Abstract
Objective
Examine the effect of off-label surfactant on mortality and morbidity in more mature and larger premature infants diagnosed with respiratory distress syndrome (RDS).
Study design
Cohort study of premature infants born at 30–36 weeks, birth weight > 2 kg, and a diagnosis of RDS. We compared the odds of mortality and morbidity between infants who were exposed vs unexposed to surfactant. We used a treatment effects model to balance covariates between groups.
Results
Of 54,964 included infants, 25,278 (46%) were exposed to surfactant. The frequency of mortality and morbidities were higher in the exposed group in unadjusted analyses. Following adjustment with a doubly robust treatment effects model, we found no significant treatment effect of surfactant on mortality or morbidity.
Conclusion
Surfactant exposure is not associated with reduced or increased mortality or morbidity in more mature premature infants with RDS.
Respiratory distress syndrome (RDS) due to surfactant deficiency is a common cause of mortality and long-term morbidity in premature infants. The incidence of RDS is more common in infants born <29 weeks gestational age (GA) compared with late preterm infants [1, 2]. Complications of RDS and the subsequent use of mechanical ventilation include air leak syndrome (i.e., pneumothorax and pulmonary interstitial emphysema), bronchopulmonary dysplasia (BPD), and mortality [3, 4].
Three animal derived surfactant preparations are approved by the FDA for the prevention or treatment of RDS: beractant, calfactant, and poractant alfa [5–7]. Following the widespread introduction of exogenous surfactant and antenatal steroids in the 1990s, RDS-related mortality and air leak syndrome were reduced in extremely premature infants [1, 8]. Current FDA labeling for these products includes specific GA and birth weight (BW) ranges based on inclusion criteria from randomized clinical trials of surfactants [3, 9–13]. All surfactant use in infants with GA 30–36 weeks and BW > 2 kg is considered off-label. Infants in this GA and BW range have a lower incidence of RDS-related mortality and morbidity compared with less mature and smaller infants [1, 2].
A recent study using data from the Pediatrix Medical Group found that the majority of surfactant given to premature infants is administered off-label due to its common use in more mature and larger infants [14]. While the use of surfactant to prevent and treat RDS in these larger, more mature infants is often routine, there is a paucity of data on the relative safety and effectiveness of surfactant in this population. The objective of this study was to examine the association between surfactant administration and mortality and morbidity in premature infants with GA 30–36 and BW > 2 kg. We hypothesized that surfactant use in this population is not associated with decreased mortality, air leak syndrome, pulmonary hemorrhage, or BPD.
Materials/subjects and methods
We obtained data from the Pediatrix Medical Group Clinical Data Warehouse, which prospectively captures information entered into an electronic health record system by clinicians at 392 US NICUs within the Pediatrix Medical Group between 1997 and 2016 [15]. We identified infants discharged from 2006 to 2016 with a diagnosis of RDS, based on clinical judgment by the treating neonatologist and documented in the clinical note, in the first 3 postnatal days, GA 30–36 weeks, and BW > 2 kg. We included infants who received ≥1 dose of beractant, calfactant, or poractant alfa in the first 3 postnatal days as the off-label exposure group and infants who were unexposed to surfactant during the hospitalization as the comparison group. Infants transferred to units outside of the Pediatrix Medical Group were excluded from the analysis. We excluded infants with meconium aspiration syndrome or congenital diaphragmatic hernia, infants who received more than one type of surfactant or a surfactant other than beractant, calfactant, or poractant alfa, and those who received the first dose of surfactant after the third postnatal day (Fig. 1). The study was approved by the Duke University Institutional Review Board as exempt research.
Fig. 1.
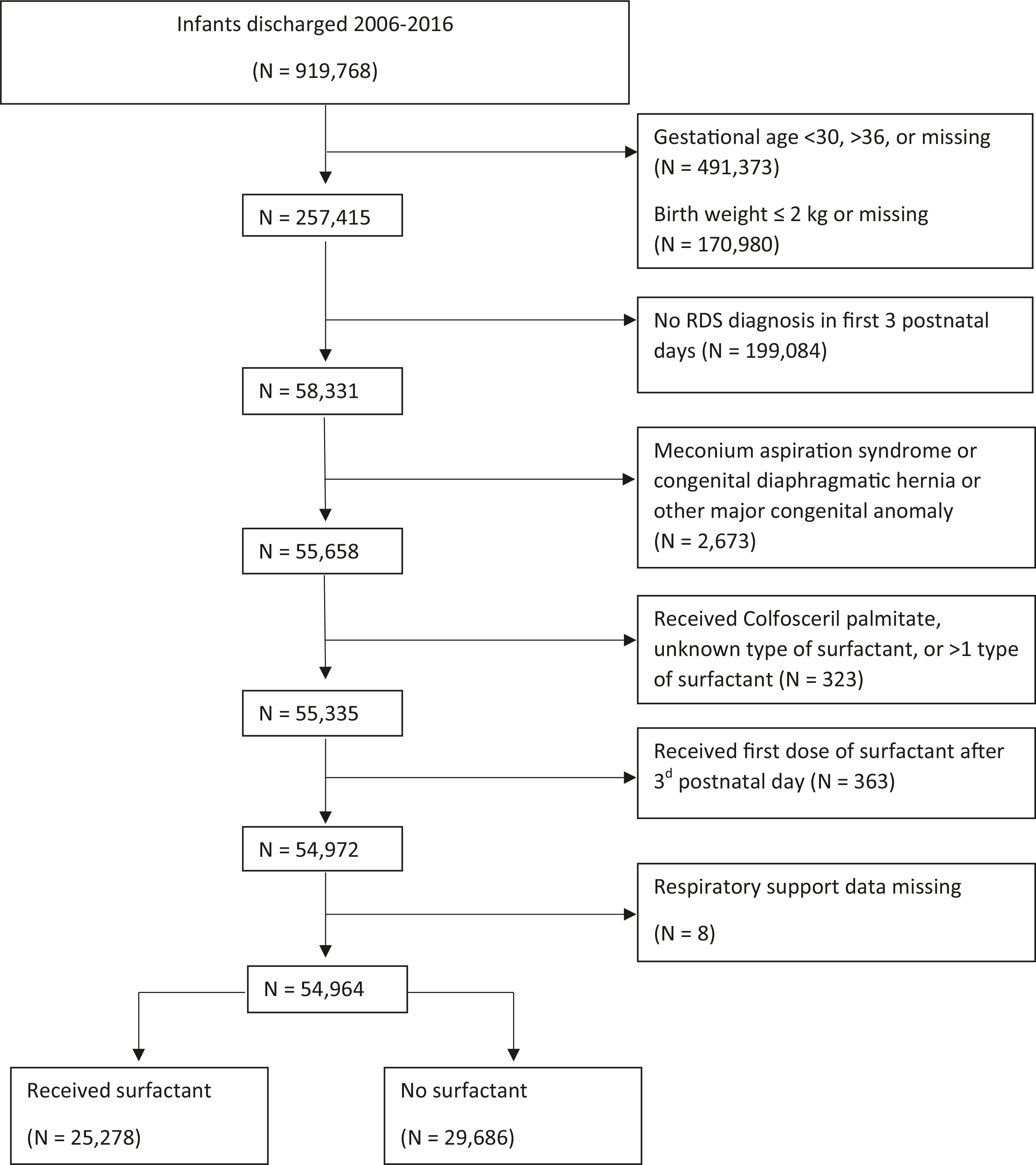
Study flow diagram of the study population, from initial cohort through exclusions.
We reported demographic and clinical variables for infants exposed and unexposed to surfactant and the frequency of all-cause mortality before discharge, air leak syndrome (pneumothorax or pulmonary interstitial emphysema) within the first 3 postnatal days, pulmonary hemorrhage within the first 3 postnatal days, and BPD. Air leak syndrome and pulmonary hemorrhage were identified based on abstraction from clinical notes. We defined BPD in infants <32 weeks GA as receipt of supplemental oxygen or respiratory support (nasal cannula, continuous positive airway pressure (CPAP), or mechanical ventilation) continuously from a corrected GA of 360/7–366/7 weeks. In infants ≥32 weeks GA, BPD was defined by the receipt of supplemental oxygen or respiratory support continuously from a postnatal age of 28–34 days [16]. We recorded the maximum fraction of inspired oxygen (FiO2) based on clinical notes in the first 3 postnatal days, as well as the highest level of respiratory support in the first 3 postnatal days from the following ascending levels: room air (no support), noninvasive support (including hood oxygen, low-flow and high-flow nasal cannula, nasal CPAP, and noninvasive positive pressure ventilation), conventional mechanical ventilation, and high-frequency mechanical ventilation.
Statistical analyses
We compared the frequency of mortality and morbidities between infants exposed and unexposed to surfactant using the chi square test for categorical variables or the Wilcoxon rank-sum test for continuous variables. We used multivariable logistic regression to compare mortality, air leak, pulmonary hemorrhage, and BPD in infants exposed and unexposed to surfactant. We presented the results as odds ratios (ORs) with 95% confidence intervals following model adjustment for the following a priori covariates: GA group (30–33 weeks/34–36 weeks), BW group (2.001–2.499 kg/ 2.500–3.499 kg/ ≥ 3.500 kg), sex (male/female), maternal race (white/black/Hispanic/other), antenatal steroids (yes/ no), cesarean section (yes/no), Apgar score at 5 min (0–10), inborn status (yes/no), prolonged rupture of membranes (yes/no), discharge year (2006–2016), and highest respiratory support level (room air/noninvasive support/conventional mechanical ventilation/high-frequency mechanical ventilation) and FiO2 (0.21–1.0) in the first 3 postnatal days.
Because key factors, such as respiratory support level and FiO2, differed substantially between treated and untreated patients, we preferentially used a treatment effects model to compare outcomes in infants exposed and unexposed to surfactant. The approach, inverse-probability-weighted regression adjustment (IPWRA), weights subjects by the inverse of the conditional probability of treatment exposure as a function of model covariates, rather than weighting subjects equally (as in conventional regression analysis). This approach yields estimated coefficients based on a sample in which the treatment group has clinical features similar to the untreated group, and thus isolates the effect of treatment on the outcome of interest. The resulting average treatment effect among treated subjects (ATET) is reported as a percentage, with a negative value indicating a decreased likelihood of the outcome.
We used the same covariates for the outcome models as were used in the logistic regression models. The mortality and BPD models also included early-onset sepsis (in the first 3 postnatal days) and late-onset sepsis (after the third postnatal day). The covariates for the treatment model included GA and BW group, inborn status, and highest respiratory support and FiO2 in the first 3 postnatal days. We included interaction terms for inborn status and all other covariates. All analyses were performed using Stata (version 15.1 StataCorp, College Station, Texas). p values of <0.05 were considered significant.
Results
A total of 54,964 infants were included in the study, of whom 25,278 infants (46%) received surfactant. Infants exposed to surfactant were more likely to receive mechanical ventilation and have higher maximum FiO2 in the first 3 postnatal days compared with infants with RDS who did not receive surfactant (Table 1). Of the three surfactant types in this sample, poractant alfa was most commonly used (48%). In unadjusted analyses, infants exposed to a surfactant had higher frequency of mortality, air leak syndrome, pulmonary hemorrhage, and BPD when compared with infants not exposed to surfactant (p <0.001; Table 2). In the multivariable logistic regression models, the odds of mortality (OR 0.6; 95% CI 0.4–1.0; p = 0.035) were lower in the surfactant group. While odds for air leak syndrome (OR 1.1; 95% CI 1.1.-1.3; p = 0.002) remained higher in the surfactant group, pulmonary hemorrhage and BPD were similar between the exposed and unexposed groups (Table 3).
Table 1.
Characteristics of infants with RDS, GA 30–36 weeks, and BW> 2 kg by surfactant exposure, 2006–2016.
| Characteristic | Surfactant (N = 25,278) | No surfactant (N = 29,686) |
|---|---|---|
| Discharge year, % | ||
| 2006–2010 | 48 | 41 |
| 2011–2016 | 52 | 59 |
| GA (weeks), % | ||
| 30–33 | 24 | 23 |
| 34–36 | 76 | 77 |
| BW in kilograms, median (IQR) | 2.463 (2.220–2.775) | 2.421 (2.200–2.719) |
| Large for GA, % | 17 | 15 |
| Inborn, % | 81 | 87 |
| Cesarean section, % | 66 | 63 |
| Maternal race/ethnicity, % | ||
| White | 69 | 61 |
| Black | 9 | 13 |
| Hispanic | 19 | 22 |
| Other | 3 | 4 |
| Male sex, % | 66 | 62 |
| Antenatal steroids, % | 30 | 33 |
| Highest level of respiratory support on postnatal day 0–2, % | ||
| Room air, no support | <1 | 5 |
| Noninvasive supporta | 48 | 86 |
| Conventional MV | 49 | 9 |
| High-frequency MV | 3 | 1 |
| Max FiO2 on postnatal day 0–2, median (IQR) | 35 (30–50) | 30 (21–35) |
| Type of surfactant received, % | ||
| Beractant | 30 | N/A |
| Calfactant | 22 | |
| Poractant alfa | 48 | |
GA gestational age, BW birth weight, IQR interquartile range, FiO2 fraction of inspired oxygen.
Nasal CPAP, noninvasive positive pressure ventilation, high-flow nasal cannula.
Table 2.
Frequency of outcomes in infants with RDS, GA 30–36 weeks, and BW > 2 kg by surfactant exposure.
| Outcome | Surfactant (N = 25,278) | No surfactant (N = 29,686) | p value |
|---|---|---|---|
| Mortality, % | 0.4 | 0.1 | <0.001 |
| Air leaka, % | 8.9 | 4.8 | <0.001 |
| Pulmonary hemorrhage, % | 0.3 | 0.1 | <0.001 |
| Any mechanical ventilation, % | 52 | 9 | <0.001 |
| Total ventilator days, median (5–95th percentile) | 1 (0–6) | 0 (0–2) | <0.001 |
| BPD, % | 1.2 | 0.5 | <0.001 |
BPD bronchopulmonary dysplasia.
Air leak includes pneumothorax or pulmonary interstitial emphysema.
Table 3.
Logistic regression and treatment effects models for outcomes in infants with RDS, GA 30–36 weeks, and BW > 2 kg exposed to surfactant.
| Unadjusted OR (95% CI) | Adjusteda OR (95% CI) | Treatment effects |
|||
|---|---|---|---|---|---|
| ATETb 95% CI | p value | Implied OR | |||
| Mortality | 3.7 (2.5–5.6) | 0.6 (0.4–1.0) | −0.2% −0.5 to 0.04% | 0.11 | 0.7 |
| Air leak | 1.9 (1.8–2.1) | 1.1 (1.1–1.3) | −0.2% −1.0 to 0.7% | 0.73 | 1.0 |
| Pulmonary hemorrhage | 3.6 (2.3–5.5) | 0.7 (0.4–1.2) | −0.2% −0.5 to 0.007% | 0.06 | 0.6 |
| BPD | 2.7 (2.1–3.3) | 1.2 (0.9–1.6) | 0.06%−0.3 to 0.4% | 0.77 | 1.1 |
Adjusted for gestational age group, birth weight group, sex, maternal race, antenatal steroids, mode of delivery, Apgar at 5 min, inborn, prolonged rupture of membranes, discharge year, and respiratory support and FiO2 in first 3 postnatal days. The death and BPD models also adjust for early-onset and late-onset sepsis.
ATET average treatment effects among treated infants.
Bold values indicate statistical significant p <0.05.
In our treatment effects model, the ATET was small and not statistically significant for any of the outcomes. The ORs implied by the treatment effects are reported to permit comparison with the results of the logistic regression models (Table 3). The raw and inverse-probability weighted standardized differences and variance ratios for each treatment covariate in the model for mortality are shown in Table 4. Although the weighted variance ratio for early-onset sepsis is slightly elevated, the overall pattern is one of well-balanced covariates. The weighted standardized differences and variance ratios are substantively identical to the results in the models for all other outcomes reported in Table 3.
Table 4.
Standardized differences and variance ratios for covariates in the treatment effects model of mortality.
| Standardized differences |
Variance ratios |
|||
|---|---|---|---|---|
| Raw | Weighted | Raw | Weighted | |
| Gestational age groupa | −0.032 | 0.015 | 1.04 | 0.98 |
| Birth weight groupb | 0.086 | −0.006 | 1.04 | 1.00 |
| Male | 0.091 | 0.063 | 0.95 | 0.96 |
| Maternal race (white race is reference) | ||||
| Black | −0.103 | 0.002 | 0.77 | 1.00 |
| Hispanic | −0.103 | 0.008 | 0.86 | 1.01 |
| Other | −0.058 | −0.007 | 0.75 | 0.96 |
| Antenatal steroids | −0.076 | −0.022 | 0.95 | 0.98 |
| Cesarean section | 0.057 | 0.026 | 0.97 | 0.98 |
| Apgar score at 5 min | −0.193 | 0.01 | 2.22 | 1.00 |
| Inborn | −0.210 | −0.009 | 1.50 | 1.01 |
| Prolonged rupture of membranes | −0.071 | −0.001 | 0.80 | 1.00 |
| Discharge year | −0.206 | −0.024 | 0.99 | 0.96 |
| Highest respiratory support on PND 0–2 | 1.07 | −0.020 | 2.08 | 1.00 |
| Highest FiO2 on PND 0–2 | 0.498 | −0.023 | 1.76 | 1.04 |
| Early sepsis | 0.023 | 0.029 | 1.45 | 1.62 |
| Late sepsis | 0.021 | 0.004 | 1.48 | 1.06 |
A standardized difference close to 0 and a variance ratio close to 1 indicate the covariates are well balanced between treatment groups.
PND postnatal day.
Gestational age groups: 30–33 weeks/34–36 weeks.
Birth weight groups: 2.001–2.400 kg/2.500–3.499 kg/≥3.500 kg.
Discussion
In our cohort, surfactant exposure was not associated with a statistically significant difference in mortality, air leak syndrome, pulmonary hemorrhage, or BPD. While infants receiving surfactant had higher unadjusted frequencies of mortality and morbidity, the results of our doubly robust treatment effects model consistently demonstrated no significant differences in the risks of mortality or morbidity between groups. The multivariable logistic regression model demonstrated decreased odds of mortality in infants exposed to surfactant compared with unexposed infants after adjusting for covariates reflecting respiratory disease severity. However, the treatment effects model produces more reliable (i.e., less biased) results than the multivariable logistic regression model to address our study objective because it better isolates the effect of surfactant from the influence of measured covariates on outcomes. These results suggest that surfactant in more mature and larger premature infants with RDS may not decrease mortality or air leak syndrome.
Exogenous surfactant is commonly used to treat RDS by reducing the elevated surface tension at the air liquid interface of alveoli. This reduction in surface tension decreases atelectasis, promotes lung expansion, and improves gas exchange, resulting in significant improvement in mortality and air leak syndrome [5–7]. The use of surfactant to treat and prevent RDS remains an active area of research, with efforts to characterize the comparative benefits of natural vs synthetic surfactant [17], early vs delayed selective use [18], single dose vs multiple doses [19], nebulized/aerosolized delivery systems [20–22], and late (>3 days postnatal age) surfactant therapy [23, 24]. There are limited data on the safety and effectiveness of surfactant in more mature, larger premature infants, who comprise the majority of surfactant use [14]. Several cohort studies in late preterm infants have demonstrated a reduction in supplemental FiO2 and improvement in partial pressure of oxygen (PaO2) following surfactant administration, but inconsistent evidence of reduced mortality or air leak syndrome [25–27].
One explanation for our findings is that clinical care in critically ill neonates has dramatically improved since randomized, clinical trials of surfactant were conducted in the 1980s and 1990s. In addition to exogenous surfactant, advances in the management of neonatal RDS include the increased use of antenatal steroids, avoidance of ventilator-induced lung injury through preferential noninvasive respiratory support, improvements in synchronized assisted ventilation, and a focus on optimum fluid management and thermoregulation [28–31]. Infants in our sample were born in an era in which these practices have become the standard of care in managing premature infants. This has contributed to reduced RDS mortality in all BW categories and current mortality is low in premature infants with BW > 1500 g [32]. It is possible that the treatment effect of surfactant is less appreciable in contemporary neonatal practice where the incidence of mortality and morbidity in our study population is low.
Another possible explanation for our findings is that surfactant administration is less effective in more mature and larger premature infants with RDS. This may occur because the dose of surfactant is not the same for more mature infants due to additional airway and alveolar development, which could reduce efficacy and explain the difference in safety signals compared with smaller infants [33, 34]. Another possibility for the apparent lack of effect on morbidity and mortality is that neonatologists may not be using surfactant in an effort to mitigate these uncommon complications when administering surfactant to infants with higher GA. Instead, clinicians may be using surfactant to reduce exposure to high FiO2 and mechanical ventilation, even in infants who are at very low risk of the outcomes reported in our study.
As with any medication, there are potential risks associated with surfactant administration. Exogenous surfactants are generally viewed as safe and well tolerated; however, transient adverse events associated with beractant, calfactant, or poractant alfa administration in premature infants include bradycardia, hypotension, hypertension, endotracheal tube blockage, oxygen desaturation, apnea, hypocarbia, and hypercarbia [5–7]. Of the three surfactant products, only the calfactant label lists the frequency of common adverse reactions associated with dosing: cyanosis (65%), airway obstruction (39%), bradycardia (34%), reflux of surfactant into endotracheal tube (21%), requirement for manual ventilation (16%), and reintubation (3%) [6]. This information is based on infants enrolled in the surfactant trials. The incidence and severity of these adverse events in more mature and larger premature infants are not known.
The strengths of our study include the use of prospectively collected data on a large sample of infants to address a clinical question with limited available data. Our analysis plan isolated the treatment effects of surfactants while accounting for greater severity of illness in infants exposed to surfactants. Limitations to our study include the use of clinical diagnoses abstracted from the electronic medical record, which may reduce diagnosis reliability. The indication for surfactant use in our cohort is unclear based on the available information. Furthermore, while our approach using IPWRA reduces the risk of bias in the estimated treatment effects, it is possible there were unmeasured confounders related to either the treatment or the outcome for which we did not account in our analysis. In conclusion, we found that infants with GA 30–36 weeks, BW > 2 kg, and RDS exposed to beractant, calfactant, or poractant alfa in the first 3 postnatal days had outcomes similar to those of infants not exposed to surfactant. Because the majority of surfactant use is administered off-label in this more mature and larger population, prospective studies may be useful in further informing the safety and efficacy of surfactant in this population.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibbard JU, Wilkins I, Sun L, Gregory K, Hoffman M, et al. Respiratory morbidity in late preterm births. JAMA. 2010;304:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polin RA, Carlo WA. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133:156–63. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AH. The new bronchopulmonary dysplasia. Curr Opin Pediatr. 2011;23:167–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AbbVie, Inc. Survanta [package label] AbbVie Inc, North Chicago, IL; 2013. [Google Scholar]
- 6.ONY, Inc. Infasurf [package label] ONY, Inc, Amherst, NY; 2011. [Google Scholar]
- 7.Chiesi Farmaceutici. Curosurf [package label] Chiesi Farmaceutici, Parma, Italy; 2014. [Google Scholar]
- 8.Koivisto M, Marttila R, Kurkinen-Raty M, Saarela T, Pokela ML, Jouppila P, et al. Changing incidence and outcome of infants with respiratory distress syndrome in the 1990s: a population-based survey. Acta Paediatr. 2004;93:177–84. [DOI] [PubMed] [Google Scholar]
- 9.Bhat R, Dziedzic K, Bhutani VK, Vidyasagar D. Effect of single dose surfactant on pulmonary function. Crit Care Med. 1990;18: 590–5. [DOI] [PubMed] [Google Scholar]
- 10.Couser RJ, Ferrara TB, Ebert J, Hoekstra RE, Fangman JJ. Effects of exogenous surfactant therapy on dynamic compliance during mechanical breathing in preterm infants with hyaline membrane disease. J Pediatr. 1990;116:119–24. [DOI] [PubMed] [Google Scholar]
- 11.Gitlin JD, Soll RF, Parad RB, Horbar JD, Feldman HA, Lucey JF, et al. Randomized controlled trial of exogenous surfactant for the treatment of hyaline membrane disease. Pediatrics. 1987;79:31–7. [PubMed] [Google Scholar]
- 12.Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane database Syst Rev. 2000;2:Cd000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suresh GK, Soll RF. Overview of surfactant replacement trials. J Perinatol. 2005;25 Suppl 2:S40–4. [DOI] [PubMed] [Google Scholar]
- 14.Taylor G, Jackson W, Hornik CP, Koss A, Mantena S, Homsley K, et al. Surfactant administration in preterm infants: drug development opportunities. J Pediatr. 2019;208:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spitzer AR, Ellsbury DL, Handler D, Clark RH. The Pediatrix BabySteps Data Warehouse and the Pediatrix QualitySteps improvement project system–tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70. [DOI] [PubMed] [Google Scholar]
- 16.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soll RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2001;2:Cd000144. [DOI] [PubMed] [Google Scholar]
- 18.Yost CC, Soll RF. Early versus delayed selective surfactant treatment for neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2000;2:Cd001456. [DOI] [PubMed] [Google Scholar]
- 19.Soll R, Ozek E. Multiple versus single doses of exogenous surfactant for the prevention or treatment of neonatal respiratory distress syndrome. Cochrane Database Syst Rev. 2009;1: Cd000141. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Latif ME, Osborn DA. Nebulised surfactant in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev. 2012;10:Cd008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minocchieri S, Berry CA, Pillow JJ. Nebulised surfactant to reduce severity of respiratory distress: a blinded, parallel, randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2019; 104:F313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sood BG, Cortez J, Kolli M, Sharma A, Delaney-Black V, Chen X. Aerosolized surfactant in neonatal respiratory distress syndrome: phase I study. Early Hum Dev. 2019;134:19–25. [DOI] [PubMed] [Google Scholar]
- 23.Ballard RA, Keller RL, Black DM, Ballard PL, Merrill JD, Eichenwald EC, et al. Randomized trial of late surfactant treatment in ventilated preterm infants receiving inhaled nitric oxide. J Pediatr. 2016;168:23–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laughon M, Bose C, Moya F, Aschner J, Donn SM, Morabito C, et al. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics. 2009;123:89–96. [DOI] [PubMed] [Google Scholar]
- 25.Dani C, Mosca F, Vento G, Tagliabue P, Picone S, Lista G, et al. Effects of surfactant treatment in late preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med. 2018;31:1259–66. [DOI] [PubMed] [Google Scholar]
- 26.Surmeli-Onay O, Korkmaz A, Yigit S, Yurdakok M. Surfactant therapy in late preterm infants: respiratory distress syndrome and beyond. Turk J Pediatr. 2012;54:239–46. [PubMed] [Google Scholar]
- 27.Wang H, Gao X, Liu C, Yan C, Lin X, Dong Y, et al. Surfactant reduced the mortality of neonates with birth weight 1500g and hypoxemic respiratory failure: a survey from an emerging NICU network. J Perinatol. 2017;37:645–51. [DOI] [PubMed] [Google Scholar]
- 28.Saccone G, Berghella V. Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Guo L, Chi C, Wang X, Guo L, Wang W, et al. Mechanical ventilation modes for respiratory distress syndrome in infants: a systematic review and network meta-analysis. Crit Care. 2015;19:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 Update. Neonatology. 2017; 111:107–25. [DOI] [PubMed] [Google Scholar]
- 31.Schmolzer GM, Kumar M, Pichler G, Aziz K, O'Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamath BD, Macguire ER, McClure EM, Goldenberg RL, Jobe AH. Neonatal mortality from respiratory distress syndrome: lessons for low-resource countries. Pediatrics. 2011;127: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Konishi M, Fujiwara T, Naito T, Takeuchi Y, Ogawa Y, Inukai K, et al. Surfactant replacement therapy in neonatal respiratory distress syndrome. A multi-centre, randomized clinical trial: comparison of high-versus low-dose of surfactant TA. Eur J Pediatr. 1988;147:20–5. [DOI] [PubMed] [Google Scholar]
- 34.Davis DJ, Barrington KJ, Canadian Pediatric Society, Fetus and Newborn Committee. Recommendations for neonatal surfactant therapy. Paediatr Child Health. 2005;10:109–16. [PMC free article] [PubMed] [Google Scholar]


