Abstract
We aimed to investigate whether month of birth is associated with blood pressure (BP) and prevalent hypertension in adults from a region with frost‐free days of <150 days and average temperatures − 13°C in winter, Xinjiang, China. We analyzed data for 6158 subjects from several surveys. We divided participants into April to August (n = 2624) and September to March (n = 3534) groups, based on length of maternal exposure to cold months, and analyzed BP, prevalent hypertension, and related factors. Diastolic BP in total subjects and systolic and diastolic BP in male subjects born between April and August were significantly higher than in those born between September and March. In sensitivity analysis, untreated males born between April and August showed significantly higher systolic and diastolic BP than did their counterparts. Subjects born between April and August showed significantly higher prevalence of hypertension (31.3% vs 27.8%, P = .003), and isolated systolic (23.3% vs 20.8%, P = .018) and diastolic hypertension (24.5% vs 21.4%, P = .004), than those born between September and March, which is similar for men. Birth between April and August showed 1.68 (95% CI: 1.06‐2.67, P = .027)‐fold increased odds for the prevalence of hypertension, independent of gender, age, body mass index, waist circumference, cigarette consumption, alcohol intake, and family history, compared with their counterparts. In conclusion, maternal exposure to cold spells during pregnancy may be associated with the increased risk of hypertension in offspring later in life, particularly among males, suggesting the involvement of maternal cold exposure during pregnancy in offspring hypertension development.
Keywords: blood pressure, cold exposure, hypertension, maternal exposure, month of birth
1. INTRODUCTION
The prevalence of hypertension continues to increase worldwide, implying a huge potential burden of future cardiovascular disease. 1 The underlying mechanisms of hypertension are complex and multi‐factorial, where environmental factors play important roles such as exposure to low temperatures (cold spells). 2 , 3 In addition, recent research has demonstrated that exposure to unfavorable environmental stimuli early in life is another important determinant of the risk of hypertension and associated conditions during adulthood. 4 , 5 , 6
The developmental origin of health and diseases suggest that some serious health problems later in life might have originated early in life during fetus development and early postnatal period. 4 Season of birth, indicative of different maternal exposure to environmental factors during pregnancy, can be used to examine the associations between early‐life exposures and later‐life health outcomes, which is true since decades ago there were strong seasonal variations in nutrition and lifestyle, especially in developing countries. For instance, maternal exposure to cold spells in certain periods of pregnancy might have important correlations to consequent risk of developing diabetes mellitus in later life of offsprings. 5 , 6 Possible triggering factors may include decreased sunlight, physical activity, and maternal nutritional status such as vitamin D during pregnancy, which tend to change geographically and seasonally. 7 , 8 It has been also shown that a number of abovementioned environmental factors influence renal development, particularly nephron number. 9 A reduction in nephron number, well associated with hypertension, is observed even when the maternal exposure is induced by restriction of only one nutrient. 10 Nephrogenesis in the human begins by the 10th week postconception and ends by the 34th week, and therefore, the window for potential exposure is wide. Based on above evidence, it might be reasonable to hypothesize that maternal exposure to cold spell in certain periods of pregnancy may increase the risk of BP elevation and of hypertension development later in life of offspring. Nonetheless, the strength of the association between the maternal exposure to cold spells and subsequent hypertension remains unclear. 11
Exposures in early life, in terms of cold spell during pregnancy, are predictable in Xinjiang, China, where cold months, accounting for over half of a year, are longer and well‐defined. Furthermore, hypertension is highly prevalent in Xinjiang, affecting 35.01% of adults aged ≥35 years and 40.7% of those ≥45 years. 12 , 13 Therefore, the climate of Xinjiang, specially rural northern part, 14 provides a better setting for assessing the influence of cold spells on human health outcome. The aim of the present study was to investigate whether the adulthood BP and the prevalent hypertension are influenced by maternal exposure to cold spell during pregnancy.
2. METHODS AND MATERIALS
2.1. Site
Located in the hinterland of the Eurasia continent (73°40′–96°23′E and 34°25′–48°10′N), Xinjiang is divided into three distinct sub‐regions (northern, southern, and eastern part) according to its natural settings and climate patterns. 15 Xinjiang belongs to alpine regions due to its higher altitude and latitude (Figure 1) and northern part has frost‐free days of <150 days and has the lowest temperature, with annual average temperatures between 4‐8°C and − 13°C in winter, and as low as −51.5°C in Fuyun, Altay. 16
FIGURE 1.
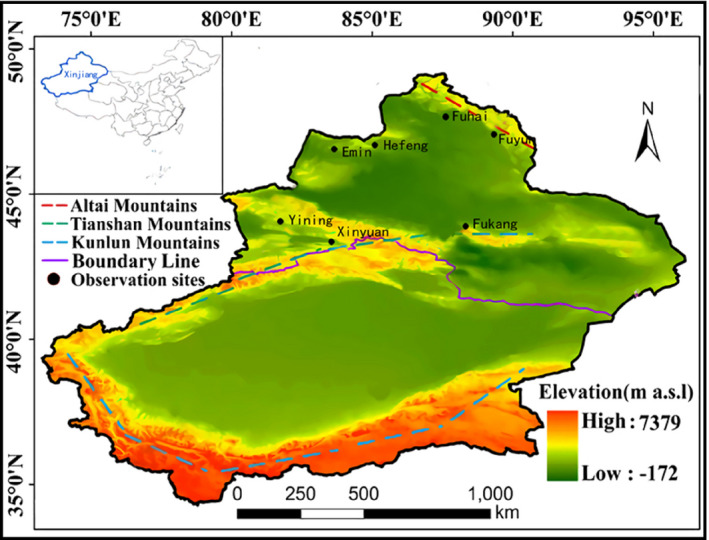
Observation sites in the study, north Xinjiang, northwest China
2.2. Study population
Data for the current study were extracted from several surveys for hypertension in northern and southern Xinjiang conducted between 1998 and 2015. 12 , 13 In brief, we used a stratified sampling method to select a representative sample of the general adult population, based on the government record of registered residence.
Considering the differences in climatic features of southern and northern Xinjiang and the aim of the study, we selected the subjects aged 19‐55 years who were born and living in northern Xinjiang. Subjects with premature birth by questionnaire and missing information on birth date were excluded. Exposure group was defined as subjects who were born between April and August and control group as those born between September and coming March, based on the length of maternal exposure to cold spells during pregnancy in Northern Xinjiang as given in Figure 2, using similar methods from Gabriele Doblhammer, et al 17
FIGURE 2.
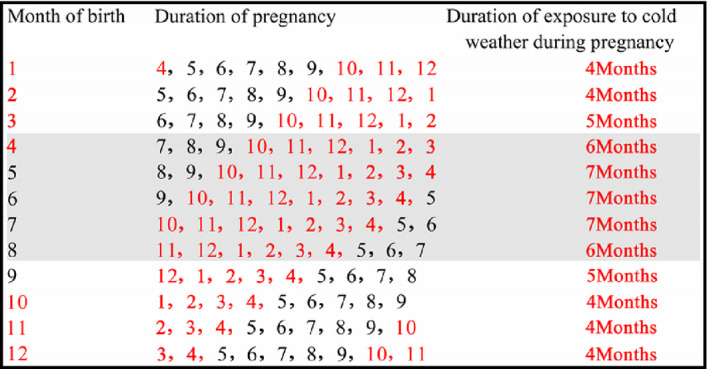
Duration of maternal exposure to cold spells corresponding to the month of birth
The study was approved by the ethics committee at People's Hospital of Xinjiang Uygur Autonomous Region with approval number: 2015049.
2.3. Definition
Hypertension is defined as systolic BP (SBP) ≥140 mm Hg, and/or diastolic BP (DBP) ≥90 mm Hg, and/or previous physician‐diagnosed hypertension, and/or use of anti‐hypertensive medicine within previous 2 weeks, regardless of BP readings. Isolated systolic hypertension (ISH) is defined as an average SBP ≥140 mm Hg and DBP <90 mm Hg, and an isolated diastolic hypertension (IDH) as SBP <140 mm Hg and DBP ≥90 mm Hg using the similar definition in the previous studies. 18 Alcohol intake is defined as consuming alcoholic beverage at least once per week in the past month and cigarette consumption as participants who have smoked at least 20 packets of cigarettes in their lifetime and currently smoke cigarettes. 19 Overweight and general obesity is defined as body mass index (BMI) 24.0‐27.9 and BMI ≥28 kg/m2 and abdominal obesity as having a waist circumference (WC) ≥90 cm for men and ≥85 cm for women. 20
2.4. Data collection
Data were collected by physicians and trained nurses from Hypertension Institute of Xinjiang, based on study protocol using a standard questionnaire in face‐to‐face interviews. Questions assessed demographic characteristics (date of birth, ethnicity, age, and sex), lifestyle risk factors (cigarette consumption and alcohol intake), and hypertension‐related information (whether it was previously diagnosed by a doctor ? whether anti‐hypertensive agents are being taken for the last 2 weeks?). Physical examination was conducted, including BP measurement. Three BP measurements were taken using a mercury sphygmomanometer in 1998‐2008 and an electronic sphygmomanometer in 2015 via a standardized procedures after a rest of at least 5 minutes from the unclothed right arm of the person in a sitting position at intervals of at least 1 minutes. Body weight, height, and WC were measured using standard methods. BMI was calculated by dividing weight by height squared (kg/m2).
2.5. Statistical analysis
Continuous variables including BP, age, WC, and BMI were expressed as mean ± standard deviation (SD) if normally distributed and comparison among groups was performed using Student's t test. The categorical variables, including sex and age groups and prevalence of hypertension, were summarized as n (%) and analyzed using the chi‐square test. We performed sensitivity analysis while comparing the BP between two groups by excluding the subjects who were taking anti‐hypertensive agents within previous 2 weeks of investigation. Univariate and multivariate logistic regression analysis was performed to assess whether birth between April and August is a related factor for presence of hypertension. All statistical analyses were performed with SPSS statistical software, version 19.0 (Chicago, IL, USA), and the significance level was set at P = .05.
3. RESULTS
3.1. Participants
The enrollment procedure of study subjects was given in Figure 3. Complete data for a total of 6158 individuals (women: 56.4%) were analyzed including 2624 (42.6%) subjects born between April and August.
FIGURE 3.
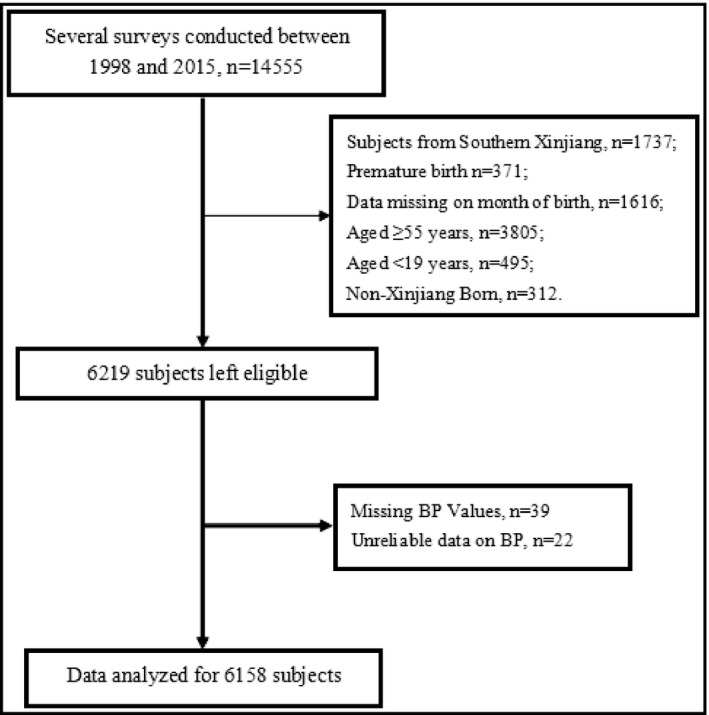
Selection procedure of the study population
Table 1 presents the characteristics of the two groups. Subjects born between April and August had significantly older age (40.4 ± 8.9 vs 39.8 ± 9.0, P = .014), compared to subjects born between September and March. Gender and ethnic group composition, family history for hypertension, BMI, WC, cigarette consumption, and alcohol intake status did not show significant differences between the two groups.
TABLE 1.
Characteristics of study participants
| Total | April to August | September to March | P | |
|---|---|---|---|---|
| N | 6158 | 2624 (42.6) | 3534 (57.4) | |
| Gender (n,%) | ||||
| Men | 2686 (43.6) | 1144 (43.6) | 1542 (43.6) | .978 |
| Women | 3472 (56.4) | 1480 (56.4) | 1992 (56.4) | |
| Age (years) | 40.0 ± 9.0 | 40.4 ± 8.9 | 39.8 ± 9.0 | .014 |
| Age groups (n, %) | ||||
| 19‐29 y | 865 (14.0) | 344 (13.1) | 521 (14.7) | .003 |
| 30‐39 y | 1911 (31.0) | 767 (29.2) | 1144 (32.4) | |
| 40‐49 y | 2289 (37.2) | 1030 (39.3) | 1259 (35.6) | |
| 50‐55 y | 1093 (17.7) | 483 (18.4) | 610 (17.3) | |
| Year of birth | ||||
| Before 1960s | 1072 (17.4) | 469 (17.9) | 603 (17.1) | .003 |
| 1960s | 2139 (34.7) | 974 (37.1) | 1165 (33.0) | |
| 1970s | 1726 (28.0) | 688 (26.2) | 1038 (29.4) | |
| 1980s | 814 (13.2) | 329 (12.5) | 485 (13.7) | |
| 1990s | 407 (6.6) | 164 (6.3) | 243 (6.9) | |
| Ethnic groups | ||||
| Han | 1772 (28.8) | 732 (27.9) | 1040 (29.4) | .380 |
| Mongolian | 847 (13.8) | 375 (14.3) | 472 (13.4) | |
| Hui | 314 (5.1) | 128 (4.9) | 186 (5.3) | |
| Kazakh | 2594 (42.1) | 1124 (42.8) | 1470 (41.6) | |
| Uygur | 571 (9.3) | 245 (9.3) | 326 (9.2) | |
| Others | 60 (1.0) | 20 (0.8) | 40 (1.1) | |
| Family history | 1328 (31.9) | 576 (32.5) | 752 (31.4) | .266 |
| Body mass index (kg/m2) | 25.7 ± 4.2 | 25.8 ± 4.2 | 25.7 ± 4.2 | .499 |
| Waist circumference (cm) | 85.7 ± 11.7 | 85.6 ± 11.5 | 85.7 ± 12.0 | .843 |
| Cigarette consumption (n,%) | 698 (22.7) | 301 (23.3) | 397 (22.3) | .124 |
| Alcohol intake (n,%) | 280 (54.2) | 113 (54.3) | 167 (54.0) | .950 |
Values are presented as number (%), Mean ± standard deviation.
As shown in Table 2, SBP was significantly higher in male subjects born between April and August than in those born between September and March (128.8 ± 19.3 vs 127.3 ± 18.6 mm Hg, P = .047). Whole subjects born between April and August showed significantly higher DBP, compared to those born between September and March (80.3 ± 14.4 vs 79.4 ± 14.1 mm Hg, P = .010), which was the same for male subjects (82.2 ± 14.0 vs 80.6 ± 13.4 mm Hg, P = .002), while divided by gender.
TABLE 2.
Comparison of blood pressure between two groups and sensitivity analysis by excluding those taking anti‐hypertensive agents
| April to August | September to March | P | |
|---|---|---|---|
| Total subjects | |||
| Systolic blood pressure (mm Hg) | |||
| All(n = 6158) | 127.0 ± 21.8 | 126.2 ± 21.6 | .168 |
| Men (n = 2686) | 128.8 ± 19.3 | 127.3 ± 18.6 | .047 |
| Women (n = 3472) | 125.7 ± 23.5 | 125.4 ± 23.6 | .773 |
| Diastolic blood pressure (mm Hg) | |||
| All(n = 6158) | 80.3 ± 14.4 | 79.4 ± 14.1 | .010 |
| Men (n = 2686) | 82.2 ± 14.0 | 80.6 ± 13.4 | .002 |
| Women (n = 3472) | 78.8 ± 14.6 | 78.5 ± 14.6 | .691 |
| Subjects not treated | |||
| Systolic blood pressure (mm Hg) | |||
| All(n = 5943) | 125.9 ± 21.0 | 125.7 ± 21.2 | .624 |
| Men (n = 2608) | 128.0 ± 19.2 | 126.5 ± 18.0 | .034 |
| Women (n = 3335) | 124.4 ± 22.2 | 125.1 ± 23.3 | .824 |
| Diastolic blood pressure (mm Hg) | |||
| All(n = 5943) | 79.6 ± 14.1 | 79.0 ± 13.9 | .204 |
| Men (n = 2608) | 81.3 ± 13.7 | 79.9 ± 12.9 | .008 |
| Women (n = 3335) | 78.3 ± 14.3 | 78.4 ± 14.6 | .438 |
Values are presented as mean ± standard deviation.
In further sensitivity analysis by excluding those taking anti‐hypertensive agents, untreated men subjects born between April and August showed significantly higher SBP (128.0 ± 19.2 vs 126.5 ± 18.0 mm Hg, P = .034) and DBP (81.3 ± 13.7 vs 79.9 ± 12.9 mm Hg, P = .008) than did those born between September and March.
As shown in Table 3, subjects born between April and August showed significantly higher presence of hypertension (31.3% vs 27.8%, P = .003), and isolated systolic (23.3% vs 20.8%, P = .018) and diastolic hypertension (24.5% vs 21.4%, P = .004), compared to those born between September and March. While divided by gender, male subjects born between April and August showed significantly higher presence of hypertension (35.1% vs 28.5%, P <.001), and isolated systolic (24.7% vs 20.0%, P = .003) and diastolic hypertension (28.6% vs 23.0%, P = .001), compared to those born between September and March.
TABLE 3.
Prevalence of total, isolated systolic and diastolic hypertension in both sex, men and women subjects by the month of birth (n, %)
| All | Men | Women | |
|---|---|---|---|
| Hypertension (n, %) | |||
| April to August | 822 (31.3) | 402 (35.1) | 420 (28.4) |
| September to March | 982 (27.8) | 440 (28.5) | 542 (27.2) |
| P | .003 | <.001 | .446 |
| Isolated systolic hypertension (n, %) | |||
| April to August | 612 (23.3) | 283 (24.7) | 329 (22.2) |
| September to March | 735 (20.8) | 308 (20.0) | 427 (21.4) |
| P | .018 | .003 | .575 |
| Isolated diastolic hypertension (n, %) | |||
| April to August | 642 (24.5) | 327 (28.6) | 315 (21.3) |
| September to March | 756 (21.4) | 354 (23.0) | 402 (20.2) |
| P | .004 | .001 | 0427 |
Values are presented as number (%).
In Table S1, we compared the treatment rates of hypertension and the number and type of anti‐hypertensive agents between two groups, whereas we did not observe significant differences.
3.2. Multivariable risk assessment
In Table 4, univariate and multivariate logistic regression analysis showed that those born between April and August revealed significantly higher odds ratio (OR) for the presence of hypertension (OR = 1.68, 95% CI: 1.06, 2.67, P = .027), independent of gender, age, BMI, WC, cigarette consumption, alcohol intake, and family history, compared with those born between September and March.
TABLE 4.
Univariate and multivariate logistic regression analysis for effects of month of birth on the presence of hypertension in total participants
| Models | OR | 95% CI | R2 | P | Adjusted variables |
|---|---|---|---|---|---|
| Model 1 | |||||
| September to March | Ref | Unadjusted model. | |||
| April to August | 1.19 | (1.06,1.32) | 0.002 | .003 | |
| Model 2 | |||||
| September to March | Ref | Model 1 plus gender | |||
| April to August | 1.19 | (1.06,1.32) | 0.004 | .003 | |
| Model 3 | |||||
| September to March | Ref | Model 2 plus age | |||
| April to August | 1.13 | (1.00,1.27) | 0.143 | .036 | |
| Model 4 | |||||
| September to March | Ref | Model 3 plus BMI | |||
| April to August | 1.14 | (1.01,1.29) | 0.200 | .034 | |
| Model 5 | |||||
| September to March | Ref | Model 4 plus waist circumference. | |||
| April to August | 1.25 | (1.05,1.48) | 0.233 | .011 | |
| Model 6 | |||||
| September to March | Ref | Model 5 plus cigarette consumption. | |||
| April to August | 1.37 | (1.09,1.72) | 0.253 | .006 | |
| Model 7 | |||||
| September to March | Ref | Model 6 plus alcohol intake | |||
| April to August | 1.62 | (1.03,2.56) | 0.185 | .037 | |
| Model 8 | |||||
| September to March | Ref | Model 7 plus family history | |||
| April to August | 1.68 | (1.06,2.67) | 0.203 | .027 | |
Abbreviations: 95% CI, 95% confidence interval; BMI, body mass index.
4. DISCUSSION
Season of birth, indicative of exposure to environmental factors, can be used to examine associations between early‐life exposures and later‐life health outcomes, which is true since there are strong seasonal variations in nutrition in developing countries, especially decades ago. 21 Main results of the current study encompass that SBP in men and DBP in total subjects are significantly higher in the ones born between April and August than in those born between September and March; subjects born between April and August show significantly higher presence of hypertension and isolated systolic and diastolic hypertension, compared to those born between September and March; and birth between April and August revealed significantly higher risk for the presence of hypertension, independent of well‐established risk factors.
Subjects of the current study are divided into two groups as April to August group and September to March group, since it is considered that individuals born between April and August often experience as long as 6‐7 months' maternal exposure to cold spells during their pregnancy, based on local climatic features, and using similar methods from Gabriele Doblhammer, et al 17 Studying the relationship between maternal exposure to cold spells during pregnancy and BP and presence of hypertension in the offspring provides new insights into hypertension and provides evidence for the prevention of adult hypertension from the embryonic stage. Mothers who give birth in September to coming March may have had access to plentiful food and fresh fruits and vegetables and higher daily activity throughout most of their pregnancy, with profound implications for clinical practice and public health policy. A half of the variation in human longevity may be due to genetic factors and early‐life factors. 17 To create a good intrauterine environment for the fetus to reduce the incidence of adult hypertension is of great significance to the quality of life of society, families, and individuals. That is, choosing specific months or plentiful supplements for key nutrients can be considered the alternative when a mother from regions like Xinjiang with longer cold periods within a year is planning to get pregnancy. This may be the option before we explore a better solution when considering the difficulty to predict how changes in the current climate will affect the epidemiology and prognosis of hypertension in the highly industrialized era. 22
Precise mechanisms responsible for the early‐life exposure programming of adult life hypertension are not yet thoroughly characterized, whereas suggested ones include the following. First, seasonal conditions around the period of birth are demonstrated to significantly determine birthweight, which is used as a proxy for fetal conditions in the majority of population studies. 11 That is, maternal exposure to cold spell during pregnancy is associated with predominantly indoor lifestyle and leads to low birthweight. 23 , 24 Lower birthweights, a well‐established risk factor for hypertension, 25 , 26 , 27 are observed in newborns exposed to cold weather around birth. 28 From the evidence currently available, it has been initially concluded that seasonality of birth has been associated with high SBP per se, 29 and with other contributors of BP including obesity, dyslipidemia, and insulin resistance. 30 , 31 , 32 Second, nephrogenesis in the human begins by the 10th week postconception and ends by the 34th week, which is influenced even when the maternal exposure is induced by restriction of only one nutrient. 10 Availability of cereals, vegetables, fruits, and animal proteins and important factors for nephrogenesis varies significantly according to the season especially in developing countries with longer cold months. 5 , 21 The decreased number of nephrons is one of the important determinants of elevated BP. The kidneys of Australian Aboriginals contain 30% fewer nephrons than those of non‐indigenous Australians, explaining in part why Australian Aborigines in remote areas have a much greater incidence of hypertension than non‐Aboriginal peoples. 33 Third, other contributors of BP including temperature, infections, sunlight photoperiod productions of vitamin D, and maternal lifestyle such as physical activity also change seasonally. 34 Mounting literature has confirmed that vitamin D deficiencies of parents translate into enhanced susceptibility to hypertension in their children. 35 , 36 Furthermore, maternal exposure to cold spells and changes in nutrients and lifestyles intervenes with BP through changing DNA methylation status of the offspring. Small observed changes in methylation status can lead to large phenotypic differences that contribute to altered susceptibility to certain diseases later in life. 6 , 7 , 8 , 9 , 10 , 11 , 17 , 23 , 24 , 25 , 26 , 27 , 33 , 36 However, further studies are warranted to explore the underlying mechanisms that link season of birth and hypertension in adulthood.
We observed a gender inconsistency of the relationship between maternal exposure to cold spells during pregnancy and elevated BP later in life of offspring. Current study design is unable to explain the observation, whereas growth patterns of girls and boys in utero are different. A sex‐specific impact of environmental factors on nephrogenesis has been suggested. Animal models show restriction of nutrients in the maternal diet during gestation reduce the nephron number in male, but not in female, offspring. 37 , 38 It can also be possibly explained by endocrinological differences between men and women later in life. Indeed, we excluded subjects aged ≥55 years, indicating that majority of the women are in pre‐menopausal status, which may have blunted the effects of the parameter of interest on BP due to gender‐specific protective role of estrogen against hypertension. Furthermore, previous studies focusing on the birth body weight and CVD have also shown these kind of gender differences. 27
Current study is strengthened by the well‐defined pattern of cold spells in northern Xinjiang, by the inclusion of randomly selected larger sample size and of different ethnic groups while considering the genetic background, and by the exclusion of those with premature birth to avoid, at least in part, the bias from the low birthweight and those aged ≥55 years in order to avoid confounding effects of atherosclerosis due to aging and post‐menopausal hormone changes. In addition, we selected subjects for hypertension from 7 counties of Altay, Tacheng, and Yili, where within the last 50 years the annual mean temperature is 3.1‐12°C, the lowest temperature is −12 to −51.15°C, and the mean of frost‐free days is 135‐160 days, which has provided us an ideal setting for the study. 39 Admittedly, current study contains some limitations. First, based on cross‐sectional nature, we cannot draw casual relationship between birth of month and elevated BP. However, it may be sufficient to put forward a hypothesis. Second, as the good predictors of nutritional, developmental and health status of the fetal condition, the birthweight and/or Apgar scores may have provided good explanation for the current findings if available. Unfortunately, we failed to collect relevant data, since subjects were enrolled to the study after 19‐55 years of birth and the study was conducted in a less developed rural region in China, where the system for collecting the data like birthweight or Apgar score did not exist in 20‐50 years ago. This may have brought some bias to the current results. Nonetheless, we have excluded subjects with premature birth, which may have lowered the bias from this aspect, at least in part. Third, we also failed to collect data on important predictors of hypertension such as salt intake. Previous studies show that this is a region with population characterized by high salt intake, 40 and it is unknown to what extent the salt intake would affect our current findings. Nonetheless, salt intake and/or salt sensitivity is a feature of whole community or the population living in a certain region, more pronounced in elderly population. And we selected the diverse local adult population aged <55 years using stratified sampling method. This characteristic, specially sampling method, may have lowered the potential effects of salt intake on our results.
In conclusion, maternal exposure to cold spells during pregnancy may be associated with the increased risk of hypertension in offspring later in life, suggesting the involvement of maternal cold exposure during pregnancy in hypertension development of offspring.
CONFLICT OF INTEREST
All the authors declared to have no conflict of interest.
AUTHOR CONTRIBUTIONS
NL conceived the project. LC, MH, ZB, and JZ collected the data. LC performed the statistical analysis and generated the figures and tables. LC and MH drafted the manuscript with help from NL and LW. LW assisted in the interpretation of the data. ML, DZ, SA, XY, and JH gave important suggestions and did significant changes. NL, LC, and MH contributed equally to this work and shares first co‐authorship.
Supporting information
Tab S1
Li N, Cai L, Heizhati M, et al. Maternal exposure to cold spells during pregnancy is associated with higher blood pressure and hypertension in offspring later in life. J Clin Hypertens. 2020;22:1884–1891. 10.1111/jch.14015
Li, Cai and Heizhati these authors contributed equally to this work and share first co‐authorship.
Funding information
This study is supported by Key technology integration research and application of hypertension prevention and control in pastoral areas of northern Xinjiang (grant no. 2017B03015).
REFERENCES
- 1. Zhou M, Wang H, Zhu J, et al. Cause‐specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the global burden of disease study 2013. Lancet. 2016;387(10015):251‐272. [DOI] [PubMed] [Google Scholar]
- 2. Kang Y, Han Y, Guan T, et al. Clinical blood pressure responses to daily ambient temperature exposure in China: An analysis based on a representative nationwide population. Sci Total Environ. 2020;705:135762. [DOI] [PubMed] [Google Scholar]
- 3. Hermann JM, Rosenbauer J, Dost A, et al. Seasonal variation in blood pressure in 162 135 patients with Type 1 or Type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2016;18(4):270‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and dis‐ ease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vaiserman AM, Khalangot MD, Carstensen B, et al. Seasonality of birth in adult type 2 diabetic patients in three Ukrainian regions. Diabetologia. 2009;52(12):2665‐2667. [DOI] [PubMed] [Google Scholar]
- 6. Si J, Yu C, Guo Y, et al. Season of birth and the risk of type 2 diabetes in adulthood: a prospective cohort study of 0.5 million chinese adults. Diabetologia. 2017;60(5):836‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lapillonne A. Vitamin D deficiency during pregnancy may impair maternal and fetal outcomes. Med Hypotheses. 2010;74(1):71‐75. [DOI] [PubMed] [Google Scholar]
- 8. Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289(1):F8‐F28. [DOI] [PubMed] [Google Scholar]
- 9. Mesquita FF, Gontijo JA, Boer PA. Maternal undernutrition and the offspring kidney: from fetal to adult life. Braz J Med Biol Res. 2010;43(11):1010‐1018. [DOI] [PubMed] [Google Scholar]
- 10. Paixão AD, Alexander BT. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol Reprod. 2013;89(6):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schreier N, Moltchanova E, Forsén T, Kajantie E, Eriksson JG. Seasonality and ambient temperature at time of conception in term‐born individuals influences on cardiovascular disease and obesity in adult life. Int J Circumpolar Health. 2013;72:21466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yao X, Frommlet F, Zhou L, et al. The prevalence of hypertension, obesity and dyslipidemia in individuals of over 30 years of age belonging to minorities from the pasture area of Xinjiang. BMC Public Health. 2010;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li N, Wang H, Yan Z, Yao X, Hong J, Zhou L. Ethnic disparities in the clustering of risk factors for cardiovascular disease among the Kazakh, Uygur, Mongolian and Han populations of Xinjiang: a cross‐sectional study. BMC Public Health. 2012;12:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu J, Chen Y, Li W, Liu Z, Wei C, Tang J. Understanding the complexity of temperature dynamics in Xinjiang, China, from multitemporal scale and spatial perspectives. ScientificWorldJournal. 2013;2013:259248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo B, Chen Z, Guo J, Liu F, Chen C, Liu K. Analysis of the nonlinear trends and non‐stationary oscillations of regional precipitation in xinjiang, northwestern china, using ensemble empirical mode decomposition. Int J Environ Res Public Health. 2016;13(3):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Z, Zhang H, Krause CM, Cobb NS. Climate change and human activities: a case study in Xinjiang, China. Climatic Change. 2010;99(3):457‐472. [Google Scholar]
- 17. Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci USA. 2018;98(5):2934‐2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Xing F, Liu R, et al. Isolated diastolic hypertension associated risk factors among Chinese in Anhui Province, China. Int J Environ Res Public Health. 2015;12(4):4395‐4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation. 2018;137(22):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 20. Chen CM, Kong LZ. For China obesity problem working group. Chinese adults guidelines for the prevention and control of overweight and obesity. Beijing: People's Medical Publishing House; 2006. [Google Scholar]
- 21. Vaiserman AM. Early‐life nutritional programming of Type 2 diabetes: experimental and Quasi‐experimental evidence. Nutrients. 2017;9(3):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park S, Kario K, Chia YC, et al. The influence of the ambient temperature on blood pressure and how it will affect the epidemiology of hypertension in Asia. J Clin Hypertens (Greenwich). 2020;22(3):438‐444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martyn CN, Barker DJ, Jespersen S, Greenwald S, Osmond C, Berry C. Growth in utero, adult blood pressure, and arterial compliance. Br Heart J. 1995;73(2):116‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elter K, Ay E, Uyar E, Kavak ZN. Exposure to low outdoor temperature in the midtrimester is associated with low birth weight. Aust N Z J Obstet Gynaecol. 2004;44(6):553‐557. [DOI] [PubMed] [Google Scholar]
- 25. Lackland DT. Fetal and early life determinants of hypertension in adults: implications for study. Hypertension. 2004;44(6):811‐812. [DOI] [PubMed] [Google Scholar]
- 26. Gunnarsdottir I, Birgisdottir BE, Benediktsson R, Gudnason V, Thorsdotti I. Association between size at birth, truncal fat and obesity in adult life and its contribution to blood pressure and coronary heart disease; study in a high birth weight population. Eur J Clin Nutr. 2004;58(5):812‐818. [DOI] [PubMed] [Google Scholar]
- 27. Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch‐up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18(7):815‐831. [DOI] [PubMed] [Google Scholar]
- 28. Lockett GA, Soto‐Ramírez N, Ray MA, et al. Association of season of birth with DNA methylation and allergic disease. Allergy. 2016;71(9):1314‐1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith AD, Crippa A, Woodcock J, Brage S. Physical activity and incident type 2 diabetes mellitus: a systematic review and dose‐response meta‐analysis of prospective cohort studies. Diabetologia. 2016;59(12):2527‐2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vaiserman AM. Early‐life exposure to substance abuse and risk of type 2 diabetes in adulthood. Curr Diab Rep. 2015;15(8):48. [DOI] [PubMed] [Google Scholar]
- 31. Chodick G, Flash S, Deoitch Y, Shalev V. Seasonality in birth weight: review of global patterns and potential causes. Hum Biol. 2009;81(4):463‐477. [DOI] [PubMed] [Google Scholar]
- 32. Banegas JR, Rodríguez‐Artalejo F, de la Cruz JJ, Graciani A, Villar F, del Rey‐Calero J. Adult men born in spring have lower blood pressure. J Hypertens. 2000;18(12):1763‐1766. [DOI] [PubMed] [Google Scholar]
- 33. Hoy WE, Kincaid‐Smith P, Hughson MD, et al. CKD in aboriginal Australians. Am J Kidney Dis. 2010;56(5):983‐993. [DOI] [PubMed] [Google Scholar]
- 34. Vaiserman A, Khalangot M. Similar seasonality of birth in type 1 and type 2 diabetes patients: a sign for common etiology? Med Hypotheses. 2008;71(4):604‐605. [DOI] [PubMed] [Google Scholar]
- 35. Meems LM, Mahmud H, Buikema H, et al. Parental vitamin D deficiency during pregnancy is associated with increased blood pressure in offspring via Panx1 hypermethylation. Am J Physiol Heart Circ Physiol. 2016;311(6):H1459‐H1469. [DOI] [PubMed] [Google Scholar]
- 36. van Ballegooijen AJ, Gansevoort RT, Lambers‐Heerspink HJ, et al. Plasma 1,25‐dihydroxyvitamin d and the risk of developing hypertension: the prevention of renal and vascular end‐stage disease study. Hypertension. 2015;66(3):563‐570. [DOI] [PubMed] [Google Scholar]
- 37. Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin‐angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49(4):460‐467. [DOI] [PubMed] [Google Scholar]
- 38. Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction: role of nephrogenesis. Kidney Int. 2004;65(4):1339‐1348. [DOI] [PubMed] [Google Scholar]
- 39. Gan Y, Li H, Ma Y, Liu X. Analysis and Research on the trend of climate change in Northern Xinjiang in the past 50 years. Yunnan Geo Environ Res. 2012;24(6):80‐86. [Google Scholar]
- 40. Liu L, Liu L, Ding Y, et al. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO cardiovascular diseases and alimentary comparison (CARDIAC) study. Hypertens Res. 2001;24(3):315‐322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1


