Abstract
The prevalence of erectile dysfunction (ED) is above 40% in both Asian and non‐Asian male populations after the age of 40 years. The prevalence of ED among hypertensive patients is approximately double than that in normotensive population. Pelvic arterial insufficiency is the predominant cause of ED in men aged over 50 years. Stenosis in any segment of the iliac–pudendal–penile arterial system, which is considered an erectile‐related arterial axis, could lead to ED. Pharmacotherapy with lifestyle modification is effective in alleviating sexual dysfunction, yet a substantial number of patients still develop ED. Given the established applicability of angioplasty for the entire iliac–pudendal–penile arterial system, penile duplex ultrasound, and pelvic computed tomography angiography could be considered as the routine screening tools in ED patients with poor response to phosphodiesterase‐5 inhibitors. Endovascular therapy for pelvic arterial insufficiency‐related ED has been shown to be a safe and effective treatment option in patients who have anatomically suitable vessels and functionally significant stenoses. Clinical improvement was achieved in over 60% of patients at one year following pelvic angioplasty in the PERFECT registry from Taiwan. A 30%‐40% restenosis rate in distal internal pudendal and penile arteries remains a hurdle. Angioplasty for pelvic arterial occlusive disease could be considered as a viable approach to arteriogenic ED.
Keywords: Asian patients, atherosclerosis, hypertension, sexual dysfunction, vascular disease
1. INTRODUCTION
Erectile dysfunction (ED) is defined as a consistent or recurrent inability to attain or sustain an erection that is required for successful vaginal intercourse. 1 ED is a common condition that affects the quality of life of a male patient with his partner. There are complex neurovascular mechanisms underlying normal erectile function. These include neurochemical stimulation and end‐organ responses (vasculature and penile body), required for erection initiation and maintenance. Herein, we reviewed literature on the relationship between ED and hypertension and further focused on the recent development of applying pelvic computed tomography angiography (CTA) and endovascular therapy for diagnosis and management of pelvic arterial insufficiency‐related ED.
2. EPIDEMIOLOGY AND ETIOLOGY
In general, ED is prevalent in more than 50% of men aged between 45 and 75 years. Currently, more than 150 million people globally have ED, and this number is predicted to increase to 322 million by the year 2025. 2 A survey of 1150 men aged 50‐80 years from five Asian countries (Hong Kong, Malaysia, Philippines, Singapore, and Thailand) showed the prevalence of ED being 63%. 3 Other studies also showed that the prevalence of ED was above 40% in Asian men aged over 40 years. 4 , 5 , 6
The first step of disease management is to identify the underlying etiology, including vasculogenic, endocrinologic, neurologic, drug‐related, psychologic, or mixed causes as well as end‐organ disease. Levels of testosterone and other related hormones (prolactin, follicular‐stimulating hormone, luteinizing hormone, estrogen, etc) and penile Doppler examination may aid in the differential diagnosis of ED. 7 Recurrent ulcers and/or gangrene at glans penis indicate the presence of severe pelvic arterial occlusive disease. 8 Yet the majority of patients with pelvic arterial insufficiency‐related ED do not develop recognizable changes in the morphology of penis.
Erectile dysfunction severity can be objectively measured using the International Index of Erectile Function 5 (IIEF‐5) questionnaire or complete IIEF. 9 The validated IIEF‐5 questionnaire evaluates self‐reported indicators of male sexual function, encompassing erectile strength, orgasm, desire, satisfaction with intercourse, and overall satisfaction. In clinical trial settings, a difference in the IIEF‐5 score of ≥ 4 is considered a minimal clinically important difference. 9
Vasculogenic causes of ED include endothelial dysfunction involving impaired smooth muscle relaxation, arterial insufficiency resulting from proximal atherosclerotic stenosis, or venous leak causing inability to trap blood in the corpus cavernosa. Arterial insufficiency is the predominant cause of ED in men over 50 years of age. Stenosis in any segment of the iliac–pudendal–penile arterial system, which is considered an erectile‐related arterial axis, could lead to ED. Previous angiographic studies, including a total of 629 patients (mostly Caucasians) with ED, revealed that approximately 75% of arterial stenoses were located in the common penile and internal pudendal arteries. 10 , 11 Since 2012, we developed the pelvic CTA reconstruction program. Based on this reconstruction program, we can diagnose pelvic arterial stenosis non‐invasively. 12 We divided the iliac–pudendal–penile arterial system into the following 8 segments in reconstructed pelvic CTA (Figure 1 and Table 1): zone 1, common iliac artery; zone 2, internal iliac artery; zone 3, anterior division of the internal iliac artery; zone 4A, proximal internal pudendal artery (from the origin of the internal pudendal artery to the 90‐degree turn close to the ischial spine at lateral projection); zone 4B, distal internal pudendal artery (from the 90‐degree turn to the origin of the perineal scrotal artery); zone 5A, common penile artery; zone 5B, dorsal penile artery and cavernosal artery; and zone 6, accessory pudendal artery (any artery anastomosed with the common penile artery or its branches). With this nomenclature system, the researchers and interventionists can communicate easily in this novel field for endovascular therapy. Among more than 1500 patients with ED screened by non‐invasive multi‐detector pelvic CTA in Taiwan, we found that an almost identical 75% of atherosclerotic stenoses were located in the common penile and internal pudendal arteries in patients with ED. 13 These findings demonstrate that the distributions of pelvic arterial stenotic lesions in patients with ED were similar between Asian and Caucasian populations.
Figure 1.
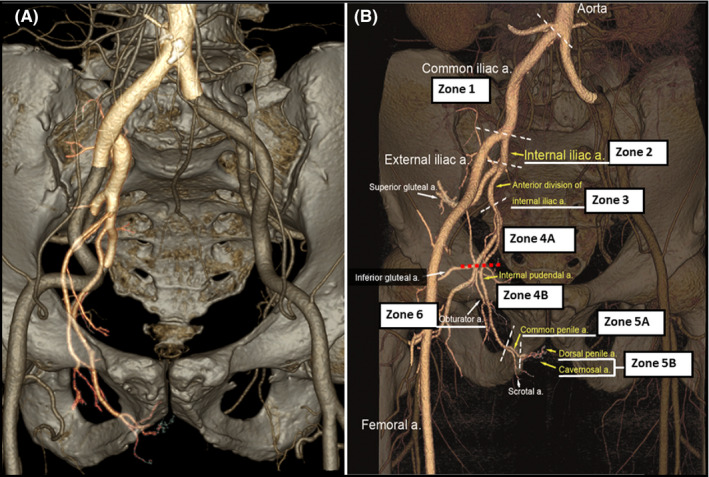
The 8‐zone segmentation scheme of the iliac–pudendal–penile arterial system shown in reconstructed pelvic computed tomography angiography
Table 1.
The 8‐zone segmentation scheme of the iliac–pudendal–penile arterial system
| Zone | Vessel diameter (mm) a |
|---|---|
| Zone 1: Common iliac artery | 9.2 ± 1.2 |
| Zone 2: Internal iliac artery | 6.8 ± 1.0 |
| Zone 3: Anterior division of internal iliac artery | 4.1 ± 1.1 |
| Zone 4A: Proximal internal pudendal artery | 2.7 ± 0.3 |
| Zone 4B: Distal internal pudendal artery | 2.3 ± 0.4 |
| Zone 5A: Common penile artery | 1.8 ± 0.3 |
| Zone 5B: Distal penile arteries | 1.3 ± 0.2 |
| Zone 6: Accessory pudendal artery | 2.0 ± 0.4 |
Values are mean ± SD.
Based on data from 284 patients with invasive pelvic angiography.
3. RISK FACTORS FOR ERECTILE DYSFUNCTION IN HYPERTENSIVE PATIENTS
Erectile dysfunction shares several risk factors with hypertension and cardiovascular diseases, such as aging, obesity, metabolic syndrome, chronic comorbid conditions, and tobacco use. 14 Bacon et al 15 stated that exercise was associated with a lower risk of ED, while obesity, smoking, prolonged hours of sitting (such as watching television, laptop, or mobile), and presence of chronic comorbidities were associated with a higher risk. Another study reported that weight loss and increased exercise improved erectile function in approximately one‐third of obese men with ED. 16
Erectile dysfunction has also been shown to be a harbinger for the development of coronary artery disease and adverse cardiovascular events. 17 ED generally precedes the manifestation of coronary artery disease by 3‐5 years. 18 Interventions including lifestyle modification and pharmacotherapy, which aim to reduce risk factors (high blood pressures, high low‐density lipoprotein cholesterol, high hemoglobin A1c, etc), are vital in the management of both ED and hypertension/cardiovascular health.
4. RELATIONSHIP BETWEEN ERECTILE DYSFUNCTION AND HYPERTENSION
The prevalence of ED among hypertensive patients is approximately double than that among the normotensive population in both Asian and non‐Asian populations. 4 , 19 Previous studies showed odds ratios in the range of 1.30‐2.79 for ED in hypertensive patients. 20 Nevertheless, ED remains underdiagnosed and undertreated in hypertensive patients. 21 For the first time ever, ED was included in the 2013 European Society of Hypertension/European Society of Cardiology Guidelines for the management of arterial hypertension 22 because hypertension can unmask the possibility of ED.
Sperling et al 23 revealed that hypertensive patients with controlled blood pressure had a lower prevalence of ED. In contrast, Doumas et al reported that ED prevalence in untreated hypertensive patients was lower than that in treated hypertensive patients. 24 Therefore, it is necessary to review which antihypertensive drugs are more suitable for patients with ED. Angiotensin II receptor blockers, angiotensin‐converting enzyme inhibitors, calcium antagonists, alpha‐adrenergic blockers, and vasodilating beta‐blockers have neutral or even beneficial effects on ED, whereas thiazide diuretics, non‐vasodilating beta‐blockers, and mineralocorticoid receptor antagonists are known to exacerbate ED. 22
A cross‐sectional and observational study conducted among 1007 hypertensive men who were treated with a beta‐blocker for at least 6 months showed an ED prevalence as high as 71%. 20 This effect was higher with first‐generation beta‐blockers such as propranolol or second‐generation beta‐blockers such as atenolol or metoprolol than with vasodilating third‐generation beta‐blockers. Nebivolol is a third‐generation beta‐blocker that produces peripheral vasodilation, blocking alpha‐adrenergic receptors, and inducing nitric oxide release. In an open‐label, prospective study, which included hypertensive men who were treated with first‐ and second‐generation beta‐blockers, 68% patients showed improvement in erectile function when their treatment was switched to nebivolol. 25 Furthermore, mineralocorticoid receptor antagonists, mainly spironolactone, frequently result in ED and decrease libido. The association between thiazide diuretics and ED has been widely described in studies.
A prospective study, which included 3500 hypertensive men, was performed to investigate the effect of valsartan on sexual function measured by the IIEF‐5. In previously untreated patients, the reported ED significantly reduced from 65% to 45% (p < .0001); whereas patients who were initially treated with other drugs but then switched to valsartan, the reported ED reduced from 75% to 53%. 26
5. ERECTILE DYSFUNCTION MANAGEMENT
5.1. Imaging studies
An intracavernosal injection of a vasodilator with Doppler ultrasound imaging is the most commonly used non‐invasive diagnostic tool for vasculogenic ED. 27 This tool can aid in distinguishing arterial insufficiency and venous leak from other causes of ED. 28 However, this tool does not provide information about the topography and pathology of the entire iliac–pudendal–penile arterial system proximal to the cavernosal artery. Further, Doppler ultrasound may cause psychological stress resulting from penile injection. Previous studies assessing the diagnostic accuracy of pelvic CTA have shown that CTA can clearly delineate the anatomic features of the internal pudendal artery but not the common penile artery, of which the diameter is often smaller than 2 mm. 29 , 30 In our previous study, we extended the diagnostic capability of multi‐detector CTA down to the level of the common penile artery and its major branches by combining it with a 64‐detector row computed tomography scanner, without intracavernosal injection of a vasodilator. This progress broadens the clinical application of pelvic CTA as a non‐invasive screening tool for arteriogenic ED.
Pelvic CTA can provide a clear roadmap to guide interventional therapy and the optimal projection angles for visualizing the lesions, which greatly reduces the time consumed and the amount of contrast material used during an interventional procedure. Another advantage of CTA is that it can detect calcification or thrombus in stenotic vascular lesions. It is worth mentioning that there was no calcification or thrombus in any penile arterial stenotic lesion in our study. 13 , 31 This finding suggests that the tissue characteristics of all these lesions were favorable for endovascular therapy.
According to our observation based on pelvic CTA in Asian population (Taiwan) and those based on invasive angiography mainly in Caucasian populations, 32 , 33 the prevalence of obstructive (diameter stenosis of ≥50%) lesions in the iliac–pudendal–penile arterial system was generally more than 70% in men with ED aged older than 50 years. With regard to obstructive lesions, 60% were located in the distal internal pudendal and common penile arteries within the pelvic floor, with no racial differences. The average diameter of artery in the iliac–pudendal–penile system varies from 10 mm, for the common iliac artery, to 1.0 mm, for the cavernosal artery (Table 1). 7 Owing to improvement in technology and understanding of pelvic anatomical characteristics, all these arteries are now accessible for endovascular therapy.
5.2. Lifestyle modification
Randomized clinical trials have shown that lifestyle modification provided clinical benefit for improving ED. 34 Smoking cessation, weight reduction and maintenance, regular physical exercise, moderation of alcohol consumption, and dietary changes are the common lifestyle modification measures to reduce hypertension, risk of ED, and risk of hypertension‐related cardiovascular complications. A Mediterranean diet is helpful for patients with ED and hypertension. 35 Moderation of alcohol and salt consumption should also be recommended. 35
5.3. Pharmacotherapy
The 2018 American Urological Association Erectile Dysfunction Guidelines recommend a shared decision‐making model when clinicians discuss treatment options with patients with ED. Most clinicians usually select phosphodiesterase‐5 (PDE5) inhibitors, as these drugs increase penile arterial blood flow and improve endothelial function. PDE5 inhibitors are known for their efficacy, ease of use, and favorable side‐effect profile. An important factor associated with successful PDE5 inhibitor therapy is the instruction and counseling on proper use, including the onset of action of the drug and taking medication on an empty stomach. However, the use of PDE5 inhibitors is contraindicated in men taking nitrates. PDE5 inhibitors should be used cautiously in hypertensive patients receiving an alpha‐adrenergic blocker due to an increased risk of hypotension.
According to previous studies, approximately 50% of ED patients showed a poor response to PDE5 inhibitors or had a contraindication to their use. Alternatively, they are instructed to use a vacuum constriction device, receive an intrapenile injection of prostaglandin, or undergo implantation of a penile prosthesis. 36 , 37 Endovascular therapy is still considered an option in the context of clinical studies, even though the clinical safety and efficacy data of endovascular therapy for arteriogenic ED is reassuring.
6. ENDOVASCULAR THERAPY FOR ARTERIAL INSUFFICIENCY‐RELATED ED
A novel minimally invasive approach targeting arterial insufficiency may provide a treatment option for patients with arteriogenic ED. The study conducted by us and other investigators 13 , 31 , 33 , 38 , 39 provides reassuring evidence about the safety and feasibility of endovascular revascularization for obstructive disease in these arteries (Table 2).
Table 2.
Endovascular treatment of erectile dysfunction
| Study | n | Arterial stenosis | Technique | Follow‐up period | Success rate |
|---|---|---|---|---|---|
| Castaneda‐Zunga 1982 | 2 | Internal iliac | PTA | 18 months | 2/2 (100%) |
| Van Unnik 1984 | 1 | External iliac | PTA | N/A | 1/1 (100%) |
| Goldwasser 1985 | 1 | Internal iliac | N/A | N/A | 1/1 (100%) |
| Dewar 1985 | 30 |
70% aorto‐iliac 47% internal iliac |
PTA | N/A | 10/33 (33%) |
| Angelini 1985 | 5 | Internal iliac | PTA | 2‐18 months | 4/5 (80%) |
| Valji 1988 | 3 | N/A | PTA | N/A | N/A |
| Urigo 1994 | 23 |
65% internal iliac 13% internal pudendal |
N/A | N/A |
15/23 (65%) 3/3 (100%) |
| Rogers 2011 | 30 | Internal pudendal | PTA and DESs | 3 months |
68.2% had improvement in IIEF‐5 score ≥ 4 |
| Wang 2014 | 20 |
59% Common penile 38% Dorsal penile 3% Cavernosal |
PTA | 6 months |
Clinical success a 12/20 (60%) |
| Wang 2016 | 22 |
73% Common penile 24% Dorsal penile 3% Cavernosal |
PTA | 12 months |
Clinical success a 11/22 (50%) |
| Wang 2018 | 182 |
19% internal iliac 47% internal pudendal 29% penile/cavernosal 5% accessory pudendal |
PTA/stents | 12 months |
Any improvement in IIEF‐5 score ≥ 4 within 12 months 134/182 (74%) Clinical success a 112/182 (62%) |
Abbreviations: IIEF, International Index on Erectile Function; N/A, not available; PTA, percutaneous transluminal balloon angioplasty.
Clinical success was defined as improvement in the IIEF‐5 score ≥ 4 or normalization of erectile function (IIEF‐5 ≥ 22).
The ZEN trial (Zotarolimus‐Eluting Peripheral Stent System for the Treatment of Erectile Dysfunction in Males with Suboptimal Response to PDE5 Inhibitors) evaluated the safety and efficacy of endovascular therapy in patients with drug‐refractory ED and internal pudendal artery disease. A total of 30 patients (out of 383 subjects screened) with ED were enrolled. 39 Procedural success was 100%, with no major adverse events (procedure‐related death, perineal hematoma, gangrene or necrosis, or the need for subsequent perineal, penile, or anal surgery) through 6‐month follow‐up. The improvement in IIEF‐5 score of ≥4 at 6 months, the primary endpoint, was achieved in 59.3% and 69.6% of patients by intention‐to‐treat and per‐protocol analyses, respectively. The peak systolic velocity of the cavernosal arteries as assessed by duplex ultrasound increased, from baseline, by 14.4 ± 10.7 cm/s at 30 days and 22.5 ± 23.7 cm/s at 6 months. Angiographic binary restenosis was reported in 11 (34%) of 32 lesions at 6 months. The relatively high binary restenosis rate indicates that unknown aspects are involved in the application of DES to the pelvic vasculature.
Given the anatomic variations and the degree of diffuse disease noted in the ZEN trial, our team initiated the pelvic revascularization for arteriogenic erectile dysfunction (PERFECT) program to explore and establish the role of endovascular therapy for pelvic arterial occlusive disease. In the PERFECT‐1 study, we used CTA to assess the safety and feasibility of balloon angioplasty for isolated penile artery stenoses (unilateral stenosis ≥ 70% or bilateral stenoses ≥ 50%) in patients with drug‐refractory ED. Twenty‐five patients were enrolled, and 20 patients (mean age 61 years; range 48‐79 years) underwent balloon angioplasty. Three patients had bilateral penile artery stenoses. Procedural success was achieved in all 23 penile arteries, with an average balloon size of 1.6 mm (range 1.00 to 2.25 mm). The average IIEF‐5 score improved from 10.0 ± 5.2 at baseline to 15.2 ± 6.7 (p < .001) at 1 month and 15.2 ± 6.3 (p < .001) at 6 months. Clinical success (change in IIEF‐5 score ≥ 4 or normalization of erectile function defined as IIEF‐5 ≥ 22) was achieved in 15 (75%), 13 (65%), and 12 (60%) patients at 1, 3, and 6 months, respectively. There were no major adverse events immediately after the procedure and through 6‐month follow‐up. Despite the lack of angiographic follow‐up or the use of a control group in this study, we could conclude that penile artery angioplasty is safe and can show clinically significant improvement in erectile function in 60% of patients with ED and isolated penile artery stenosis. 13
In the PERFECT‐2 study, 31 penile artery balloon angioplasty not only was proven safe but also achieved sustained improvement in erectile function in 50% of ED patients with isolated penile artery stenoses 12 months after angioplasty. More than a third of penile artery lesions (reference vessel diameter 1.0‐2.2 mm) exhibited restenosis on the 8‐month CTA. The development of restenosis was related to baseline lesion characteristics including lesion length, minimal lumen diameter, and reference vessel diameter. It is worthwhile mentioning that there were no adverse events or clinical worsening throughout the 12‐month follow‐up period.
In 2018, we reported results of all 182 patients with ED who underwent endovascular therapy and CTA follow‐up at 8 months in the PERFECT registry. 40 The mean age was 62.6 ± 7.9 years (range, 42‐83 years) with 334 obstructive segmental lesions (1.8 lesions/patient) and an average IIEF‐5 score of 9.1 ± 4.4. One hundred and twelve (34%) obstructive lesions were treated with stenting in addition to balloon angioplasty. At 8 months, the CT angiographic binary restenosis occurred in 102 lesions (102/334, 31%) and 76 patients (76/182, 42%). For lesions located in the proximal internal pudendal artery and above, binary restenosis rate was 3.5% (4/113), whereas for lesions located in the distal internal pudendal artery and beyond, binary restenosis rate was 44% (98/221). Sustained clinical success in erectile function was achieved in 62% of patients at 12 months, with an overall improvement in IIEF‐5 of 5.7 ± 4.7 (p < .001). Among patients not developing any binary restenosis, 82% (87/106) achieved sustained clinical success in erectile function, whereas for those with binary restenosis, 33% (25/76) achieved sustained improvement in erectile function. There were no adverse events except two cases with restenosis experiencing transient worsening of erectile function during follow‐up. The low restenosis rate (<4%) for lesions located in the proximal internal pudendal and iliac arteries and the above 80% sustained clinical success rate in patients not developing restenosis are encouraging. However, for lesions located in the distal internal pudendal and penile arteries, the around 40% restenosis rate remains a hurdle. Further refinement of intervention strategies, like direct stenting and use of intravascular imaging, has been evaluated to solve this unmet need.
7. CONCLUSIONS
Erectile dysfunction is highly prevalent in patients with hypertension and leads to reduced quality of life, with no racial differences. This condition is associated with several modifiable risk factors including hypertension, obesity, lack of exercise, metabolic syndrome, chronic comorbid conditions, and cigarette smoking. ED may be the initial sign of endothelial dysfunction and subclinical arterial stenosis. Pharmacotherapy with lifestyle modification is shown to be effective in improving and/or restoring sexual function in men, yet a substantial number of patients remain symptomatic ED. Given the established applicability of angioplasty for the entire iliac–pudendal–penile arterial system, penile duplex ultrasound, and pelvic CTA could be considered as the routine screening tools in ED patients with poor response to PDE5 inhibitors. Anatomically suitable vessels and functionally significant stenoses for interventions can be confirmed by penile duplex ultrasound assessment and pelvic CTA or invasive angiographic study. Endovascular therapy for arterial insufficiency‐related ED has been shown to be a safe and effective treatment option in selected patients who have been evaluated by a multidisciplinary team for all potential causes of ED.
CONFLICT OF INTEREST
TD Wang has received honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Medtronic, Menarini, Novartis, Omron, Pfizer, Sanofi, and Servier. YC Chia has received honorarium and sponsorship at attending conferences and seminars from Boeringher‐Ingelheim, Pfizer, Omron, Servier, and Xepa‐Sol and an investigator‐initiated research grant from Pfizer. Peera Buranakitjaroen have no conflict of interest, except a research grant from Pfizer (Thailand) Limited, as mentioned in the paper. CH Chen reports personal fees from Novartis, Sanofi, Daiichi Sankyo, SERVIER, Bayer, and Boehringer Ingelheim Pharmaceuticals, Inc HM Cheng received speakers honorarium and sponsorship to attend conferences and CME seminars from Eli Lilly and AstraZeneca; Pfizer Inc; Bayer AG; Boehringer Ingelheim Pharmaceuticals, Inc; Daiichi Sankyo, Novartis Pharmaceuticals, Inc; SERVIER; Co., Pharmaceuticals Corporation; Sanofi; TAKEDA Pharmaceuticals International; Menarini Co., Ltd.; and served as an advisor or consultant for ApoDx Technology, Inc JG Wang reports having received research grants from Chendu Di‐Ao and Omron, and lecture and consulting fees from AstraZeneca, Novartis, Omron, Servier, and Takeda. K Kario reports research grants from Omron Healthcare, Fukuda Denshi, A&D, Pfizer Japan, and honoraria from Omron Healthcare. All other authors report no potential conflicts of interest in relation to this article.
AUTHOR CONTRIBUTIONS
Tzung‐Dau Wang and Chih‐Kuo Lee: Substantial contributions to the conception and design of the work; acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Yook‐Chin Chia, Kelvin Tsoi, Peera Buranakitjaroen, Chen‐Huan Chen, Hao‐Min Cheng, Jam Chin Tay, Boon Wee Teo, Yuda Turana, Guru Prasad Sogunuru, and Ji‐Guang Wang: Drafting the work and revising it critically for important intellectual content. Kazuomi Kario: contributions to the conception or design of the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published.
Wang T‐D, Lee C‐K, Chia Y‐C, et al; the HOPE Asia Network . Hypertension and erectile dysfunction: The role of endovascular therapy in Asia. J Clin Hypertens. 2021;23:481–488. 10.1111/jch.14123
Tzung‐Dau Wang and Chih‐Kuo Lee shared the first author.
REFERENCES
- 1. Hernández‐Cerda J, Bertomeu‐González V, Zuazola P, Cordero A. Understanding erectile dysfunction in hypertensive patients: the need for good patient management. Vasc Health Risk Manag. 2020;16:231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 3. Li MK, Garcia LA, Rosen R. Lower urinary tract symptoms and male sexual dysfunction in Asia: a survey of ageing men from five Asian countries. BJU Int. 2005;96(9):1339‐1354. [DOI] [PubMed] [Google Scholar]
- 4. Kang SY, Lee JA, Sunwoo S, et al. Prevalence of sexual dysfunction and associated risk factors in middle‐aged and elderly Korean men in primary care. J Sex Res. 2016;53(9):1165‐1178. [DOI] [PubMed] [Google Scholar]
- 5. Buranakitjaroen P, Phoojaroenchanachai M, Saravich S. Prevalence of erectile dysfunction among treated hypertensive males. J Med Assoc Thai. 2006;89(Suppl 5):S28‐S36. [PubMed] [Google Scholar]
- 6. Tan JK, Hong CY, Png DJ, Liew LC, Wong ML. Erectile dysfunction in Singapore: prevalence and its associated factors–a population‐based study. Singapore Med J. 2003;44(1):20‐26. [PubMed] [Google Scholar]
- 7. Vlachopoulos C, Jackson G, Stefanadis C, Montorsi P. Erectile dysfunction in the cardiovascular patient. Eur Heart J. 2013;34(27):2034‐2046. [DOI] [PubMed] [Google Scholar]
- 8. Vijayan P. Gangrene of the penis in a diabetic male with multiple amputations and follow up. Indian J Urol. 2009;25(1):123‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosen RC, Allen KR, Ni X, Araujo AB. Minimal clinically important differences in the erectile function domain of the International Index of Erectile Function scale. Eur Urol. 2011;60(5):1010‐1016. [DOI] [PubMed] [Google Scholar]
- 10. Gray RR, Keresteci AG, St Louis EL, et al. Investigation of impotence by internal pudendal angiography: experience with 73 cases. Radiology. 1982;144(4):773‐780. [DOI] [PubMed] [Google Scholar]
- 11. Schwartz AN, Freidenberg D, Harley JD. Nonselective angiography after intracorporal papaverine injection: an alternative technique for evaluating penile arterial integrity. Radiology. 1988;167(1):249‐253. [DOI] [PubMed] [Google Scholar]
- 12. Wang TD, Lee WJ, Chen WJ, Chen MF. TCT‐72 prevalence and distribution of obstructive pelvic arterial lesions by computed tomographic angiography in 476 patients with erectile dysfunction: implications for endovascular therapy. J Am Coll Cardiol. 2015;66(15 Supplement):B32. [Google Scholar]
- 13. Wang TD, Lee WJ, Yang SC, et al. Safety and six‐month durability of angioplasty for isolated penile artery stenoses in patients with erectile dysfunction: a first‐in‐man study. EuroIntervention. 2014;10(1):147‐156. [DOI] [PubMed] [Google Scholar]
- 14. Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294(23):2996‐3002. [DOI] [PubMed] [Google Scholar]
- 15. Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow‐up study. Ann Intern Med. 2003;139(3):161‐168. [DOI] [PubMed] [Google Scholar]
- 16. Esposito K, Giugliano F, Di Palo C, et al. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978‐2984. [DOI] [PubMed] [Google Scholar]
- 17. Nadir Karakulak U, Okutucu S, Lokman U, et al. Evaluation of erectile dysfunction and left ventricular diastolic parameters in lead exposed workers. Acta Cardiol Sin. 2019;35(1):75‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manolis A, Doumas M. Antihypertensive treatment and sexual dysfunction. Curr Hypertens Rep. 2012;14(4):285‐292. [DOI] [PubMed] [Google Scholar]
- 19. Naya Y, Mizutani Y, Ochiai A, et al. Preliminary report of association of chronic diseases and erectile dysfunction in middle‐aged men in Japan. Urology. 2003;62(3):532‐536. [DOI] [PubMed] [Google Scholar]
- 20. Cordero A, Bertomeu‐Martínez V, Mazón P, et al. Erectile dysfunction in high‐risk hypertensive patients treated with beta‐blockade agents. Cardiovasc Ther. 2010;28(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 21. Viigimaa M, Vlachopoulos C, Lazaridis A, Doumas M. Management of erectile dysfunction in hypertension: tips and tricks. World J Cardiol. 2014;6(9):908‐915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159‐2219. [DOI] [PubMed] [Google Scholar]
- 23. Sperling J, Nilsson PM. Does early life programming influence arterial stiffness and central hemodynamics in adulthood? J Hypertens. 2020;38(3):481‐488. [DOI] [PubMed] [Google Scholar]
- 24. Nilsson PM, Lurbe E, Laurent S. The early life origins of vascular ageing and cardiovascular risk: the EVA syndrome. J Hypertens. 2008;26(6):1049‐1057. [DOI] [PubMed] [Google Scholar]
- 25. Doumas M, Tsakiris A, Douma S, et al. Beneficial effects of switching from beta‐blockers to nebivolol on the erectile function of hypertensive patients. Asian J Androl. 2006;8(2):177‐182. [DOI] [PubMed] [Google Scholar]
- 26. Düsing R. Effect of the angiotensin II antagonist valsartan on sexual function in hypertensive men. Blood Press. 2003;12(Sup2):29‐34. [DOI] [PubMed] [Google Scholar]
- 27. Meller SM, Stilp E, Walker CN, Mena‐Hurtado C. The link between vasculogenic erectile dysfunction, coronary artery disease, and peripheral artery disease: role of metabolic factors and endovascular therapy. J Invasive Cardiol. 2013;25(6):313‐319. [PubMed] [Google Scholar]
- 28. Sikka SC, Hellstrom WJ, Brock G, Morales AM. Standardization of vascular assessment of erectile dysfunction: standard operating procedures for duplex ultrasound. J Sex Med. 2013;10(1):120‐129. [DOI] [PubMed] [Google Scholar]
- 29. Philip F, Shishehbor M, Desai M, Schoenhagen P, Ellis S, Kapadia S. Characterization of internal pudendal artery atherosclerosis using aortography and multi‐detector computed angiography. Catheter Cardiovasc Interv. 2013;82(4):E516‐E521. [DOI] [PubMed] [Google Scholar]
- 30. Kawanishi Y, Lee KS, Kimura K, Kojima K, Yamamoto A, Numata A. Feasibility of multi‐slice computed tomography in the diagnosis of arteriogenic erectile dysfunction. BJU Int. 2001;88(4):390‐395. [DOI] [PubMed] [Google Scholar]
- 31. Wang TD, Lee WJ, Yang SC, et al. Clinical and imaging outcomes up to 1 year following balloon angioplasty for isolated penile artery stenoses in patients with erectile dysfunction: the PERFECT‐2 study. J Endovasc Ther. 2016;23(6):867‐877. [DOI] [PubMed] [Google Scholar]
- 32. Shishehbor MH, Philip F. Endovascular treatment for erectile dysfunction. J Am Coll Cardiol. 2012;60(25):2628. [DOI] [PubMed] [Google Scholar]
- 33. von Allmen RS, Nguyen DP, Birkhäuser FD, et al. Lesion pattern in patients with erectile dysfunction of suspected arterial origin: an angiographic study. J Endovasc Ther. 2016;23(1):76‐82. [DOI] [PubMed] [Google Scholar]
- 34. Gupta BP, Murad MH, Clifton MM, Prokop L, Nehra A, Kopecky SL. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta‐analysis. Arch Intern Med. 2011;171(20):1797‐1803. [DOI] [PubMed] [Google Scholar]
- 35. Estruch R, Ros E, Salas‐Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279‐1290. [DOI] [PubMed] [Google Scholar]
- 36. Wespes E, Amar E, Hatzichristou D, Montorsi F, Pryor J, Vardi Y. Guidelines on erectile dysfunction. Eur Urol. 2002;41(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 37. Montague DK, Jarow JP, Broderick GA, et al. Chapter 1: the management of erectile dysfunction: an aua update. J Urol. 2005;174(1):230‐239. [DOI] [PubMed] [Google Scholar]
- 38. Diehm N, Do DD, Keo HH, et al. Early recoil after balloon angioplasty of erection‐related arteries in patients with arteriogenic erectile dysfunction. J Endovasc Ther. 2018;25(6):710‐715. [DOI] [PubMed] [Google Scholar]
- 39. Rogers JH, Goldstein I, Kandzari DE, et al. Zotarolimus‐eluting peripheral stents for the treatment of erectile dysfunction in subjects with suboptimal response to phosphodiesterase‐5 inhibitors. J Am Coll Cardiol. 2012;60(25):2618‐2627. [DOI] [PubMed] [Google Scholar]
- 40. Wang TD. Commentary: angioplasty of internal pudendal and penile arteries for arteriogenic erectile dysfunction: reassuring, but the jury is still out. J Endovasc Ther. 2018;25(6):716‐718. [DOI] [PubMed] [Google Scholar]


