Abstract
Background:
Stigmatizing attitudes towards people who use drugs (PWUD) impact their access and retention in health care. Current measures of PWUD stigma in medical settings are limited. Therefore, we developed and validated the Medical Provider Stigma Experienced by PWUD (MPS-PWUD) scale.
Methods:
As part of an ongoing clinical trial, we recruited HCV RNA positive people who inject drugs in New York City. Based on 164 participants, principal component analysis (PCA) was conducted on fifteen stigma items answered on a 5-point Likert scale. We evaluated internal consistency using Cronbach’s alpha coefficient and assessed construct validity by comparing stigma levels with willingness to communicate health concerns with medical providers and likelihood to seek HCV treatment.
Results:
PCA identified a 9-item scale with two components of stigmatization that explained 60.8% of the total variance and overall high internal consistency (alpha=0.90). The enacted stigma (alpha=0.90) consisted of 6 scale items related to the medical providers’ stigmatizing actions or perceptions. The internalized stigma component (alpha=0.84) included 3 scale items related to PWUD’s shame or drug use disclosure. As hypothesized, higher levels of either stigma were associated with less likelihood to openly communicate with medical providers (p <0.005). Participants with a higher level of enacted stigma were less likely to seek HCV treatment (p=0.011).
Conclusions:
The validated MPS-PWUD scale could help healthcare providers, harm reduction services and researchers measure stigma experienced by PWUD in medical settings in efforts to minimize the impact of stigma on limiting access to and retention of care for PWUD.
Keywords: Stigma in medical settings, people who use drugs, stigma scale
1. Introduction
Drug use is heavily stigmatized. This stigma is mainly due to prejudice and negative perceptions and attitudes towards people who use drugs (PWUD) that are associated with moral views on substance use (Clark, 2011; Denning, 2000; Levine, 1978; Musto, 2002; NIDA, 2007). Within these views, PWUD are perceived to be morally flawed individuals, responsible, and only to be blamed for their drug related-problems and experiences (Gowan, 2010; NIDA, 2007). Drug use and its negative consequences are perceived to be a reflection of bad choices made by PWUD (Clark, 2011; Denning, 2000; Gowan, 2010; Levine, 1978). Additionally, drug-related stigma is driven and facilitated by various factors including, cultural norms, prejudice, lack of awareness, and fear of social (safety) and economic ramifications (Stangl et al., 2019).
Among the general population, there is greater stigmatization of drug addiction in comparison to other conditions, such as mental illness. Although the public may recognize PWUD’s difficult lived experiences, this population tends to be viewed as more blameworthy for their drug use and dangerous because of it (Corrigan et al., 2009; Ormston et al., 2010). To gain deeper insight into general stigma associated with drug use, Palamar et al. (2011) developed and validated scales to evaluate perceived stigma towards PWUD and general stigmatization related to illicit drug use (marijuana, powder cocaine, ecstasy, and the nonmedical use of amphetamines and opioids) among a general, nonclinical sample of 1,000 PWUD. After assessing two stigma factors, the negative characteristics associated with PWUD (e.g. morally wrong, weak-minded, dishonest) and the perceived stigma of the general population towards PWUD, results indicated that individuals who were less exposed to PWUD reported greater perceived stigma and general stigmatization towards this population.
PWUD also experience stigmatization from the healthcare sector. For example, some people who inject drugs (PWID) have reported that they felt ignored when offering suggestions or input related to their health status and condition (Clements et al., 2015). In order to become more informed about drug use stigma from the perspective of PWUD, Smith et al. (2016) tested the reliability and validity of the Substance Use Stigma Mechanisms Scale (SU-SMS) which was informed by the Stigma Framework. This framework identifies measurable stigma mechanisms which are a reflection of an individual’s distinct psychological responses to their own experiences surrounding drug use, including enacted stigma, anticipated stigma, and internalized stigma (Earnshaw and Chaudoir, 2009). Both enacted and anticipated stigma involve experiences of discrimination, stereotyping, and/or prejudice from others in the past or present. Enacted refers to these experiences in the past or present while anticipated refers to PWUD’s expectation of these experiences in the future. Internalized stigma refers to endorsing negative feelings and beliefs and applying them to the self (Earnshaw et al., 2013). Findings validated the use of the SU-SMS in identifying enacted, anticipated, and internalized stigma as distinct, measurable stigma experiences, and established family and health care providers as two distinct sources of drug use stigma.
Another study also found that among 32 self-identified PWID in New York City (NYC), the majority of participants (78.1%) reported at least one instance of stigma in a prior healthcare experience, 23 participants (71.9%) reported enacted healthcare stigma and 19 participants (59.4%) reported experiencing anticipated healthcare stigma (Muncan et al., 2020).
Drug use stigma from medical providers can have an impact on testing and treatment for PWUD. Stigma from healthcare professionals may result in less willingness to treat this population, due to the perception that PWUD bare the sole responsibility of their drug use and should be able to control it (i.e. stop using drugs if they decide to do so), Therefore, they are uniquely responsible for health conditions associated with their drug use. (Brickman et al., 1982; Corrigan, 2000; Corrigan et al., 2003; Strauser et al., 2009; Weiner et al., 1988). Compared to patients with diabetes and depression, healthcare professionals reported having negative attitudes towards patients with substance use disorder (SUD) and were found to have a lower regard for working with patients with SUD (Gilchrist et al., 2011). Nurses also reported experiencing little motivation and low satisfaction in caring for SUD patients, further perceiving them as emotionally challenging and potentially unsafe (Ford, 2011). As summarized by Goodyear et al. (2020), health care providers have been reluctant to provide Hepatitis C treatment to PWID due to concerns such as questioning PWID’s capacity for adherence to medication regimens (Grebely et al., 2017; Krook et al., 2007), risk of HCV re-infection (Asher et al., 2016; Grebely et al., 2017; Lazarus et al., 2017), a presumed lack of motivation to engage in HCV treatment (Litwin et al., 2019; Treloar et al., 2010), and history of substance use (Boerekamps et al., 2018; Litwin et al., 2019).
In turn, this stigma may also impact how PWUD respond to and engage in medical treatment. Specific studies have demonstrated SUD patients were less likely to complete their treatment when they reported discrimination by health professionals (Brener et al., 2010). One study found PWID were discouraged from participating in various substance use treatments, including methadone maintenance treatment and recovery support services (i.e. sober living and Narcotics Anonymous) due to the general public stigma that equates methadone treatment with illicit drug use (Paquette et al., 2018).
Drug use stigma in medical settings has received less research attention in comparison to other stigmatized mental and physical illnesses, such as schizophrenia and HIV (Goffman, 2009). However, it deserves more attention, as it may lead to negative health outcomes that are both related and unrelated to an individual’s drug use. In this paper, we present the Medical Provider Stigma Experienced by PWUD (MPS-PWUD) scale that directly inquires about drug use stigma and its impact on interactions with medical providers. Although Palamar et al. (2011) and Smith et al. (2016) focused exclusively on the PWUD perspective when validating their stigma scales, they did not include how PWUD experience drug use stigma nor how it impacts their interactions with medical providers. Our aim is to create and validate a drug use stigma scale that explicitly focuses on the PWUD perspective and how stigma affects their experience in health care settings. We do so by creating questions that specifically inquire about how interactions with medical providers may be affected by drug use. We believe this scale may benefit healthcare-related prevention strategies aimed at serving PWUD, by informing staff of existing stigmatizing behaviors and their impact within healthcare settings.
2. Methods
2.1. Study population and design
Baseline data of 167 participants were collected in NYC from July 2017 to March 2020 for Accessible Care, a randomized-controlled study of HCV treatment-related outcomes. Participants were recruited at physical venues, such as needle exchange programs in NYC and via peer referrals and online media advertisements. Three participants were excluded because they did not respond to any of the stigma items, resulting in 164 participants being included in the analysis.
Eligible participants were 18 years or older, were HCV RNA positive, had not received HCV treatment in the previous 6 months, had injected illicit drugs in the 90-day period prior to enrollment, and provided written, informed consent. Following eligibility screening, participants were randomly assigned to receive either low-threshold care co-located at a needle exchange program or usual care (linkage to HCV care providers with experience serving PWID). All participants completed an interviewer-assisted baseline questionnaire that took approximately 60–90 minutes to complete and inquired about socio-demographics, mental and physical health history, illicit drug use, and treatment history, among others. Additionally, the questionnaire included stigma-related questions and participants’ self-efficacy to openly discuss medical concerns with providers.
Through principal component analysis (PCA), one or more underlying stigma components were identified, as well as the components’ shared variance in participants’ responses to items related to drug stigma. We evaluated statistical significance at the 5% level. SPSS® version 25 was used to conduct all the data analyses including descriptive statistics and bivariate analyses (IBM Corp, 2017).
2.2. Content validity
Fifteen items in the questionnaire assessed participants’ experiences and perceptions of stigma about their drug use while interacting with medical providers. The 15 items were developed from investigators’ subject matter expertise, experience working with PWUD, and knowledge of general quantitative and qualitative literature on stigma. These items were adapted from various scales focused on health-related stigma and interaction with medical providers, including one related to obesity stigma (Luborsky et al., 1996; Wadden et al., 2000). The questions covered areas such as medical providers stigmatizing PWUD and behavior related to drug use. They also included PWUD efforts to either not disclose drug use to medical providers or actively hide their drug use.
2.3. Variable Definition
In creating the stigma scale, we inquired about the nature of participants’ interactions with their medical providers which include doctors, nurses, and other healthcare workers. In the questionnaire, “regular” for drug use and drug injection was defined as using drugs three or more times per week for at least one month. We also inquired about participants’ perceptions of how they were treated by medical providers due to their drug use. The 15 stigma questionnaire items were rated on a 5-point scale (1=strongly disagree, 2=disagree, 3=neither agree nor disagree, 4=agree, and 5=strongly agree). Additionally, the questionnaire included three measures of self-efficacy in interacting with medical providers. These measures asked how confident participants were to ask their medical provider about their illness concerns, to discuss openly with their medical provider any personal problems that may be related to their illness, and to work out difficulties with their medical provider when they arise. These three measures were originally rated on a scale from 0 (cannot do at all) to 10 (certain to do). To secure enough N per category and facilitate conceptual understanding, we collapsed the scores into 4 groups: 0 to 2 (not confident at all), 3 to 5 (somewhat confident), 6 to 8 (confident), and 9 to 10 (very confident).
2.4. Construct validity
PCA was used to identify underlying constructs from the 15 stigma-related items. Using an orthogonal rotation assumes uncorrelated constructs and may distort findings (Matsunaga, 2010). Therefore, a promax rotation was used to interpret the components as shown by a moderately high correlation (0.659) between the 2 components in the final solution. Eigenvalues (components > 1.0) and component loadings (items > 0.600 on one component and < 0.300 on any other components) informed the selection of the final solution.
We conducted additional assessments for construct validity under the hypotheses that higher stigma levels would be correlated with lower levels of patient self-efficacy to discuss concerns with their medical provider and a lesser likelihood of seeking HCV treatment. Because of the ordinal level and the skewedness of the self-efficacy measures, as well as the stigma scale, the association of the three self-efficacy measures with the stigma scale was assessed using the Spearman’s rho correlation coefficient. For similar reasons, the nonparametric Mann-Whitney U test was used to compare the mean values of the stigma scale between those who sought HCV treatment and those who did not.
2.5. Reliability
We evaluated the internal consistency of the items extracted from the PCA using the Cronbach’s alpha coefficient. Since we were interested in the stigma level of the participants before they were engaged in the Accessible Care study activities, we focused on the baseline data for this analysis. Because the analysis was only performed on the cross-sectional baseline data, test-retest reliability was not assessed.
3. Results
3.1. Sample description
Table 1 presents participants’ sociodemographic characteristics, drug use, drug treatment, and HCV testing and treatment experiences. The sample included 79% male, 59% Hispanic, 32% non-Hispanic white, and 5% non-Hispanic black, with a mean age of 42.3 years. The majority (68%) of the sample had an annual income of less than or equal to $10,000, 92% had experienced lifetime homelessness, and 5% were employed (full- or part-time) at the time the study took place. Furthermore, 95% of participants had been arrested at least once in their lifetime and 16% had been arrested in the past 90 days.
Table 1.
Sample Characteristics (N=164)
| Sociodemographics | ||||
|
mean (s.d.) |
||||
| Age (years) | 42.3 (10.5) | |||
|
n (%) |
||||
| Gender | Male | 130 (79.3) | ||
| Female | 33 (20.1) | |||
| Race / Ethnicity | Hispanic | 96 (58.5) | ||
| Non-Hispanic White | 52 (31.7) | |||
| Non-Hispanic Black | 8 (4.9) | |||
| Other | 8 (4.9) | |||
| Estimated annual household income | <=$10,000 | 107 (68.2) | ||
| $10,001 to $25,000 | 30 (19.1) | |||
| > $25,000 | 20 (12.7) | |||
| Current employment status | Full time on the book | 6 (3.7) | ||
| Part time on the book | 2 (1.2) | |||
| Odd job, off the book, or Seasonal | 17 (10.4) | |||
| Disabled for work | 17 (10.4) | |||
| Unemployed | 121 (73.8) | |||
| Homelessness | Ever Homeless | 150 (91.5) | ||
| Homeless in the past 90 days | 92 (56.1) | |||
| Arrested | Ever Arrested | 156 (95.1) | ||
| Arrested in the past 90 days | 25 (15.2) | |||
| HCV Testing and Care | ||||
| Ever previously tested for HCV? | 163 (99.4) | |||
| HCV+ result the last time tested? (out of 163 tested) | 156 (95.7) | |||
| Ever sought HCV care (out of 156 tested HCV+)? | 42 (26.9) | |||
| Hard drug use in the past 90 days | ||||
| Used at least once n (%) | Regular * Used n (%) | Injected at least once n (%) | Regular * Injected n (%) | |
| Any drugs | 164 (100.0) | 132 (80.5) | 164 (100.0) | 125 (76.2) |
| Heroin | 116 (70.7) | 79 (48.2) | 114 (69.5) | 78 (47.6) |
| Cocaine | 74 (45.1) | 45 (27.4) | 66 (40.2) | 44 (26.8) |
| Speedball | 57 (34.8) | 40 (24.4) | 55 (33.5) | 40 (24.4) |
| Crack | 36 (22.0) | 9(5.5) | 6 (3.7) | 3(1.8) |
| Methamphetamine | 10 (6.1) | 4 (2.4) | 8 (4.9) | 4 (2.4) |
we define regular as 3 or more times a week for at least one month
All participants had injected drugs in the past 90 days. The majority of participants (81%) reported engaging in regular drug use in the past 90 days and 76% reported regular drug injection. The three hard drugs participants reported using the most were heroin (71%), cocaine (45%), and speedballs (35%). All but one participant had been previously tested for HCV prior to the study and 156 (96%) participants had tested HCV positive. Out of these 156 participants, only 42 (27%) ever sought HCV treatment.
3.2. Item analysis
Table 2 presents the descriptive statistics for the 15 drug use stigma items. Across all 15 items, missing data are less than 3% which is small enough to be inconsequential (Schafer, 1999). The majority of participants expressed that they either agree or strongly agree with 11 of the 15 items, with 81% as the highest percentage of agreement; this reflects an overall high level of experienced stigma related to drug use. In contrast, most participants (62%) expressed that they disagree or strongly disagree with feeling that their medical provider is afraid of them. About half of them (51%) expressed disagreement regarding not feeling welcome at the medical provider’s office. For item 15, “I feel respected by my doctor/medical provider”, the wording of the statement indicated reversed direction between agreement and stigma level. Less than half of the sample (44%) agreed or strongly agreed with the statement, indicating that only a minority of participants felt respected by their medical provider.
Table 2.
Frequencies for 15 stigma questionnaire items and factor loadings and final decisions from Principal Components Analysis (PCA)
| Number (percent) of responses to item | PCA loading by component* | Resulting Component | ||||||
|---|---|---|---|---|---|---|---|---|
| Strongly Disagree | Disagree | Neither Agree nor Disagree | Agree | Strongly Agree | Enacted Stigma | Internalized stigma | ||
| 1 Doctors/medical providers have said critical or insulting things to me about my drug use | 8 (4.9) | 61 (37.4) | 9(5.5) | 49 (30.1) | 36 (22.1) | 0.941 * | −0.153 | Enacted |
| 2 I have been very upset by comments that doctors/medical providers have made about my drug use | 7 (4.3) | 61 (37.2) | 12 (7.3) | 41 (25.0) | 42 (25.6) | 0.976 * | −0.110 | Enacted |
| 3 I feel that I have been treated disrespectfully by the medical profession because of my drug use | 10 (6.1) | 62 (37.8) | 8 (4.9) | 37 (22.6) | 47 (28.7) | 0.887 * | −0.013 | Enacted |
| 4 Doctors/medical providers have tried to scare me into quitting drugs by warning me about health risks associated with being a drug user | 8 (4.9) | 39 (23.8) | 8 (4.9) | 59 (36.0) | 50 (30.5) | 0.49. | 0.243 | — |
| 5 I feel that I cannot speak freely with doctors/medical providers about my drug use | 17 (10.4) | 51 (31.3) | 10 (6.1) | 33 (20.2) | 52 (31.9) | 0.511 | 0.243 | — |
| 6 I feel that doctors/medical providers don’t treat drug users as nicely as they do non drug users | 10 (6.1) | 33 (20.1) | 7 (4.3) | 36 (22.0) | 78 (47.6) | 0.539 | 0.241 | — |
| 7 Doctors/medical providers have told me I need to quit using drugs without my ask ing them | 4(2.5) | 25 (15.3) | 11 (6.7) | 58 (35.6) | 65 (39.9) | 0.390 | 0.436 | — |
| 8 If I relapsed, doctors/medical providers criticized me for not trying harder | 11 (6.8) | 57 (35.2) | 20 (12.3) | 30 (18.5) | 44 (27.2) | 0.766 * | 0.127 | Enacted |
| 9 I feel that most doctors/medical providers don’t understand how difficult it is to be a drug user | 7 (4.4) | 20 (12.5) | 4(2.5) | 46 (28.8) | 83 (51.9) | 0.291 | 0.525 | — |
| 10 When I go for medical care, I feel as though I try to hide my drug use | 6 (3.7) | 38 (23.3) | 7 (4.3) | 42 (25.8) | 70 (42.9) | −0.144 | 0.941* | Internalized |
| 11 When I go for medical care, I feel that I am embarrassed or ashamed about being a drug user | 7 (4.3) | 35 (21.5) | 12 (7.4) | 48 (29.4) | 61 (37.4) | −0.132 | 0.933 * | Internalized |
| 12 I do not want to disclose my drug use to a doctor/medical provider | 8 (4.9) | 33 (20.1) | 16 (9.8) | 40 (24.4) | 67 (40.9) | −0.079 | 0.823 * | Internalized |
| 13 I feel my doctor/medical provider is afraid of me | 31 (19.1) | 69 (42.6) | 26 (16.0) | 15 (9.1) | 21 (13.0) | 0.651* | −0.040 | Enacted |
| 14 I do not feel welcome at the doctor’s/medical provider’s office | 17 (10.4) | 66 (40.5) | 25 (15.3) | 23 (14.1) | 32 (19.6) | 0.734* | 0.001 | Enacted |
| 15 I feel respected by my doctor/medical provider | 20 (12.4) | 23 (14.3) | 49 (29.2) | 55 (34.2) | 16 (9.9) | −0.346 | 0.059 | — |
Items were retained within the component under which the loading is bolded and with asterisk; items with unbolded loadings (4–7 and 9, 15) were omitted from final scales
3.3. Construct validity
PCA results from the 15 drug use-related stigma items yielded a 2-component solution, with each component having an eigenvalue greater than 1. The 2-component solution retained 9 of the 15 items, which accounted for 60.8% of the total variance. Based on the description of the retained items, the two components that were identified from the overall scale were labeled as “enacted” and “internalized” stigma. Similar to the Stigma Framework, “enacted stigma” describes prejudicial experiences stemming from medical providers’ treatment of participants due to their drug use, while “internalized stigma” describes respondents’ embarrassment about their drug use, efforts to hide it, and inclination not to disclose drug use to medical providers (Earnshaw and Chaudoir 2009; Earnshaw et al., 2013). Table 2 shows loadings for the enacted and internalized components. The enacted component was comprised of 6 items (1–3, 8, 13–14) with loadings ranging from 0.651 to 0.976. These 6 items accounted for 52.6% of the variance and had an eigenvalue of 7.89. The internalized component was comprised of 3 items (10–12) with loadings ranging from 0.823 to 0.941. These 3 items accounted for 8.2% of the variance and had an eigenvalue of 1.2. Six items (4–7, 9, 15) were not included in the overall stigma scale based on their low loadings for both components.
The overall scale and the enacted and internalized stigma subscale scores were calculated by averaging the retained drug use stigma items. The scores for the stigma scale and subscales had a range of 4, with a minimum of 1 and a maximum of 5. The overall scale of the 9 items had a mean value of 3.32 with a standard deviation of 0.99. The enacted stigma subscale had a lower mean value (mean=3.10, std=1.08) than that of internalized stigma subscale (mean=3.78, std=1.13).
Table 3 presents the response distribution of the three self-efficacy items related to interactions with medical providers. These items address participant confidence in asking about illness concerns, openly discussing medical problems related to their illness, and working out difficulties when they arise. The items were scored ranging from 0 to 10, which were collapsed into 4 groups. The Spearman’s rho correlation coefficient with the overall stigma scale and its 2 components are also displayed in Table 3, as well as the average score of the scales for each group of the self-efficacy items. All 3 measures of patient self-efficacy were significantly inversely correlated with the overall stigma scale, and with both the enacted and internalized stigma subscales. As hypothesized, the results suggest that higher stigma experienced by PWID is associated with lower self-efficacy in interacting with medical providers (i.e. asking about illness concerns, openly discussing medical problems related to illness, and working out difficulties when they arise).
Table 3:
| On a scale of 0–10 based on how confident you are... | Overall Scale | Enacted Stigma | Internalized Stigma | |||||
| Mean (SD) =3.32 (0.99) | Mean (SD) = 3.10 (1.08) | Mean (SD) = 3.78 (1.13) | ||||||
| Ask your doctor things about your illness that concerns you? | Mean (SD) | rs | p-value | rs | p-value | rs | p-value | |
| 7.9 (2.7) | −0.256 | 0.001 | −0.218 | 0.005 | −0.255 | 0.001 | ||
| Rating | n (%) | Average score (SD) | Average score (SD) | Average score (SD) | ||||
| Not confident at all: | 0 to 2 | 12 (7.4) | 3.93 (0.81) | 3.66 (0.97) | 4.47 (0.64) | |||
| Somewhat confident: | 3 to 5 | 22 (13.6) | 3.52 (1.02) | 3.33 (1.16) | 3.89 (1.16) | |||
| Confident: | 6 to 8 | 37 (22.8) | 3.52 (0.88) | 3.24 (1.04) | 4.10 (0.83) | |||
| Very confident: | 9 to 10 | 91 (56.2) | 3.11 (1.01) | 2.92 (1.08) | 3.50 (1.20) | |||
| Discuss openly with your doctor any personal problems that may be related to your illness? | Mean (SD) | rs | p-value | rs | p-value | rs | p-value | |
| 7.7 (2.9) | −0.267 | 0.001 | −0.217 | 0.005 | −0.285 | < 0.001 | ||
| Rating | n (%) | Average score (SD) | Average score (SD) | Average score (SD) | ||||
| Not confident at all: | 0 to 2 | 15 (9.2) | 3.92 (0.73) | 3.68 (0.86) | 4.38 (0.64) | |||
| Somewhat confident: | 3 to 5 | 22 (13.5) | 3.54 (1.03) | 3.30 (1.12) | 4.02 (1.18) | |||
| Confident: | 6 to 8 | 39 (23.9) | 3.49 (0.92) | 3.19 (1.13) | 4.07 (0.91) | |||
| Very confident: | 9 to 10 | 87 (3.4) | 3.10 (1.00) | 2.91 (1.04) | 3.47 (1.19) | |||
| Work out difficulties with our doctor when they arise? | Mean (SD) | rs | p-value | rs | p-value | rs | p-value | |
| 7.7 (2.8) | −0.270 0.001 | −0.233 0.003 | −0.251 0.001 | |||||
| Rating | n (%) | Average score (SD) | Average score (SD) | Average score (SD) | ||||
| Not confident at all: | 0 to 2 | 15 (9.3) | 3.81 (0.85) | 3.57 (0.97) | 4.27 (0.78) | |||
| Somewhat confident | 3 to 5 | 21 (13.0) | 3.75 (0.97) | 3.62 (1.02) | 4.02 (1.21) | |||
| Confident: | 6 to 8 | 43 (26.5) | 3.39 (0.99) | 3.08 (1.14) | 4.00 (1.01) | |||
| Very confident: | 9 to 10 | 83 (51.2) | 3.11 (0.97) | 2.91 (1.03) | 3.51 (1.16) | |||
Self-efficacy with medical provider items score: 0=Cannot do at all, 10=Certain to do
Stigma score (1–5: higher score, higher stigma level)
rs = Spearman’s rho correlation coefficient
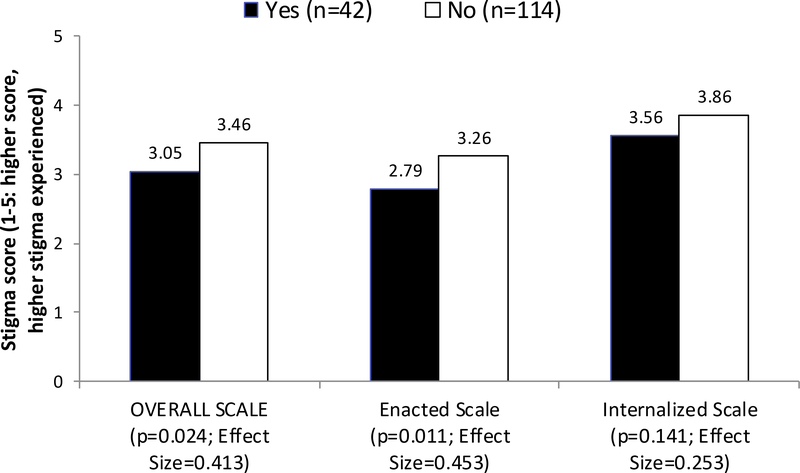
Among those who previously tested positive for HCV, the mean values of the overall stigma scale and the enacted stigma subscale were significantly higher for participants who did not seek HCV treatment (n=114) than those who did (n=42, see Figure 1). The stigma scores were 3.46 vs. 3.05 for the overall scale (p=0.024) and 3.26 vs. 2.79 for the enacted subscale (p=0.011). There were no significant mean differences between groups for the internalized stigma scores (3.86 vs. 3.56).
Figure 1.
Stigma score of HCV+ participants (N=156): Sought HCV treatment vs. Did not seek HCV treatment
3.4. Internal consistency
Cronbach’s alpha indicated strong reliability for the overall stigma scale (0.91), the enacted stigma subscale (0.90), and the internalized stigma subscale (0.84) (Gliem and Gliem, 2003). Removing any of the nine items would decrease the reliability of the overall scale. Therefore, the items selected have optimal reliability for creating the stigma scale.
4. Discussion
Findings from this study report generally high levels of drug use stigma in medical settings among our sample of PWID. For example, a majority of participants have reported that their medical provider had said critical or insulting things to them about their drug use (52%) and felt disrespected by their medical provider due to their drug use (51%). These high levels have been similarly reported among PWUD by Lloyd (2013) and Van Boekel et al. (2013) based on their systematic reviews, and by Clements et al. (2015) based on their narrative interviews with PWUD.
Based on fifteen items about various drug-related stigmas, we identified a 9-item scale with very good construct validity and internal reliability. This scale includes 2 subscales: a 6-item scale concerning enacted stigma, which includes personal experiences of drug-related stigma from medical providers (e.g. derogatory comments, being treated disrespectfully, not feeling welcome at the medical provider’s office) and a 3-item scale concerning internalized drug stigma, which applies negative feelings about drug use to oneself, such as the desire to hide and not disclose drug use to medical providers and shame about using drugs. Internal reliability was strong for the overall stigma scale and both the enacted and internalized stigma subscales. Furthermore, content and construct validity for the overall scale and subscales provided sufficient confidence for the stigma scales to be valid and reliable assessment tools.
The Medical Provider Stigma Experienced by PWUD (MPS-PWUD) scale has immediate applications for assessing stigma experienced by PWUD in medical settings. This scale addresses an urgent need given the alarming increase in complications related to the opioid epidemic across the United States, such as HCV infections (Zibbell et al., 2015; Zibbell et al., 2018), overdose (Suryaprasad et al., 2014), and endocarditis (Lankenau et al., 2015). The scale could be easily incorporated into research protocols to ascertain drug-related stigma experiences by PWUD in medical settings. It could be used by healthcare providers, service providers, and advocacy organizations as a quick screening tool that would allow for an immediate assessment of drug-related stigma that could potentially affect PWUD’s interactions with medical providers and their successful follow-up treatment.
Data collected with the MPS-PWUD from different groups of PWUD could inform drug-related stigma experienced in medical settings by the drug consumed (e.g. crack, versus methamphetamines or heroin), and/or route of administration (e.g. oral, intranasal, or intravenous), and its potential impact on PWUD’s access to and retention in medical services. Increased awareness of the stigma generated in medical settings could improve engagement and retention of PWUD in health care and curb drug-related epidemics (e.g. HCV). The MPS-PWUD could also contribute to designing stigma prevention strategies such as raising awareness regarding stigmatizing practices in medical settings or among specific providers and allow organizations that serve PWUD to monitor stigma levels over time. Finally, the MPS-PWUD could facilitate tailoring programs aiming at reducing drug-related stigma in hospitals and medical offices serving PWUD (e.g. HIV and HCV treatment, substance use disorder treatment, etc.).
To our knowledge, there is a paucity of validated stigma scales measuring stigma experienced by PWUD in medical settings, including how that stigma is felt and impacts PWUD. The MPS-PWUD also explicitly inquires about the stigma as a direct consequence of respondents’ drug use. Therefore, we believe that the newly validated MPS-PWUD scale provides valuable practical and research contributions. The MPS-PWUD and the SU-SMS (Smith et al., 2016) were designed for related, but somewhat different purposes. The MPS-PWUD assesses stigma towards PWUD due to their drug use in medical settings and its impact on communication with medical providers, while the SU-SMS assesses a generalized stigma (not specifically due to drug use) towards “pill shoppers” and other PWUD by family members and healthcare workers. The MPS-PWUD scale was validated using a sample of PWID who were injecting at the time of enrollment, while the two subsamples used in validating the SU-SMS were PWUD in methadone maintenance treatment and HIV-positive engaged in HIV clinical care and/or Buprenorphine replacement therapy.
Although both scales present enacted and internalized stigma derived from Stigma Framework (Smith et al., 2016), the MPS-PWUD enacted subscale includes six items inquiring about stigma from medical providers towards PWUD versus three items in the SU-SMS, of which none of them inquire about stigmatizing actions explicitly due to drug use. The SU-SMS internalized subscale presents six items endorsing negative feelings about drug use towards oneself. However, in contrast with the three internalized stigma items in the MPS-PWUD, none of the six items inquired how the internalized stigma impacts PWUD’s behavior in medical settings. We believe assessing enacted stigma explicitly as a consequence of drug use and inquiring about a wider array of enacted stigmatizing actions by medical providers makes the MPS-PWUD a more suited instrument to assess medical provider stigma towards PWUD.
We also found that drug use stigma may have a direct impact on PWID self-efficacy to communicate with medical providers. The overall scale and both subscales indicate that higher levels of stigma are significantly associated with diminished ability to communicate illness concerns, to discuss personal problems related to illness, and to work out difficulties with medical providers. Hence, stigma in medical settings may diminish trust and comfort with the medical provider, which could lead to detrimental health care consequences. Our results also indicated that drug use stigma may affect PWID’s decision to seek HCV treatment. The overall scale score and enacted subscale score were significantly higher for the group who did not seek treatment. The cross-sectional nature of this analysis calls for further investigation into how stigma impacts care-seeking behavior among PWUD, and whether healthcare visits lead to stigmatizing experiences. Such experiences could prevent PWUD from engaging in future health care that serves both the patient and public health efforts aimed at disease prevention and elimination.
This work highlights several limitations and improvements for future efforts on this topic. Social desirability may be an issue in measuring stigma, but we do not know what the magnitude or the direction of the bias may be in the context of a research study focused on PWID. Although the sample was within the recommended size for PCA analysis (Osborne and Costello, 2004), the number of participants was on the lower end of the recommended analysis size. Therefore, the subsample for the analysis and the relatively small sample size made it challenging to observe statistical significance. Furthermore, we were unable to conduct additional construct validity analyses using meaningful variables about the group that had previously sought HCV care because it was limited to 42 people. While all participants in this study were currently injecting drugs, the stigma scale only included items that inquire about drug use in general. Revalidation among populations who do not inject drugs, use different drugs (e.g. crack, methamphetamines, cocaine, etc.), and in multiple languages would be valuable future contributions. Future versions of the scale may be strengthened by specifically inquiring about stigma generated by injection drug use. The cross-sectional baseline data did not allow us to re-administer the items to participants to assess test-retest reliability. Finally, the validation of the scale would have been strengthened if it was cross-validated with another sample of PWID. The cross-validation is to be encouraged in future studies, whether by us or other researchers. Nevertheless, the results were similar when preliminary analyses were run on a smaller sample, which reassures our confidence in the final results.
We note several strengths of this work. The MPS-PWUD is brief, uses common language, and can be self-administered, thereby minimizing respondent burden and response biases. Its brevity may be especially important for substance-using populations. We used data from a dataset that contained numerous additional variables, such as drug use history, HCV treatment, and self-efficacy measures in communicating with medical providers. This allowed us to conduct tests of construct validity and demonstrate the robustness of the results.
Finally, the MPS-PWUD scale ought to inform healthcare-related prevention strategies aimed at serving PWUD. It is not sufficient to make medical services and treatment available for PWUD without appropriate efforts to train medical staff on avoiding stigmatizing behaviors. If health care providers are engaging in stigmatizing behaviors, PWUD may not initiate or remain in medical care, undermining critical public health efforts such as HIV and HCV elimination.
Highlights:
We present a validated Medical Provider Stigma Experienced by PWUD scale
We report high levels of drug use stigma in medical settings among PWID
High stigma levels are associated with less open communication with medical providers
PWID with a high level of enacted stigma were less likely to seek HCV treatment
The MPS-PWUD scale could help measure stigma experienced by PWUD in medical settings
Acknowledgments
The authors would like to thank all the individuals who participated in this study. We would also like to thank Dr. Kelly Quinn for contributing to the analysis design and reviewing the manuscript.
Role of Funding Source
This research was supported by the National Institutes of Health (NIH) / National Institute on Drug Abuse (NIDA), Grant No. R01DA041298 (PMG & KM, PIs). Dr. SK time as supported by Grant No. K01DA048172. The content is the sole responsibility of the authors and does not necessarily reflect the official views of NIDA or NIH.
Footnotes
Author Disclosures:
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chunki Fong, CUNY Graduate School of Public Health and Health Policy, Institute for Implementation, Science in Population Health (ISPH), 55 West 125th Street, New York, NY 10027, USA.
Pedro Mateu-Gelabert, CUNY Graduate School of Public Health and Health Policy, Institute for Implementation, Science in Population Health (ISPH), 55 West 125th Street, New York, NY 10027, USA.
Courtney Ciervo, CUNY Graduate School of Public Health and Health Policy, Institute for Implementation, Science in Population Health (ISPH), 55 West 125th Street, New York, NY 10027, USA.
Benjamin Eckhardt, NYU School of Medicine, 550 First Avenue, New York, NY 10016, USA.
Yesenia Aponte-Melendez, CUNY Graduate School of Public Health and Health Policy, Institute for Implementation, Science in Population Health (ISPH), 55 West 125th Street, New York, NY 10027, USA.
Shashi Kapadia, Weill Cornell Medicine, 1305 York Ave 4th floor, New York, NY 10021, USA.
Kristen Marks, Weill Cornell Medicine, 1305 York Ave 4th floor, New York, NY 10021, USA.
References
- Asher AK, Portillo CJ, Cooper BA, Dawson-Rose C, Vlahov D, Page KA, 2016. Clinicians’ views of hepatitis C virus treatment candidacy with direct-acting antiviral regimens for people who inect drugs. Subst. Use Misuse 51, 1218–1223. 10.3109/10826084.2016.1161054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerekamps A, Newsum AM, Smit C, Arends JE, Richter C, Reiss P, 2018. High treatment uptake in human immunodeficiency virus/Hepatitis C virus-coinfected patients after unrestricted access to direct-acting antivirals in the Netherlands. Clin. Infect. Dis 66, 1352–1359. 10.1093/cid/cix1004 [DOI] [PubMed] [Google Scholar]
- Brener L, Von Hippel W, Kippax S, Preacher KJ, 2010. The role of physician and nurse attitudes in the health care of injecting drug users. Subst. Use Misuse 45, 1007–1018. 10.3109/10826081003659543 [DOI] [PubMed] [Google Scholar]
- Brickman P, Rabinowitz VC, Karuza JJ, Coates D, Cohn E, Kidder L, 1982. Models of helping and coping. Am. Psychol 37, 368–384. 10.1037/0003-066X.37.4.368 [DOI] [Google Scholar]
- Clark M, 2011. Conceptualizing Addiction: How Useful Is the Construct? Int. J. Humanit. Soc. Sci 1, 55–64. [Google Scholar]
- Clements A, Grose J, Skirton H, 2015. Experiences of UK patients with hepatitis C virus infection accessing phlebotomy: A qualitative analysis. Nurs. Health Sci 17, 214–222. 10.1111/nhs.12173 [DOI] [PubMed] [Google Scholar]
- Corrigan P, Markowitz FE, Watson A, Rowan D, Kubiak MA, 2003. An attribution model of public discrimination towards persons with mental illness. J. Health Soc. Behav 44, 162–179. [PubMed] [Google Scholar]
- Corrigan PW, 2000. Mental health stigma as social attribution: Implications for research methods and attitude change. Clin. Psychol. (New York) 7, 48–67. 10.1093/clipsy/7.1.48 [DOI] [Google Scholar]
- Corrigan PW, Kuwabara SA, O’Shaughnessy J, 2009. The Public Stigma of Mental Illness and Drug Addiction: Findings from a Stratified Random Sample. J. Soc. Work 9, 139–147. 10.1177/1468017308101818 [DOI] [Google Scholar]
- Denning P, 2000. Practicing Harm Reduction Psychotherapy: An Alternative Approach to Addictions. The Guilford Press, New York. [Google Scholar]
- Earnshaw VA, Chaudoir SR, 2009. From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behav. 13, 1160–1177. 10.1007/s10461-009-9593-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM, 2013. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 17, 1785–1795. 10.1007/s10461-013-0437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford RH, 2011. Interpersonal challenges as a constraint on care: The experience of nurses care of patients who use illicit drugs. Contemp. Nurse 37, 241–252. 10.5172/conu.2011.37.2.241 [DOI] [PubMed] [Google Scholar]
- Gilchrist G, Moskalewicz J, Slezakova S, Okruhlica L, Torrens M, Vajd R, Baldacchino A, 2011. Staff regard towards working with substance users: a European multi-centre study. Addiction 106, 1114–1125. 10.1111/j.1360-0443.2011.03407.x. [DOI] [PubMed] [Google Scholar]
- Gliem JA, Gliem RR, 2003. Calculating, Interpreting, and Reporting Cronbach’s Alpha Reliability Coefficient for Likert-Type Scales. Midwest Research-to-Practice Conference in Adult, Continuing, and Community Education. http://hdl.handle.net/1805/344. Accessed on February 16, 2020. [Google Scholar]
- Goffman E, 2009. Stigma: Notes on the management of spoiled identity: Simon and Schuster, New York. [Google Scholar]
- Goodyear T, Ti L, Carrieri P, Small W, Knight R, 2020. “Everybody living with a chronic disease is entitled to be cured”: Challenges and opportunities in scaling up access to direct-acting antiviral hepatitis C virus treatment among people who inject drugs. Int. J. Drug Policy 81, 102766. 10.1016/j.drugpo.2020.102766 [DOI] [PubMed] [Google Scholar]
- Gowan T, 2010. Hobos, Hustlers and Backsliders: Homeless in San Francisco. University of Minnesota Press, Minneapolis. [Google Scholar]
- Grebely J, Bruneau J, Bruggmann P, Harris M, Hickman M, Rhodes T, 2017. Elimination of hepatitis C virus infection among PWID: The beginning of a new era of interferon-free DAA therapy. Int. J. Drug Policy 47, 26–33. 10.1016/j.drugpo.2017.08.001 [DOI] [PubMed] [Google Scholar]
- IBM Corp., 2017. IBM SPSS Statistics for Windows, Version 25.0. IBM Corp., New York. [Google Scholar]
- Jordan A, Masson C, Mateu-Gelabert P, McKnight C, Pepper N, Bouche K, Guzman L, Kletter E, Seewald RM, Des-Jarlais DC, Sorensen JL, Perlman D, 2013. Perceptions of drug users regarding Hepatitis C screening and care: A qualitative study. Harm Reduct. J 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook AL, Stokka D, Heger B, Nygaard E, 2007. Hepatitis C treatment of opioid dependants receiving maintenance treatment: results of a Norwegian pilot study. Eur. Addict. Res 13, 216–221. 10.1159/000104884 [DOI] [PubMed] [Google Scholar]
- Lankenau SE, Kecojevic A, Silva K, 2015. Associations between prescription opioid injection and Hepatitis C virus among young injection drug users. Drugs (Abingdon Engl.) 22, 35–42. 10.3109/09687637.2014.970515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus JV, Safreed-Harmon K, Stumo SR, Jauffret-Roustide M, Maticic M , Reic T, Hep CSG, 2017. Restrictions on access to direct-acting antivirals for people who inject drugs: The European Hep-CORE study and the role of patient groups in monitoring national HCV responses. Int. J. Drug Policy 47, 47–50. 10.1016/j.drugpo.2017.05.054 [DOI] [PubMed] [Google Scholar]
- Levine H, 1978. The Discovery of Addiction: Changing Conceptions of Habitual Drunkenness in America: Part I. J. Stud. Alcohol 39, 493–506. 10.15288/jsa.1978.39.143 [DOI] [PubMed] [Google Scholar]
- Litwin AH, Drolet M, Nwankwo C, Torrens M, Kastelic A, Walcher S, Grebely J, 2019. Perceived barriers related to testing, management and treatment of HCV infection among physicians prescribing opioid agonist therapy: The C- SCOPE Study. J. Viral Hepat 26, 1094–1104. 10.1111/jvh.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C, 2013. The stigmatization of problem drug users: A narrative literature review. Drugs (Abingdon Engl.) 20, 85–95. 10.3109/09687637.2012.743506 [DOI] [Google Scholar]
- Luborsky L, Barber JP, Siqueland L, Johnson S, Najavits LM, Frank A, Daley D, 1996. The Revised Helping Alliance Questionnaire (HAq-II): Psychometric Properties. J. Psychother. Pract. Res 5, 260–271. [PMC free article] [PubMed] [Google Scholar]
- Matsunaga M, 2010. How to Factor-Analyze Your Data Right: Do’s, Don’ts, and How-To’s. Int. J. Psychol. Res. (Medellin) 3, 97–110. 10.21500/20112084.854 [DOI] [Google Scholar]
- Muncan B, Walters SM, Ezell J, Ompad DC, 2020. “They look at us like junkies”: influences of drug use stigma on the healthcare engagement of people who inject drugs in New York City. Harm Reduct. J 17, 53. 10.1186/s12954-020-00399-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musto DF, 2002. One Hundred Years of Heroin. Auburn House, Connecticut. [Google Scholar]
- National Institute on Drug Abuse, 2007. Drugs, Brains and Behavior: The Science of Addiction. National Institutes on Drug Abuse. https://www.drugabuse.gov/sites/default/files/soa_2014.pdf. Accessed on January 25, 2018. [Google Scholar]
- Ormston R, Bradshaw P, Anderson S, 2010. Scottish Social Attitudes Survey 2009: Public Attitudes to Drugs and Drug Use in Scotland. Scottish Government Social Research. https://www2.gov.scot/Resource/Doc/312459/0098738.pdf. Accessed on September 8, 2020. [Google Scholar]
- Osborne JW and Costello AB, 2004. Sample size and subject to item ratio in principal components analysis. Prac. Assess. Res. Eval 9. 10.7275/ktzq-jq66 [DOI] [Google Scholar]
- Palamar JJ, Kiang MV, Halkitis PN, 2011. Development and Psychometric Evaluation of Scales that Assess Stigma Associated With Illicit Drug Users. Subst. Use Misuse 46, 1457–1467. 10.3109/10826084.2011.596606 [DOI] [PubMed] [Google Scholar]
- Paquette CE, Syvertsen JL, Pollini RA, 2018. Stigma at every turn: Health services experiences among people who inject drugs. Int. J. Drug Policy 57, 104–110. 10.1016/j.drugpo.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, 1999. Multiple imputation: a primer. Stat. Methods in Med 8, 3–15. 10.1191/096228099671525676 [DOI] [PubMed] [Google Scholar]
- Smith LR, Earnshaw VA, Copenhaver MM, Cunningham CO, 2016. Substance use stigma: Reliability and validity of a theory-based scale for substance-using populations. Drug Alcohol Depend. 162, 34–43. 10.1016/j.drugalcdep.2016.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stangl A, Earnshaw V, Logie C, Van Brakel W, Simbayi L, Barré I, Dovidio J, 2019. The Health Stigma and Discrimination Framework: a global, crosscutting framework to inform research, intervention development, and policy on health-related stigmas. BMC Med. 17. 10.1186/s12916-019-1271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauser DR, Ciftci A, O’Sullivan D, 2009. Using attribution theory to examine community rehabilitation provider stigma. Int. J. Rehabil. Res 32, 41–47. 10.1097/MRR.0b013e328307f5b0 [DOI] [PubMed] [Google Scholar]
- Suryaprasad AG, White JZ, Xu F, Eichler BA, Hamilton J, Patel A, Bel Hamdounia S, Church DR, Barton K, Fisher C, Macomber K, Stanley M, Guilfoyle SM, Sweet K, Liu S, Iqbal K, Tohme R, Sharapov U, Kupronis BA, Ward JW, Holmberg SD, 2014. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin. Infect. Dis 59, 1411–1419. 10.1093/cid/ciu643 [DOI] [PubMed] [Google Scholar]
- Treloar C, Newland J, Rance J, Hopwood M, 2010. Uptake and delivery of hepatitis C treatment in opiate substitution treatment: perceptions of clients and health professionals. J. Viral Hepat 17, 839–844. 10.1111/j.1365-2893.2009.01250.x [DOI] [PubMed] [Google Scholar]
- van Boekel LC, Brouwers EPM, van Weeghel J, Garretsen HFL, 2013. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: Systematic review. Drug Alcohol Depend. 131, 23–35. 10.1016/j.drugalcdep.2013.02.018 [DOI] [PubMed] [Google Scholar]
- Wadden TA, Anderson DA, Foster GD, Bennett A, Steinberg C, Sarwer DB, 2000. Obese women’s perceptions of their physicians’ weight management attitudes and practices. Arch. Fam. Med 9, 854–860. 10.1001/archfami.9.9.854 [DOI] [PubMed] [Google Scholar]
- Weiner B, Perry RP, Magnusson J, 1988. An attributional analysis of reactions to stigmas. J Pers. Soc. Psychol 55, 738–748. 10.1037//0022-3514.55.5.738 [DOI] [PubMed] [Google Scholar]
- Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, Holtzman D, 2018. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 108, 175–181. 10.2105/ajph.2017.304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore-Moravian L, Serrecchia J, Blankenship S, Ward JW, Holtzman D, Centers for Disease Control and Prevention, 2015. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤30 years - Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. Morb. Mortal. Wkly. Rep 64, 453–458. [PMC free article] [PubMed] [Google Scholar]