, and how they are affected by valsartan are listed in Table 1. The targeted actions of sacubitril on sodium handling and vasodilatation are thus especially attractive in the treatment of hypertension in Asians, which, as previously mentioned, are strongly related to salt sensitivity and vascular aging (Figure 2).
Table 1.
Peptide hormones catabolized by neprilysin and their effects on blood pressures
| Name | Actions | Effect on blood pressures | Effect by valsartan |
|---|---|---|---|
| Adrenomedullin | Systemic vasodilation and new angiogenesis | Decrease | Increase levels |
| Angiotensin II | Systemic vasoconstriction and cardiovascular remodeling | Increase | Antagonism |
| Bradykinin | Systemic vasodilation and cough stimulation | Decrease | Possibly increase levels, but generally neutral |
| Endothelin‐1 | Systemic vasoconstriction | Increase | Decrease levels |
| Glucagon | Released from the α cells of pancreas; glycogen catalysis and blood glucose increase | Increase | Possibly increase levels |
| NPs | Systemic vasodilation; diuresis, and natriuresis | Decrease | Increase levels |
| Neurotensin | Regulation of luteinizing hormone and prolactin; systemic vasodilation through interaction with the dopaminergic system | Biphasic | Unknown |
| Oxytocin | Facilitation in child birth | Decrease | Possibly increase levels |
| Substance P | Cough production | Decrease | Neutral |
Figure 2.
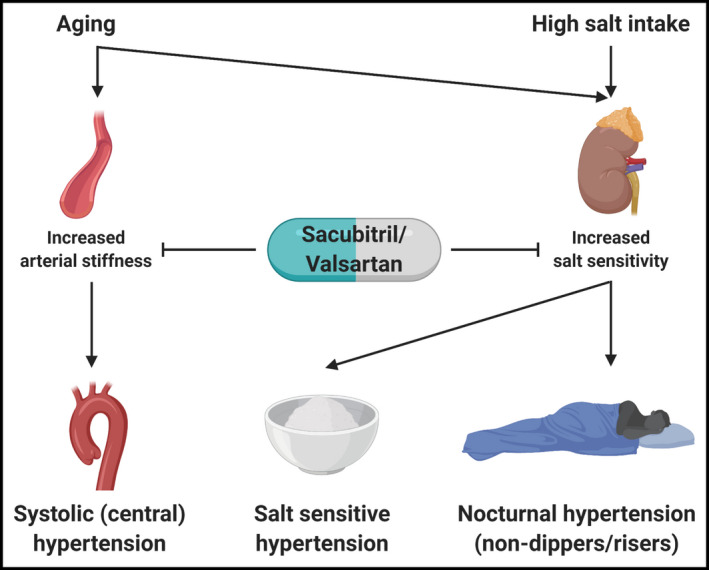
Characteristics of hypertension in Asians that are targets of sacubitril/valsartan. Created with BioRender.com
4. EFFICACY AND SAFETY OF SACUBITRIL/VALSARTAN AS AN ANTIHYPERTENSIVE AGENT
Efficacy of sacubitril/valsartan as an antihypertensive agent has been studied mostly in comparison with a single angiotensin II receptor type 1 blocker (ARB) (Tables 2 and 3). Studies have demonstrated consistent blood pressure reductions by sacubitril/valsartan that is superior to its competitors. The role of sacubitril/valsartan as add‐on therapy in uncontrolled hypertension has also been validated in selected studies. However, data comparing the effects of sacubitril/valsartan to other drug classes (namely beta blockers, thiazide diuretics, and calcium channel blockers) are lacking. Patients with certain comorbidities, such as diabetics, chronic kidney disease, and the obese, are also poorly represented in the present literature. Nonetheless, investigations are ongoing, and the body of evidence regarding the use of this drug is expected to grow.
Table 2.
Studies on sacubitril/valsartan in hypertension in Asia
| Title | Conditions | Age | Ethnicity | Enrollment | Study design | Intervention | Length of follow up | Results |
|---|---|---|---|---|---|---|---|---|
| Efficacy and Safety of LCZ696, a First‐in‐Class Angiotensin Receptor Neprilysin Inhibitor, in Asian Patients With Hypertension (Kario et al, 33 2014) |
|
≥18 | Asian | 389 | Multicenter, randomized, double‐blind | Sacubitril/valsartan 100 mg QD vs sacubitril/valsartan 200 mg QD vs 400 mg QD vs placebo | 8 weeks | Sacubitril/valsartan was superior to placebo in reduction of clinic DBP, SBP, and pulse pressures across all doses. |
| Safety and efficacy of LCZ696, a first‐in‐class angiotensin receptor neprilysin inhibitor, in Japanese patients with hypertension and renal dysfunction (Ito et al, 36 2015) |
|
≥20 | Asian | 32 | Multicenter, open‐label | Initiate with sacubitril/valsartan 100 mg QD, followed by a stepwise optional dose titration to 200 and 400 mg | 8 weeks | Geometric mean reduction in UACR was 15.1%; mean reduction in msSBP and msDBP was 20.5 ± 11.3 and 8.3 ± 6.3 mmHg. |
| Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Compared With Olmesartan in Elderly Asian Patients (≥65 Years) With Systolic Hypertension (Supasyndh et al, 35 2017) |
|
≥65 | Asian | 588 | Multicenter, randomized, double‐blind | Sacubitril/valsartan vs olmesartan, starting with 100 mg or 10 mg QD; doses were increased to sacubitril/valsartan 200 mg or olmesartan 20 mg at week 4, and then to 400 mg or 40 mg at week 10 if BP > 140/90 mmHg | 14 weeks | Sacubitril/valsartan resulted in greater reduction of msSBP than olmesartan only (−22.71 vs. −16.11 mmHg, respectively; P < .001). |
| Long‐term (52‐week) safety and efficacy of Sacubitril/valsartan in Asian patients with hypertension (Supasyndh et al, 34 2017) |
|
≥18 | Asian | 341 | Multicenter, open‐label | Start with sacubitril/valsartan 200 mg QD, increased to 400 mg if msSBP ≥ 140 mmHg or msDBP ≥ 90 mmHg after 4 weeks; after four weeks, amlodipine 5‐10 mg and hydrochlorothiazide 6.25‐25 mg were added at any visit if msSBP > 140 mmHg or msDBP > 90 mmHg | 12 months | Sacubitril/valsartan‐based regimen significantly reduced msSBP and msDBP from baseline (−24.7 and − 16.2 mmHg), with 75.3, 90.6 and 87.6% response rates in overall BP control, msSBP and msDBP |
| Efficacy and safety of sacubitril/valsartan (LCZ696) add‐on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy (Wang et al, 63 2017) |
|
≥18 | Asian | 266 | Multicenter, randomized, double‐blind | Sacubitril/valsartan 200 mg QD + amlodipine 5 mg QD vs amlodipine 5 mg QD | 8 weeks | Sacubitril/valsartan with amlodipine led to greater reductions in 24‐h SBP compared with amlodipine monotherapy from baseline (−13.9 vs −0.8 mmHg, P < .001). |
| Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT‐proBNP in Salt‐Sensitive Hypertension (Wang et al, 21 2017) |
|
≥18 | Asian | 72 | Multicenter, randomized, double‐blind | Sacubitril/valsartan 400 mg or valsartan 320 mg QD for 4 weeks, followed by a washout period of 1 to 2 weeks, then crossed over and treated for 4 weeks | 4 weeks in each treatment period | Sacubitril/valsartan was associated with a significant increase in natriuresis (adjusted treatment difference: 24.5 mmol/6 hours, 50.3 mmol/24 hours, both P < .001) and diuresis (adjusted treatment difference: 291.2 mL/6 hours, P < .001; 356.4 mL/24 hours, P = .002) on day 1, with greater reductions in office and ambulatory blood pressure on day 28. |
| Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double‐blind, 8‐week study (Huo et al, 64 2019) |
|
≥18 | Asian | 1438 | Multicenter, randomized, double‐blind | Sacubitril/valsartan 200 mg QD vs sacubitril/valsartan 400 mg QD vs olmesartan 20 mg QD | 8 weeks | Sacubitril/valsartan provided larger decreases in msSBP compared to olmesartan at week 8 (between‐treatment difference: −2.33 mmHg [95% confidence interval (CI) −4.00 to − 0.66 mmHg], P < .05 for noninferiority and superiority for 200 mg; −3.52 [−5.19 to − 1.84 mmHg], P < .001 for superiority for 400 mg). |
Table 3.
Global studies on sacubitril/valsartan in hypertension
| Title | Conditions | Age | Ethnicity | Enrollment | Study design | Intervention | Length of follow up | Results |
|---|---|---|---|---|---|---|---|---|
| Blood pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double‐blind, placebo‐controlled, active comparator study (Ruilope et al, 31 2010) |
|
18‐75 | Multi‐ethnic; 87% white | 1328 | Multicenter, randomized, double‐blind |
Sacubitril/valsartan 100 mg vs sacubitril/valsartan 200 mg vs sacubitril/valsartan 400 mg vs 80 mg valsartan vs 160 mg valsartan vs 320 mg valsartan vs 200 mg sacubitril vs placebo |
13 weeks (8 weeks of treatment) | Sacubitril/valsartan significantly decreased msDBP compared to valsartan only (mean reduction: –2.17 mm Hg, 95% CI –3.28 to –1.06; P < .0001); 200 mg of sacubitril/valsartan was superior also to 160 mg valsartan (msDBP reduction –2.97 mm Hg, 95% CI –4.88 to –1.07, P = .0023), as was 400 mg sacubitril/valsartan to 320 mg valsartan (msDBP reduction –2.70 mm Hg, –4.61 to –0.80, P = .0055). |
| Efficacy and Safety of Crystalline Valsartan/Sacubitril (LCZ696) Compared With Placebo and Combinations of Free Valsartan and Sacubitril in Patients With Systolic Hypertension: The RATIO Study (Izzo et al, 32 2017) |
|
≥18 | Multi‐ethnic; 68.4% white | 907 | Multicenter, randomized, double‐blind | Sacubitril/valsartan 400 mg QD vs free valsartan 320 mg QD with placebo or increasing doses of free sacubitril (50, 100, 200, or 400 mg QD) | 8 weeks | Sacubitril/valsartan 400 mg resulted in greater reductions in sitting office SBP and 24‐hour ambulatory SBP than with valsartan 320 mg (−5.7 and −3.4 mmHg, respectively, P < .05 each) |
| Effects of Sacubitril/Valsartan Versus Olmesartan on Central Hemodynamics in the Elderly With Systolic Hypertension: The PARAMETER Study (Williams et al, 65 2017) |
|
≥60 | Multi‐ethnic; 64.3% white | 432 | Multicenter, randomized, double‐blind | Sacubitril/valsartan 200 mg vs olmesartan 20 mg for 4 weeks, then doubled doses. If msSBP > 140 mmHg or msDBP > 90 mmHg after 12 weeks, amlodipine (2.5‐5 mg) followed by hydrochlorothiazide (6.25‐25 mg) were added every 4 weeks up to week 24 | 52 weeks | Sacubitril/valsartan resulted in greater reductions of central aortic systolic pressure than olmesartan at week 12 by a difference of − 3.7 mmHg (P = .010). At week 52, there were no differences between blood pressure parameters of the two groups; however, more patients required add‐on antihypertensive therapy with olmesartan (47%) versus sacubitril/valsartan (32%; P < .002). |
| Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: A randomized, double‐blind, 8‐week study (Cheung et al, 66 2018) |
|
≥18 | Multi‐ethnic; 57.6% white | 376 | Multicenter, randomized, double‐blind | Addition of sacubitril/valsartan 200 mg QD to uncontrolled hypertension under Olmesartan 20 mg QD | 8 weeks | Addition of sacubitril/valsartan led to superior reductions in 24‐hour mean ambulatory systolic BP vs olmesartan alone (−4.3 mm Hg vs − 1.1 mm Hg, P < .001); sacubitril/valsartan was also superior for reductions in 24‐hour mean ambulatory DBP, pulse pressures and office SBP and DBP. |
Reported in 2010, Ruilope et al 31 conducted a double‐blind study that recruited 1328 patients with mild‐to‐moderate hypertension between 18 and 75 years of age and randomly assigned them to one of eight groups: 100 mg, 200 mg, or 400 mg of sacubitril/valsartan; 80 mg, 160 mg, or 320 mg of valsartan; 200 mg of sacubitril; or placebo. Patients received eight weeks of the allocated treatment and mean sitting diastolic blood pressure were analyzed. The average reduction in mean sitting diastolic blood pressure (DBP) was significantly greater with sacubitril/valsartan across different doses (mean additional reduction: −2.2 mmHg, 95% CI −3.3 to −1.1; P < .0001). The drug was well tolerated and there were no reported cases of angioedema. A multicenter, double‐blind, randomized controlled trial by Izzo et al 32 further examined the potency and safety of 400 mg sacubitril/valsartan compared with 320 mg free valsartan in conjunction with placebo or increasing doses of free sacubitril (50, 100, 200, or 400 mg once daily) in patients over 18 years old with mild‐to‐moderate hypertension. A total of 852 patients completed the study. Primary endpoint was the change in office systolic blood pressure (SBP) from baseline to week eight. The combination of sacubitril/valsartan 400 mg resulted in greater decrease of office SBP and 24‐hour ambulatory SBP than free valsartan 320 mg (−5.7 and −3.4 mmHg, respectively, P < .05 each). Adverse event rates were similar between sacubitril/valsartan and free valsartan with placebo.
Motivated by the favorable features of Asian hypertension, several trials have been conducted in Asia, supporting the use of sacubitril/valsartan as an antihypertensive drug in these patients. Kario et al 33 were among the first to demonstrate the efficacy of sacubitril/valsartan in Asians with hypertension. Three hundred and sixty two patients with mild‐to‐moderate hypertension beyond 18 years of age completed the study and were randomized to receive sacubitril/valsartan 100 mg, 200 mg, 400 mg, or placebo for eight weeks. Primary endpoints were mean decreases in clinic DBP measured after eight weeks of treatment. After eight weeks, changes in clinic DBP were − 7.8, −7.3, and − 8.8 mmHg for sacubitril/valsartan 100, 200, and 400 mg, respectively, compared with placebo (all P < .0001). Nasopharyngitis and upper respiratory tract infections were the most frequently reported adverse events, with the former occurring equally among all treatment groups and the latter arising more often in the 100 mg group. All in all, the drug was well tolerated with minimal adverse events across all groups.
After completion of the eight‐week core study, the same group of patients were followed in a 52‐week extension to assess the long‐term safety, tolerability, and efficacy of sacubitril/valsartan. 34 In this 52‐week open‐label study, patients received 200 mg sacubitril/valsartan daily, with the dose increased to 400 mg if blood pressures remained uncontrolled after four weeks. After four weeks, amlodipine 5‐10 mg and hydrochlorothiazide 6.25‐25 mg were added every four weeks in incremental doses if blood pressure control was still inadequate. As seen in the eight‐week core study, the most frequent adverse events were nasopharyngitis (18.2%) and dizziness (8.8%). There was a case of transient angioedema that resolved spontaneously in 2.5 hours but led to withdrawal of the treatment drug. Sacubitril/valsartan provided reliable decreases in blood pressures throughout the 52 weeks, with 75.3% of patients achieving adequate blood pressure control. The majority of patients did not require add‐on amlodipine or hydrochlorothiazide.
Wang et al 21 demonstrated the impact of sacubitril/valsartan on salt‐sensitive hypertension in Asians in a randomized, double‐blind, crossover study that compared sacubitril/valsartan 400 mg with valsartan 320 mg. Seventy‐two patients with defined salt‐sensitive hypertension were involved in the study, with the primary outcome being cumulative 6‐ and 24‐hour sodium excretion after the first dosage given. Administration of sacubitril/valsartan resulted in more pronounced natriuresis and diuresis on day 1 but not on day 28. Although sodium excretion were similar between the two treatment arms by day 28, blood pressures and serum N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) levels were significantly lower in the sacubitril/valsartan group, suggesting that mechanisms to lower blood pressure and decreased cardiac chamber wall stress were not solely dependent on natriuresis. Despite daytime dosing, blood pressure reductions were more pronounced at night with both sacubitril/valsartan and valsartan. Both sacubitril/valsartan and valsartan only were well tolerated, with stable patient body weight and serum electrolytes throughout the study period.
The elderly Asian with systolic hypertension was addressed in a randomized, double‐blind study by Supasyndh et al 35 588 patients over 65 years of age with mean sitting SBP between 150 and 180 mmHg were randomized to sacubitril/valsartan or olmesartan, starting with 100 mg or 10 mg once daily, respectively. Doses were increased to sacubitril/valsartan 200 mg or olmesartan 20 mg at week 4 and then further up‐titrated to 400 mg or 40 mg at week 10 if blood pressures remained greater than 140/90 mmHg. Primary efficacy assessment was reduction of SBP at week ten. At week ten, treatment with sacubitril/valsartan resulted in greater reduction of SBP than olmesartan only (22.7 vs. 16.1 mmHg, respectively; P < .001). Sacubitril/valsartan was also superior in other secondary assessments, including mean sitting pulse pressure and mean ambulatory pulse pressure. Three patients in the sacubitril/valsartan group discontinued treatment due to serious adverse events, but were not related to the study drug. Changes in renal function and serum electrolyte levels were negligible. Both treatments were generally well tolerated.
Hypertension in the elderly is characterized not only by a greater contribution of arterial stiffness, but also decreased glomerular filtration and larger percentage of patients with comorbidities such as chronic kidney disease. In an eight‐week, multicenter, open‐label study, Ito et al 36 evaluated the efficacy and safety of sacubitril/valsartan in 31 Japanese patients over 20 years old with hypertension and moderate‐to‐severe renal dysfunction, defined as an eGFR between 15 and 60 ml/min/1.73 m2. Patients received 100 mg sacubitril/valsartan daily for two weeks, after which doses were increased to 200 mg daily if blood pressures remained greater than 130/80 mmHg. Doses were further up‐titrated to 400 mg daily if blood pressure control was still inadequate after another two weeks. Blood chemistry, including blood urea nitrogen (BUN), creatinine, sodium, potassium, and urine albumin to creatinine ratio (UACR), were analyzed. After eight weeks of treatment, patients achieved a mean reduction of mean sitting SBP and mean sitting DBP of 20.5 ± 11.3 and 8.3 ± 6.3 mm Hg, respectively. On average, UACR decreased by 15.1%. Changes in serum electrolytes, BUN, creatinine, and eGFR were insignificant. There were a total of 14 adverse events, with most being nasopharyngitis, and none were severe.
In summary, multiple studies have consistently demonstrated the superiority of sacubitril/valsartan compared with ARB in blood pressure control, with substantial evidence in Asians. This was further validated by meta‐analyses 37 , 38 , 39 , 40 that showed superior blood pressure control by sacubitril/valsartan compared with ARBs. On average, reported additional reductions of average SBP in treatment arms receiving sacubitril/valsartan (at up to 400 mg per day) ranged between 5 and 7 mmHg. Although the methods of assessment and treatment protocol varied across studies, there seems to be a trend in more prominent blood pressure reductions in trials that enrolled Asians. This possibly reflects on how sacubitril/valsartan targets mechanisms in hypertension that are particularly essential to physiology of the disease in Asians. Currently, meta‐analyses that focused on Asian data are lacking. Further investigations are needed to shed insight on this issue.
5. SPECIAL CONSIDERATIONS
As urbanization continues in the Asian society, prevalence of obesity has grown dramatically. According to the WHO, there are approximately 6.6 million children under 5 years and one in five adults who are overweight in Asia. Asian populations are generally slimmer than Caucasian, yet the prevalence of hypertension in Asia is similar to global rates. The combination of hypertension, impaired fasting glucose, dyslipidemia, and obesity denotes the metabolic syndrome. Wang et al have shown that cutoff values for both body mass index and waist circumference that correlate with the presence of hypertension and other metabolic syndrome features were lowest in East Asian populations and highest in South Asian populations. 41 This suggests that Asian populations are prone to developing obesity‐related metabolic derangements. Proposed mechanisms of obesity‐induced hypertension include excitement of the sympathetic system, activation of the RAAS, impaired pressure natriuresis, and vascular endothelial dysfunction. 42 , 43 Sacubitril/valsartan targets several of these mechanisms (Figure 1), thus posing as an appealing choice of therapy for these patients. The use of instruments, such as a bioimpedance device, to assess changes in volume status in patients treated with sacubitril/valsartan may shed insight on this issue. 44
Interestingly, over the past few years there is emerging evidence that obesity and type 2 diabetes mellitus may represent a state of “natriuretic handicap.” 45 Magnusson et al 46 followed 1828 nondiabetic patients for a median of 16 years, 301 of which developed diabetes during follow up. There was an inverse association between baseline serum ANP and the incidence of diabetes. Haufe et al 47 demonstrated how physical exertion in the obese acutely stimulates ANP secretion, whereas modest diet‐induced weight loss led to decreased NPR‐C levels, implicating decreased clearance of NPs. Khan et al 48 elucidated how, while obesity alone was associated with minor reductions in plasma NT‐proBNP levels, insulin resistance correlated with significant decreases in NT‐proBNP. NPs have been shown to involve in multiple metabolic processes, including lipolysis, lipid oxidation, and the browning of white adipose tissue. 45 , 49 , 50 , 51 In a multicenter, randomized, double‐blind study by Jordan et al, 52 92 patients with obesity and hypertension were allocated to sacubitril/valsartan 400 mg daily and amlodipine 10 mg daily. Insulin sensitivity and lipolysis were examined at baseline and at eight weeks, via hyperinsulinemic‐euglycemic glucose clamp, abdominal adipose tissue microdialysis and whole‐body glycerol tracer. After eight weeks, sacubitril/valsartan was associated with significant increases in insulin sensitivity. In a post hoc analysis of 3778 patients with known diabetes or an HbA1c ≥ 6.5% at screening in the PARADIGM‐HF trial, the decrease in HbA1c was significantly greater in patients receiving sacubitril/valsartan compared with those receiving enalapril (overall reduction 0.14%, 95% CI 0.06‐0.23, P = .006) over the full duration of follow‐up. 53
Serum BNP and NT‐proBNP levels are commonly used biomarkers in the care of patients with heart failure. They are useful in the evaluation of chronic heart failure, diagnosis of acute heart failure, and providing prognostic information. 54 , 55 , 56 Because sacubitril inhibits the degradation of NPs by neprilysin but simultaneously reduces cardiac wall stress, how sacubitril affects NP levels as a whole has elicited much debate. BNP and NT‐proBNP are products of proBNP cleavage, which occurs upstream of the actions of neprilysin. BNP is a target of neprilysin, while NT‐proBNP is eliminated by alternative mechanisms. Therefore, serum levels of BNP increase as a result of suppressed degradation with sacubitril use, but may also concurrently decrease due to improved hemodynamics. 57 , 58 , 59 The net effect of sacubitril on serum BNP levels may thus vary on a patient‐to‐patient basis and test results should be interpreted with caution. The test performance of NT‐proBNP is hardly affected by sacubitril use, and changes in serum levels more likely reflect changes in cardiac stress.
The majority of international and regional hypertension guidelines advocate early or initial use of combination antihypertensive therapy, ideally in a single pill, composed of angiotensin‐converting enzyme inhibitors or ARB and dihydropyridine calcium channel blockers or thiazide diuretics. 60 , 61 , 62 Sacubitril/valsartan offers a novel, dual class, single‐molecule property that may also be considered as first‐line therapy. Despite its many beneficial effects, the costs of sacubitril/valsartan may limit its use in hypertension, especially in developing countries. Costs and effectiveness of sacubitril/valsartan as an antihypertensive agent remain to be evaluated. In particular, natriuretic and diuretic effects of sacubitril may have significant overlap with that of thiazides, though through different molecular mechanisms. Currently, there are no studies comparing sacubitril/valsartan against the combination of a thiazide plus an ARB. Although it is known that sacubitril also exerts vasodilatory, antihypertrophic, and antifibrotic effects, whether these actions translate into benefits beyond that of thiazides in a cost‐effective manner needs further investigation.
6. FUTURE PERSPECTIVES
Sacubitril/valsartan, a first‐in‐class ARNi, has gained much attention over the past years due to its remarkable actions in heart failure with reduced ejection fraction. Studies have scrutinized its actions in various patient populations, including those with hypertension. Trials have confirmed the efficacy and short‐term safety outcomes of sacubitril/valsartan in hypertension, with the longest period for which patients were followed being 52 weeks. However, hypertension is a chronic disease that requires treatment for decades; the safety profile of sacubitril/valsartan for such an extended length of time is unknown. Neprilysin also degrades amyloid β peptides, the accumulation of which may predispose to Alzheimer disease, age‐related macular degeneration, and cerebral amyloid angiopathy. 5 , 7 This is thus the most concerning issue regarding long‐term use of sacubitril/valsartan, which necessitates further research and validation.
At present, data on the use of sacubitril/valsartan in hypertensive patients are promising. Sacubitril/valsartan is superior to valsartan and olmesartan in mild‐to‐moderate hypertension, and is safe in the elderly patients and those with chronic kidney disease. It is especially effective in those with salt‐sensitive and systolic hypertension, both of which are typical features of Asian hypertension. In addition, sacubitril/valsartan poses a novel approach to obesity and insulin resistance, with the prevalence of both increasing in Asia. Application of sacubitril/valsartan in the obese or diabetic Asians is thus an attractive topic for future research.
Conflicts of interest
CH Chen reports personal fees from Novartis, Sanofi, Daiichi Sankyo, SERVIER, and Boehringer Ingelheim Pharmaceuticals, Inc HM Cheng received speakers honorarium and sponsorship to attend conferences and CME seminars from Eli Lilly and AstraZeneca; Pfizer Inc; Bayer AG; Boehringer Ingelheim Pharmaceuticals, Inc; Daiichi Sankyo, Novartis Pharmaceuticals, Inc; SERVIER; Co., Pharmaceuticals Corporation; Sanofi; TAKEDA Pharmaceuticals International and served as an advisor or consultant for ApoDx Technology, Inc YC Chia has received honorarium and sponsorship at attend conferences and seminars from Boehringer‐Ingelheim, Pfizer, Omron, Servier, and Xepa‐Sol and an investigator‐initiated research grant from Pfizer. JG Wang reports having received research grants from Chendu Di‐Ao and Omron, and lecture and consulting fees from AstraZeneca, Novartis, Omron, Servier, and Takeda. Kario reports research grants from Omron Healthcare, Fukuda Denshi, A&D, Pfizer Japan, and honoraria from Omron Healthcare. All other authors report no potential conflicts of interest in relation to this article.
AUTHOR CONTRIBUTIONS
Conception or design of the work: DL, TDW, KK. Acquisition, analysis, and interpretation of data for the work: DL, TDW. Drafting the work: DL, TDW. Revising it critically for important intellectual content: All. Final approval of the version to be published: All. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: DL, TDW.
Lin DS‐H, Wang T‐D, Buranakitjaroen P, et al; the HOPE Asia Network . Angiotensin receptor neprilysin inhibitor as a novel antihypertensive drug: Evidence from Asia and around the globe. J Clin Hypertens. 2021;23:556–567. 10.1111/jch.14120
REFERENCES
- 1. Wehland M, Simonsen U, Buus NH, Krüger M, Grimm D. An evaluation of the fixed‐dose combination sacubitril/valsartan for the treatment of arterial hypertension. Expert Opin Pharmacother. 2020;21:1133‐1143. [DOI] [PubMed] [Google Scholar]
- 2. Park JB, Kario K, Wang J‐G. Systolic hypertension: an increasing clinical challenge in Asia. Hypertens Res. 2015;38:227‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chia Y‐C, Buranakitjaroen P, Chen C‐H, et al. Current status of home blood pressure monitoring in Asia: statement from the HOPE Asia network. J Clin Hypertens. 2017;19:1192‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan H‐Y, Lin H‐J, Chen W‐J, Wang T‐D. Prevalence, treatment, control and monitoring of hypertension: a nationwide community‐based survey in Taiwan, 2017. Acta Cardiol Sin. 2020;36:375‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kjeldsen SE, Narkiewicz K, Burnier M, Oparil S. Will we ever use angiotensin receptor neprilysin inhibition (ARNi) for the treatment of hypertension? Blood Press. 2019;28:75‐76. [DOI] [PubMed] [Google Scholar]
- 6. McMurray JJV, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993‐1004. [DOI] [PubMed] [Google Scholar]
- 7. Chrysant SG. Benefits and pitfalls of sacubitril/valsartan treatment in patients with hypertension. J Clin Hypertens. 2018;20:351‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azizi M, Rossignol P, Hulot J‐S. Emerging drug classes and their potential use in hypertension. Hypertension. 2019;74:1075‐1083. [DOI] [PubMed] [Google Scholar]
- 9. Wang T‐D, Lee Y‐H, Chang S‐S, et al. 2019 consensus statement of the taiwan hypertension society and the taiwan society of cardiology on renal denervation for the management of arterial hypertension. Acta Cardiol Sin. 2019;35:199‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kario K, Chen C‐H, Park S, et al. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension. 2018;71:375‐382. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Shi M, Dolan J, He J. Sodium sensitivity of blood pressure in Chinese populations. J Hum Hypertens. 2020;34:94‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH. Sodium bicarbonate and sodium chloride: effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens. 1990;8:663‐670. [DOI] [PubMed] [Google Scholar]
- 13. Kotchen TA, Kotchen JM. Dietary sodium and blood pressure: interactions with other nutrients. Am J Clin Nutr. 1997;65:708S‐711S. [DOI] [PubMed] [Google Scholar]
- 14. Luft FC, McCarron DA. Heterogeneity of hypertension: the diverse role of electrolyte intake. Annu Rev Med. 1991;42:347‐355. [DOI] [PubMed] [Google Scholar]
- 15. Rhee M‐Y, Yang SJ, Oh SW, et al. Novel genetic variations associated with salt sensitivity in the Korean population. Hypertens Res. 2011;34:606‐611. [DOI] [PubMed] [Google Scholar]
- 16. Katsuya T, Ishikawa K, Sugimoto K, Rakugi H, Ogihara T. Salt sensitivity of Japanese from the viewpoint of gene polymorphism. Hypertens Res. 2003;26:521‐525. [DOI] [PubMed] [Google Scholar]
- 17. Nierenberg JL, Li C, He J, et al. Blood pressure genetic risk score predicts blood pressure responses to dietary sodium and potassium: the gensalt study (genetic epidemiology network of salt sensitivity). Hypertension. 2017;70:1106‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qi H, Liu B, Guo C, et al. Effects of environmental and genetic risk factors for salt sensitivity on blood pressure in northern China: the systemic epidemiology of salt sensitivity (EpiSS) cohort study. BMJ Open. 2018;8:e023042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Z, Qi H, Liu B, et al. Genetic susceptibility to salt‐sensitive hypertension in a Han Chinese population: a validation study of candidate genes. Hypertens Res. 2017;40:876‐884. [DOI] [PubMed] [Google Scholar]
- 20. Kario K. The Sacubitril/Valsartan, a First‐in‐Class, Angiotensin Receptor Neprilysin Inhibitor (ARNI): Potential Uses in Hypertension, Heart Failure, and Beyond. Curr Cardiol Rep. 2018;20:5. [DOI] [PubMed] [Google Scholar]
- 21. Wang T‐D, Tan R‐S, Lee H‐Y, et al. Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT‐proBNP in Salt‐Sensitive Hypertension. Hypertension. 2017;69:32‐41. [DOI] [PubMed] [Google Scholar]
- 22. Cheng H‐M, Chuang S‐Y, Sung S‐H, et al. 2019 consensus of the taiwan hypertension society and taiwan society of cardiology on the clinical application of central blood pressure in the management of hypertension. Acta Cardiol Sin. 2019;35:234‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bavishi C, Messerli FH, Kadosh B, Ruilope LM, Kario K. Role of neprilysin inhibitor combinations in hypertension: insights from hypertension and heart failure trials. Eur Heart J. 2015;36:1967‐1973. [DOI] [PubMed] [Google Scholar]
- 24. Salazar J, Rojas‐Quintero J, Cano C, et al. Neprilysin: a potential therapeutic target of arterial hypertension? Curr Cardiol Rev. 2020;16:25‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Volpe M. Natriuretic peptides and cardio‐renal disease. Int J Cardiol. 2014;176:630‐639. [DOI] [PubMed] [Google Scholar]
- 26. Kun A, Kiraly I, Pataricza J, et al. C‐type natriuretic peptide hyperpolarizes and relaxes human penile resistance arteries. J Sex Med. 2008;5:1114‐1125. [DOI] [PubMed] [Google Scholar]
- 27. Jansen HJ, Mackasey M, Moghtadaei M, et al. NPR‐C (Natriuretic Peptide Receptor‐C) Modulates the Progression of Angiotensin II‐Mediated Atrial Fibrillation and Atrial Remodeling in Mice. Circ Arrhythm Electrophysiol. 2019;12:e006863. [DOI] [PubMed] [Google Scholar]
- 28. Hua R, MacLeod SL, Polina I, et al. Effects of Wild‐Type and Mutant Forms of Atrial Natriuretic Peptide on Atrial Electrophysiology and Arrhythmogenesis. Circ Arrhythm Electrophysiol. 2015;8(5):1240‐1254. [DOI] [PubMed] [Google Scholar]
- 29. Egom EE, Vella K, Hua R, et al. Impaired sinoatrial node function and increased susceptibility to atrial fibrillation in mice lacking natriuretic peptide receptor C. J Physiol (Lond). 2015;593:1127‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong PCY, Guo J, Zhang A. The renal and cardiovascular effects of natriuretic peptides. Adv Physiol Educ. 2017;41:179‐185. [DOI] [PubMed] [Google Scholar]
- 31. Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double‐blind, placebo‐controlled, active comparator study. Lancet. 2010;375:1255‐1266. [DOI] [PubMed] [Google Scholar]
- 32. Izzo JL, Zappe DH, Jia Y, Hafeez K, Zhang J. Efficacy and safety of crystalline valsartan/sacubitril (LCZ696) compared with placebo and combinations of free valsartan and sacubitril in patients with systolic hypertension: the RATIO study. J Cardiovasc Pharmacol. 2017;69:374‐381. [DOI] [PubMed] [Google Scholar]
- 33. Kario K, Sun N, Chiang F‐T, et al. Efficacy and safety of LCZ696, a first‐in‐class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double‐blind, placebo‐controlled study. Hypertension. 2014;63:698‐705. [DOI] [PubMed] [Google Scholar]
- 34. Supasyndh O, Sun N, Kario K, Hafeez K, Zhang J. Long‐term (52‐week) safety and efficacy of Sacubitril/valsartan in Asian patients with hypertension. Hypertens Res. 2017;40:472‐476. [DOI] [PubMed] [Google Scholar]
- 35. Supasyndh O, Wang J, Hafeez K, Zhang Y, Zhang J, Rakugi H. Efficacy and safety of sacubitril/valsartan (LCZ696) compared with olmesartan in elderly asian patients (≥65 years) with systolic hypertension. Am J Hypertens. 2017;30:1163‐1169. [DOI] [PubMed] [Google Scholar]
- 36. Ito S, Satoh M, Tamaki Y, et al. Safety and efficacy of LCZ696, a first‐in‐class angiotensin receptor neprilysin inhibitor, in Japanese patients with hypertension and renal dysfunction. Hypertens Res. 2015;38:269‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Q, Li L, Wang F, et al. Effect and safety of LCZ696 in the treatment of hypertension: a meta‐analysis of 9 RCT studies. Medicine. 2019;98:e16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malik AH, Aronow WS. Efficacy of sacubitril/valsartan in hypertension. Am J Ther. January 2019. 10.1097/MJT.0000000000000925 [DOI] [Google Scholar]
- 39. De Vecchis R, Soreca S, Ariano C. Anti‐hypertensive effect of Sacubitril/Valsartan: a meta‐analysis of randomized controlled trials. Cardiol Res. 2019;10:24‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao Y, Yu H, Zhao X, Ma R, Li N, Yu J. The effects of LCZ696 in patients with hypertension compared with angiotensin receptor blockers: a meta‐analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2017;22:447‐457. [DOI] [PubMed] [Google Scholar]
- 41. Wang T‐D, Goto S, Bhatt DL, et al. Ethnic differences in the relationships of anthropometric measures to metabolic risk factors in Asian patients at risk of atherothrombosis: results from the REduction of Atherothrombosis for Continued Health (REACH) Registry. Metab Clin Exp. 2010;59:400‐408. [DOI] [PubMed] [Google Scholar]
- 42. Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G. Mechanisms of obesity‐induced hypertension. Hypertens Res. 2010;33:386‐393. [DOI] [PubMed] [Google Scholar]
- 43. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity‐related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of The Obesity Society and the American Society of Hypertension. J Clin Hypertens. 2013;15:14‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tapolyai M, Faludi M, Dossabhoy NR, et al. Diuretics and bioimpedance‐measured fluid spaces in hypertensive patients. J Clin Hypertens. 2014;16:895‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moro C. Does insulin resistance trigger natriuretic peptide deficiency? EBioMedicine. 2017;17:11‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Magnusson M, Jujic A, Hedblad B, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab. 2012;97:638‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Haufe S, Kaminski J, Utz W, et al. Differential response of the natriuretic peptide system to weight loss and exercise in overweight or obese patients. J Hypertens. 2015;33:1458‐1464. [DOI] [PubMed] [Google Scholar]
- 48. Khan AM, Cheng S, Magnusson M, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community‐based studies. J Clin Endocrinol Metab. 2011;96:3242‐3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schlueter N, de Sterke A, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014;144:12‐27. [DOI] [PubMed] [Google Scholar]
- 50. Moro C. Targeting cardiac natriuretic peptides in the therapy of diabetes and obesity. Expert Opin Ther Targets. 2016;20:1445‐1452. [DOI] [PubMed] [Google Scholar]
- 51. Verboven K, Hansen D, Jocken JWE, Blaak EE. Natriuretic peptides in the control of lipid metabolism and insulin sensitivity. Obes Rev. 2017;18:1243‐1259. [DOI] [PubMed] [Google Scholar]
- 52. Jordan J, Stinkens R, Jax T, et al. Improved insulin sensitivity with angiotensin receptor neprilysin inhibition in individuals with obesity and hypertension. Clin Pharmacol Ther. 2017;101:254‐263. [DOI] [PubMed] [Google Scholar]
- 53. Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post‐hoc analysis from the PARADIGM‐HF trial. Lancet Diabetes Endocrinol. 2017;5:333‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chow SL, Maisel AS, Anand I, et al. Role of biomarkers for the prevention, assessment, and management of heart failure: a scientific statement from the American heart association. Circulation. 2017;135:e1054‐e1091. [DOI] [PubMed] [Google Scholar]
- 55. Masson S, Latini R, Anand IS, et al. Prognostic value of changes in N‐terminal pro‐brain natriuretic peptide in Val‐HeFT (Valsartan Heart Failure Trial). J Am Coll Cardiol. 2008;52:997‐1003. [DOI] [PubMed] [Google Scholar]
- 56. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. Circulation. 2017;136:e137‐e161. [DOI] [PubMed] [Google Scholar]
- 57. Vasquez N, Carter S, Grodin JL. Angiotensin receptor‐neprilysin inhibitors and the natriuretic peptide axis. Curr Heart Fail Rep. 2020;17:67‐76. [DOI] [PubMed] [Google Scholar]
- 58. Lippi G, Sanchis‐Gomar F. Monitoring B‐type natriuretic peptide in patients undergoing therapy with neprilysin inhibitors. An emerging challenge? Int J Cardiol. 2016;219:111‐114. [DOI] [PubMed] [Google Scholar]
- 59. Ibrahim NE, McCarthy CP, Shrestha S, et al. Effect of neprilysin inhibition on various natriuretic peptide assays. J Am Coll Cardiol. 2019;73:1273‐1284. [DOI] [PubMed] [Google Scholar]
- 60. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334‐1357. [DOI] [PubMed] [Google Scholar]
- 61. Umemura S, Arima H, Arima S, et al. The japanese society of hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 62. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 63. Wang J‐G, Yukisada K, Sibulo A, Hafeez K, Jia Y, Zhang J. Efficacy and safety of sacubitril/valsartan (LCZ696) add‐on to amlodipine in Asian patients with systolic hypertension uncontrolled with amlodipine monotherapy. J Hypertens. 2017;35:877‐885. [DOI] [PubMed] [Google Scholar]
- 64. Huo Y, Li W, Webb R, Zhao L, Wang Q, Guo W. Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: a randomized, double‐blind, 8‐week study. J Clin Hypertens. 2019;21:67‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Williams B, Cockcroft JR, Kario K, et al. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. 2017;69:411‐420. [DOI] [PubMed] [Google Scholar]
- 66. Cheung DG, Aizenberg D, Gorbunov V, Hafeez K, Chen C‐W, Zhang J. Efficacy and safety of sacubitril/valsartan in patients with essential hypertension uncontrolled by olmesartan: a randomized, double‐blind, 8‐week study. J Clin Hypertens. 2018;20:150‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]


