Abstract
The use of some anti‐hypertensive drugs in the current COVID‐19 pandemic has become controversial. This study investigated possible relationships between anti‐hypertensive medications use and COVID‐19 infection risk in the ambulatory hypertensive population. This is a population‐based retrospective cohort study involving 34 936 hypertensive adults >50 years in Tarragona (Southern Catalonia, Spain) who were retrospectively followed through pandemic period (from 01/03/2020 to 30/04/2020). Two data sets including demographic/clinical characteristics (comorbidities and cardiovascular medications use) and laboratory PCR codes for COVID‐19 were linked to construct an anonymized research database. Cox regression was used to calculate multivariable hazard ratios (HRs) and estimate the risk of suffering COVID‐19 infection. Across study period, 205 PCR‐confirmed COVID‐19 cases were observed, which means an overall incidence of 586.8 cases per 100 000 persons‐period. In multivariable analyses, only age (HR: 1.03; 95% CI: 1.02‐1.05; P < .001) and nursing home residence (HR: 19.60; 95% CI: 13.80‐27.84; P < .001) appeared significantly associated with increased risk of COVID‐19. Considering anti‐hypertensive drugs, receiving diuretics (HR: 1.22; 95% CI: 0.90‐1.67; P = .205), calcium channel blockers (HR: 1.29; 95%CI: 0.91‐1.82; P = .148), beta‐blockers (HR: 0.97; 95% CI: 0.68‐1.37; P = .844), and angiotensin‐converting enzyme inhibitors (HR: 0.83; 95% CI: 0.61‐1.13; P = .238) did not significantly alter the risk of PCR‐confirmed COVID‐19, whereas receiving angiotensin II receptor blockers was associated with an almost statistically significant reduction risk (HR: 0.67; 95% CI: 0.44‐1.01; P = .054). In conclusion, our data support that receiving renin‐angiotensin‐aldosterone system inhibitors does not predispose for suffering COVID‐19 infection in ambulatory hypertensive people. Conversely, receiving angiotensin II receptor blockers could be related with a reduced risk.
Keywords: angiotensin II receptor blockers, angiotensin‐converting enzyme inhibitors, anti‐hypertensive medication, COVID‐19, hypertension, SARS‐COV‐2
1. INTRODUCTION
At present, despite enormous efforts worldwide focused on the current COVID‐19 global pandemic, available clinical data on this concern are limited. 1 Most available clinical information is hospital‐based data derived from severe cases, 2 , 3 but there is scarce population or community‐based data involving a wide representative sample of infected people.
There is general accordance considering several pre‐existing conditions (ie, cardiovascular or respiratory diseases, diabetes, and obesity) as major risk conditions related with poor outcomes (need of hospitalization/ICU admission or death) in COVID‐19 patients. 2 , 3
Considering hypertension, which is the most common comorbidity in middle‐aged and older adults (who suffer the greatest burden of fatal COVID‐19 cases), a possible beneficial/harm effect using some first‐line anti‐hypertensive medications such as angiotensin‐converting enzyme inhibitors (ACEIs) and/or angiotensin II receptor blockers (ARBs) has become controversial for the management of hypertensive patients in the current COVID‐19 pandemic period. 4 , 5 , 6
Since ACE2 was identified as functional receptors to SARS‐CoV‐2, 7 it has been speculated that renin‐angiotensin‐aldosterone system (RAAS) inhibition could increase ACE2 expression and, thus, ACEIs/ARBs might be harmful in patients with COVID‐19. 4 , 5 , 6 However, most recent publications have not found evidence of harm with the continued use of RAAS inhibitors. 8 , 9 , 10 Of note, while some studies have examined the relationship between the ambulatory use of RAAS inhibitors and severe outcomes (hospital or ICU admission or death), 8 , 9 , 10 there is less evidence about the association with mild outcomes (such as COVID‐19 cases not requiring hospital admission).
In this context, the present study’s aim was to investigate population‐based incidence and risk of suffering COVID‐19 infection in a large and representative cohort (population‐based) including 34 936 ambulatory adults >50 years with hypertension, evaluating incidence and risk according to distinct anti‐hypertensive drugs use and concomitant comorbidities/underlying conditions.
2. METHODS
2.1. Design, setting, and study population
This is a retrospective cohort study including 34 936 individuals ≥50 years old with clinical diagnosis of hypertension in the region of Tarragona (a residential‐industrial urban area in Southern Catalonia, Spain, with an overall population of 210 672 all‐age inhabitants). Study subjects were all persons >50 years old registered in the 12 participant primary care centers (PCCs) managed by the Institut Català de la Salut (ICS) in the study area (Tarragonès, Alt Camp and Conca de Barberà counties) who had an ICD‐10 diagnosis code for hypertension (I10, I11, I12, or I15) coded in their electronic PCC clinical record before study started (01/03/2020). In the study region, there are 16 PCCs overall. Of them, 12 PCCs (those included in this study) are managed by the ICS, whereas the remaining 4 PCCs are managed by other providers and were not included in the present study. Reference laboratory and hospital for the 12 participating PCCs are the Hospital Universitari Joan XXIII and its Microbiological Service. Of note, in the Spanish Health System almost all inhabitants (near 98%) are affiliated to a PCC. Therefore, this study cohort represents the vast majority of hypertensive inhabitants in the study area.
Cohort members were followed from 01/03/2020 (the start of the epidemic period in the study area) until the occurrence of any study event (COVID‐19 diagnosis) or until the end of follow‐up on 30/04/2020. The study was approved by the ethical committee of the Institution (ethics committee IDIAP Jordi Gol, Barcelona, file 20/065‐PCV) and was conducted in accordance with the general principles for observational studies. 11
2.2. Data sources
The pre‐existing CAPAMIS Research Database, an institutional clinical research database previously used for other cohort studies in the study area, 12 was updated for use as the main data source in this COVID‐19 epidemiological investigation. This research database compiles data from the institutional PCCs’ clinical records system in the study area (working since the 2000s), including administrative data and clinical information coded according to the International Classification of Diseases 10th Revision (ICD‐10). It was used to identify cohort members, to establish baseline sociodemographical characteristics, comorbidities, underlying conditions, and active medications among study subjects at the start of the study (01/03/2020).
When the COVID‐19 pandemic period started in the study area, two electronic alerts including COVID‐19’s laboratory registries plus ICD‐10 codes for COVID‐19 suspicion (B34.2: unspecified coronavirus infection; B97.29: Other coronavirus as the cause of diseases classified elsewhere) were added to the electronic PCCs clinical records system and later both data sources were linked to construct an anonymized research database used for this report (which includes data from 1st March to 30 April 2020).
2.3. Outcomes
Primary outcome was the occurrence of laboratory‐confirmed COVID‐19 infection (ie, patients with a positive test for SARS‐CoV‐2 RT by PCR). For descriptive analysis, we also reported laboratory‐excluded COVID‐19 cases (PCR performed with a negative result) and presumptive cases (persons with clinical suspicion without PCR being performed).
For laboratory diagnosis of COVID‐19 by RT‐PCR, guidelines from the Health Department of the Generalitat de Catalunya were followed. 13 Briefly, from the samples collected by nasal and pharyngeal swab with transport medium for viruses and refrigerated at 4°C for a maximum of 48 hours, the RT‐PCR technique Cobas© SARS‐CoV‐2 with CE‐FDA marking was performed. 14 At the time of the study, the availability of PCR testing was scarce in the study region and PCR tests were mainly prioritized for severe case patients admitted to hospital and nursing home residences (where several outbreaks occurred). Whereas less PCR test was performed among possible cases managed as outpatient. Laboratory‐excluded cases (PCR tested with negative result) included persons with initial clinical suspicion (compatible symptomatology) but also asymptomatic persons with a history of contact with a PCR‐confirmed COVID‐19 case.
2.4. Covariates
Baseline characteristics of cohort members (age, sex, residence [community‐dwelling/nursing home], presence of pre‐existing comorbidities and medications) were established according to data registered in the electronic PCCs clinical records system on 01/03/2020.
The following comorbidities/underlying conditions were considered: neurological disease (including dementia and stroke), renal disease (including chronic renal failure), cancer (solid organ or hematological neoplasia diagnosed in the previous 5 years), systemic autoimmune rheumatic diseases (including rheumatoid arthritis and lupus), chronic respiratory disease (including chronic bronchitis/emphysema and asthma), heart disease (including congestive heart failure, coronary artery disease and other cardiopathies), atrial fibrillation, liver disease (including chronic hepatitis and cirrhosis), diabetes mellitus, hypercholesterolemia, obesity, and smoking.
Active medication treatments, coded according to the Anatomical, Therapeutic, and Chemical classification system (ATC) of the World Health Organization 15 were identified from the patient treatment plan registered in the PCC’s clinical records system, and included the following therapeutic groups: anti‐hypertensive (diuretics, beta‐blockers, calcium channel blockers, ACEIs, and ARBs), statins, anticoagulants (warfarin and new oral anticoagulant drugs), antiplatelet drugs, antidiabetic drugs (insulin, oral antidiabetic drugs), antineoplastic agents, corticosteroids, non‐steroidal anti‐inflammatory drugs, inhaled drugs for respiratory disease, antihistamines, proton‐pump inhibitors, and benzodiazepines. A comorbidity and/or medication was considered absent if it was not recorded in the electronic PCC’s clinical record (see Table S1).
2.5. Statistical analyses
Incidence rates (IRs) of PCR‐confirmed COVID‐19 were calculated per 100 000 person‐period (8.7 weeks). Age‐adjusted IRs were calculated by the standardized direct method (using the age‐structure of the Tarragona population as reference). In bivariate analyses, baseline characteristics of suffering or not a PCR‐confirmed COVID‐19 infection were compared using Chi‐squared or Fisher’s test as appropriate. Cox regression models (including all above mentioned covariates: age, sex, residence, pre‐existing comorbidities and medications use) were constructed to calculate unadjusted and multivariable‐adjusted hazard ratios (HRs) and estimate the risk of suffering COVID‐19 infection. 16 We performed a main analysis including the total study cohort (N = 34 936) and two subgroup analyses focused on community‐dwelling individuals (N = 33 991) and nursing home residents (N = 945). Statistical significance was set at P < .05 (two‐tailed). Data were performed by using IBM SPSS Statistics for Windows, version 24 (IBM Corp., Armonk, N.Y., USA).
3. RESULTS
Of the 34 936 hypertensive cohort members, 48.1% were men and mean age was 70.9 years (standard deviation: 11.3). Overall, 5173 (14.8%) did not receive any anti‐hypertensive drugs, 17 711 (50.7%) received one, 8904 (25.5%) two, 2820 (8.1%) three, and 328 (0.9%) four.
Across the study period, 855 cohort members were PCR tested. Of them, 650 (76%) presented a negative result and 205 (24%) a positive result. Additionally, 137 study subjects were coded as presumptive COVID‐19 cases (clinical suspicion without PCR performed) (Table 1).
Table 1.
Distribution of laboratory‐confirmed and laboratory‐excluded COVID‐19 cases according to baseline demographical and clinical characteristics in the study cohort (N = 34 936)
| Characteristic |
Study population (N = 34 936) n (%) |
PCR positive (N = 205) n (%) |
PCR negative (N = 650) n (%) |
P value |
|---|---|---|---|---|
| Age | <.001 | |||
| 50‐54 y | 2993 (8.6) | 9 (4.4) | 28 (4.3) | |
| 55‐59 y | 4044 (11.6) | 8 (3.9) | 42 (6.5) | |
| 60‐64 y | 4697 (13.4) | 6 (2.9) | 55 (8.5) | |
| 65‐69 y | 4994 (14.3) | 9 (4.4) | 47 (7.2) | |
| 70‐74 y | 5483 (15.7) | 12 (5.9) | 56 (8.6) | |
| 75‐79 y | 4732 (13.5) | 33 (16.1) | 98 (15.1) | |
| 80‐84 y | 3406 (9.7) | 27 (13.2) | 105 (16.2) | |
| 85‐89 y | 2872 (8.2) | 44 (21.5) | 104 (16.0) | |
| ≥90 y | 1715 (4.9) | 57 (27.8) | 115 (17.7) | |
| Sex | .618 | |||
| Men | 16805 (48.1) | 83 (40.5) | 276 (42.5) | |
| Women | 18131 (51.9) | 122 (59.5) | 374 (57.5) | |
| Community‐dwelling | 33991 (97.3) | 102 (49.8) | 421 (64.8) | <.001 |
| Nursing home residence | 945 (2.7) | 103 (50.2) | 229 (35.2) | |
| Nº comorbidities | .276 | |||
| 0 | 7235 (20.7) | 31 (15.1) | 94 (14.5) | |
| 1 | 10705 (30.6) | 49 (23.9) | 125 (19.2) | |
| 2 | 8780 (25.1) | 53 (25.9) | 177 (27.2) | |
| 3 | 4852 (13.9) | 32 (15.6) | 116 (17.8) | |
| 4 or more | 3364 (9.6) | 40 (19.5) | 138 (21.2) |
P values were calculated by using Pearson’s Chi square test for categorical variables (sex and residence) or Chi square trend for ordinal variables (age and number of comorbidities).
Of the 205 PCR‐confirmed COVID‐19 cases, 83 (40.5%) occurred in men. By age, 23 (11.2%) occurred in 50‐64 years, 54 (26.3%) in 65‐79 years, and 128 (62.4%) in people aged 80 years or older. By residence, 102 (49.8%) occurred in community‐dwelling individuals and 103 (50.2%) in nursing home residents. This means an overall IR of 586.8 PCR‐confirmed COVID‐19 cases per 100 000 persons‐period (195.9 in 50‐64 years vs 355.2 in 65‐79 years vs 1601.4 in 80 years or older; 493.9 in men vs 672.9 in women; 300.1 in community‐dwelling vs 10 899.5 in nursing home residents). According to the number of concomitant comorbidities (apart from hypertension), crude IRs were 428.5, 457.7, 603.6, 659.5, and 1189.1 for persons with zero, one, two, three, and four or more comorbidities, respectively.
The most prevalent co‐existing comorbidities among the 205 hypertensive patients with COVID‐19 were chronic heart disease (41.5%) and hypercholesterolemia (41.5%) followed by diabetes (33.7%), obesity (32.2%), and neurological disease (21.5%).
Table 2 shows study population, number of cases, and specific IRs (unadjusted and age‐adjusted) by distinct comorbidities and medications’ use.
Table 2.
Absolute number of cases and incidence rates for PCR‐confirmed COVID‐19 cases according to baseline demographical and clinical characteristics in the total study cohort (N = 34 936)
| Characteristic |
Study population (N = 34 936) n (%) |
PCR‐confirmed COVID‐19 cases (n = 205) | |||
|---|---|---|---|---|---|
|
No. of cases n (%) |
P value | Incidence Rates | |||
| Crude | Age‐adjusted | ||||
| Sex | |||||
| Men | 16805 (48.1) | 83 (40.5) | .029 | 493.9 | 449.5 |
| Women | 18131 (51.9) | 122 (59.5) | 672.9 | 457.3 | |
| Community‐dwelling | 33991 (97.3) | 102 (49.8) | <.001 | 300.1 | 272.5 |
| Nursing home residence | 945 (2.7) | 103 (50.2) | 10899.5 | 4227.5 | |
| Neurological disease | 1686 (4.8) | 44 (21.5) | <.001 | 2609.8 | 934.5 |
| Renal disease | 3938 (11.3) | 39 (19.0) | <.001 | 990.4 | 942.2 |
| Cancer in past 5 y | 3851 (11.0) | 31 (15.1) | .060 | 805.0 | 617.6 |
| Rheumatic disease | 461 (1.3) | 2 (1.0) | .665 | 433.8 | 522.9 |
| Respiratory disease | 4128 (11.8) | 42 (20.5) | <.001 | 1017.4 | 757.3 |
| Cardiac disease | 10097 (28.9) | 85 (41.5) | <.001 | 841.8 | 545.5 |
| Atrial fibrillation | 2960 (8.5) | 36 (17.6) | <.001 | 1216.2 | 679.9 |
| Liver disease | 638 (1.8) | 3 (1.5) | .697 | 470.2 | 401.9 |
| Hypercholesterolemia | 16522 (47.3) | 85 (41.5) | .094 | 541.5 | 390.2 |
| Diabetes | 9829 (28.1) | 69 (33.7) | .078 | 702.0 | 508.2 |
| Smoking | 4473 (12.8) | 12 (5.9) | .003 | 268.3 | 564.1 |
| Obesity | 14416 (41.3) | 66 (32.2) | .008 | 457.8 | 396.2 |
| Diuretics | 7495 (21.5) | 81 (39.5) | <.001 | 1080.7 | 674.2 |
| Beta‐blockers | 7723 (22.1) | 48 (23.4) | .651 | 621.5 | 502.1 |
| ACEIs | 15332 (43.9) | 77 (37.6) | .067 | 502.2 | 401.7 |
| ARBs | 8541 (24.4) | 33 (16.1) | .005 | 386.4 | 325.1 |
| Calcium channel blockers | 6201 (17.7) | 44 (21.5) | .163 | 709.6 | 579.9 |
| Statins | 11328 (32.4) | 44 (21.5) | .001 | 388.4 | 342.0 |
| Insulin | 2330 (6.7) | 23 (11.2) | .009 | 987.1 | 920.1 |
| Oral antidiabetic | 7943 (22.7) | 41 (20.0) | .349 | 516.2 | 373.5 |
| Warfarin | 1788 (5.1) | 18 (8.8) | .017 | 1006.7 | 1065.2 |
| New oral anticoagulant | 1233 (3.5) | 12 (5.9) | .070 | 973.2 | 466.9 |
| Antiplatelet drugs | 6779 (19.4) | 55 (26.8) | .007 | 811.3 | 517.2 |
| Inhaled respiratory therapy | 3728 (10.7) | 38 (18.5) | <.001 | 1019.3 | 706.8 |
| Antineoplastic agents | 859 (2.5) | 7 (3.4) | .375 | 814.9 | 681.6 |
| Corticosteroids | 780 (2.2) | 3 (1.5) | .455 | 384.6 | 524.5 |
| NSADs | 1650 (4.7) | 4 (2.0) | .061 | 242.4 | 234.8 |
| Benzodiazepines | 7471 (21.4) | 57 (27.8) | .025 | 763.0 | 482.9 |
| Antihistamines | 1579 (4.5) | 3 (1.5) | .035 | 190.0 | 148.3 |
| Proton‐Pump Inhibitors | 11807 (33.8) | 99 (48.3) | <.001 | 838.5 | 541.2 |
Incidence was calculated per 100,000 persons‐period (8.7 wk).
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; NSADs, non‐steroidal anti‐inflammatory drugs.
Considering anti‐hypertensive medication use, greater incidence rates (age‐adjusted) appeared among those receiving diuretics (674.2), calcium channel blockers (579.9), and beta‐blockers (502.1), whereas lower incidence appeared among those receiving ACEIs (401.7) and ARBs (325.1). Figure 1 shows Kaplan‐Meier curves showing survival time free of COVID‐19 in patients with and without use of ARBs (P = .006 in Log Rank [Mantel‐Cox] test).
Figure 1.
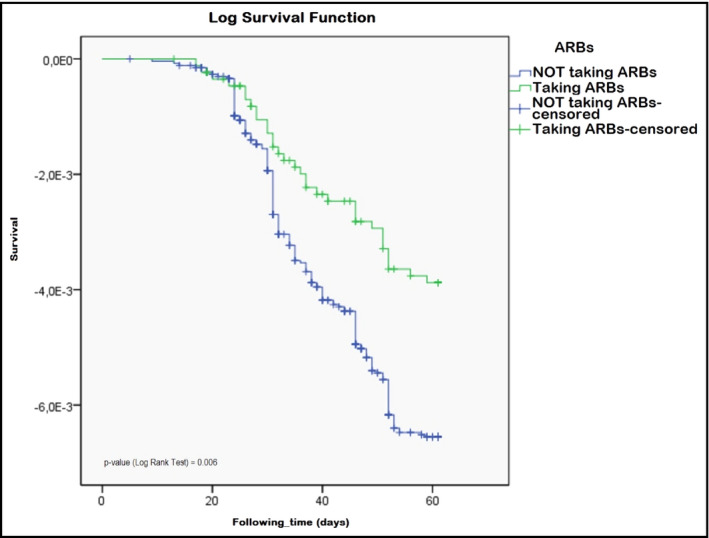
Kaplan‐Meier curves (survival free of COVID‐19 infection) comparing patients taking vs not taking angiotensin II receptor blockers (ARBs) treatment
Table 3 shows univariate and multivariate analyses assessing risk for suffering COVID‐19 by underlying conditions and medications use in the total study cohort. In the multivariable analysis, only age (HR: 1.03; 95% CI: 1.02‐1.05; P < .001), and nursing home residence (HR: 19.60; 95% CI: 13.80‐27.84; p < .001) were significantly associated with an increased risk of COVID‐19. Considering anti‐hypertensive drugs, receiving diuretics (HR: 1.22; 95% CI: 0.90‐1.67; P = .205), calcium channel blockers (HR: 1.29; 95%CI: 0.91‐1.82; P = .148), beta‐blockers (HR: 0.97; 95% CI: 0.68‐1.37; P = .844), and angiotensin‐converting enzyme inhibitors (HR: 0.83; 95% CI: 0.61‐1.13; P = .238) did not significantly alter the risk of COVID‐19, whereas receiving angiotensin II receptor blockers was associated with an almost statistically significant reduction risk (HR: 0.67; 95% CI: 0.44‐1.01; P = .054). No other cardiovascular medications were associated with increased or decreased adjusted risk for suffering COVID‐19 in the total study cohort.
Table 3.
Cox regression unadjusted and multivariable‐adjusted analyses estimating risk to suffer PCR‐confirmed COVID‐19 in the total study cohort (N = 34 936)
| Characteristic | Cox regression analysis (n = 205 COVID‐19 cases) | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Sociodemographical | ||||
| Age | 1.09 (1.08‐1.11) | <.001 | 1.03 (1.02‐1.05) | <.001 |
| Sex | ||||
| Women | 1.36 (1.03‐1.80) | .029 | 0.83 (0.61‐1.13) | .229 |
| Nursing home residence | 38.07 (28.95‐50.06) | <.001 | 19.60 (13.80‐27.84) | <.001 |
| Comorbidities | ||||
| Neurological disease | 5.44 (3.90‐7.59) | <.001 | 1.27 (0.88‐1.83) | .211 |
| Renal disease | 1.85 (1.31‐2.63) | .001 | 0.82 (0.57‐1.20) | .311 |
| Cancer in past 5 y | 1.44 (0.98‐2.11) | .061 | 1.08 (0.72‐1.61) | .721 |
| Rheumatic disease | 0.74 (0.18‐2.97) | .668 | 0.83 (0.20‐3.47) | .795 |
| Respiratory disease | 1.93 (1.37‐2.71) | <.001 | 1.39 (0.88‐2.20) | .161 |
| Cardiac disease | 1.75 (1.32‐2.30) | <.001 | 1.00 (0.73‐1.36) | .981 |
| Atrial fibrillation | 2.31 (1.61‐3.31) | <.001 | 0.99 (0.57‐1.72) | .975 |
| Liver disease | 0.80 (0.26‐2.50) | .700 | 0.94 (0.30‐2.96) | .910 |
| Hypercholesterolemia | 0.79 (0.60‐1.04) | .094 | 0.85 (0.63‐1.14) | .283 |
| Diabetes | 1.30 (0.97‐1.73) | .079 | 1.34 (0.85‐2.11) | .210 |
| Smoking | 0.42 (0.24‐0.76) | .004 | 0.81 (0.44‐1.50) | .497 |
| Obesity | 0.68 (0.50‐0.91) | .675 | 0.96 (0.71‐1.31) | .794 |
| Chronic medications use | ||||
| Diuretics | 2.40 (1.81‐3.17) | <.001 | 1.22 (0.90‐1.67) | .205 |
| Beta‐blockers | 1.08 (0.78‐1.49) | .648 | 0.97 (0.68‐1.37) | .844 |
| ACEIs | 0.77 (0.58‐1.02) | .068 | 0.83 (0.61‐1.13) | .238 |
| ARBs | 0.59 (0.41‐0.86) | .006 | 0.67 (0.44‐1.01) | .054 |
| Calcium channel blockers | 1.27 (0.91‐1.77) | .166 | 1.29 (0.91‐1.82) | .148 |
| Statins | 0.57 (0.41‐0.79) | .001 | 0.85 (0.58‐1.25) | .412 |
| Insulin | 1.77 (1.15‐2.73) | .010 | 1.15 (0.68‐1.93) | .602 |
| Oral antidiabetic | 0.85 (0.60‐1.20) | .348 | 0.78 (0.47‐1.27) | .316 |
| Warfarin | 1.79 (1.10‐2.90) | .019 | 1.22 (0.65‐2.31) | .533 |
| New oral anticoagulant | 1.71 (0.95‐3.06) | .073 | 1.09 (0.50‐2.38) | .824 |
| Antiplatelet drugs | 1.53 (1.12‐2.08) | .007 | 1.13 (0.78‐1.64) | .517 |
| Inhaled respiratory therapy | 1.91 (1.35‐2.72) | <.001 | 1.12 (0.70‐1.80) | .643 |
| Antineoplastic agents | 1.40 (0.66‐2.98) | .378 | 1.45 (0.65‐3.26) | .364 |
| Corticosteroids | 0.65 (0.21‐2.03) | .458 | 0.48 (0.15‐1.55) | .220 |
| NSADs | 0.40 (0.15‐1.08) | .071 | 0.91 (0.33‐2.49) | .856 |
| Benzodiazepines | 1.42 (1.05‐1.93) | .025 | 1.15 (0.84‐1.58) | .380 |
| Antihistamines | 0.31 (0.10‐0.98) | .046 | 0.39 (0.12‐1.21) | .103 |
| Proton‐Pump Inhibitors | 1.83 (1.39‐2.41) | <.001 | 1.09 (0.80‐1.50) | .578 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CIs, confidence intervals; HRs, hazard ratios and were calculated for those who had the condition as compared who had not the condition; NSADs, non‐steroidal anti‐inflammatory drugs.
In subgroup analysis restricted to community‐dwelling hypertensive people, 523 people were PCR tested. Of them, 421 (80.5%) presented a negative result and 102 (19.5%) a positive result. In the multivariable analysis, chronic respiratory disease (HR: 2.47; 95% CI: 1.25‐4.87; P = .009) was the unique concomitant comorbidity that emerged associated with a significant increased risk for suffering COVID‐19. Receiving ARBs emerged also at decreased risk but the result did not reach statistical significance (HR: 0.71; 95% CI: 0.42‐1.19; P = .189). Receiving other anti‐hypertensive drugs or other medications did not significantly alter the risk for suffering COVID‐19 neither (see Table S2).
In subgroup analysis restricted to 945 nursing home residents with hypertension (Table S3), a total of 332 persons were PCR tested. Of them, 229 were excluded by a PCR negative result and 103 were PCR‐confirmed COVID‐19 cases. In the multivariable analysis, receiving ARBs appeared also associated with an almost significant reduction risk (HR: 0.51; 95% CI: 0.25‐1.01; P = .053).
4. DISCUSSION
We constructed a rapid population‐based retrospective cohort study to explore possible protective/harm effects of pre‐existing medical conditions (including common comorbidities and medications use) among middle‐aged and older adults with hypertension in Tarragona (Southern Catalonia, Spain), a region with an intermediate intensity of COVID‐19 pandemic across March‐April 2020. 17 The study provides unusual population‐based incidence and multivariable‐adjusted data involving the general adult population ≥50 years with diagnosis of hypertension. Recognizing several limitations (as discussed below), data may conform an acceptable basis in assessing potential health benefits/harms using common anti‐hypertensive and cardiovascular medications in relation to the current COVID‐19 pandemic.
During the study period, more than 2% of overall cohort members were PCR tested (with 205 positive and 650 negative results). Assuming these data and considering that PCR tests were scarcely available in the study area for patients with less severe symptoms, the true incidence of COVID‐19 was logically underestimated (although it may be selectively more or less underestimated depending on the distinct subgroups). Nevertheless, considering the relatively low number of presumptive cases (clinical suspicion alone without PCR performed), our data also suggest that the overall number of the infected population (definitive plus presumptive) may be considerably lower than speculated. 17
In this study, crude incidence rates by concomitant comorbidities largely reflect the excess baseline‐risk profile related with the great number of cases observed among elderly persons and, especially, nursing home residents (where several outbreaks occurred and accounted for approximately fifty percent of overall COVID‐19 cases). The greater number of cases observed among elderly persons may be also related with the fact that this age group usually suffers from the more severe forms and therefore was more tested than younger individuals. A similar interpretation may be also applied to the great number of cases observed among persons with multiple comorbidities.
Considering anti‐hypertensive medications use, greater COVID‐19 incidence (crude and age‐adjusted) appeared among those receiving diuretics or calcium channel blockers, whereas lower incidence appeared among those receiving ACEIs and ARBs (who suffered the lowest COVID‐19 incidence).
After multivariable adjustments, apart from nursing home residence and increasing age (which increased approximately a 3% for each year the risk of PCR‐confirmed COVID‐19), only concomitant chronic respiratory disease emerged independently associated with a significant greater risk assessing community‐dwelling individuals. Most of the analyzed comorbidities (eg, cancer, cardiac or respiratory disease, and diabetes) were related with an increased risk of COVID‐19 in the unadjusted analysis (HRs greater than one), although this association did not reach statistical significance after multivariable adjustment. There is general agreement about considering these conditions as major risk conditions related with poor prognosis in hospitalized COVID‐19 patients 2 , 3 , 18 but there is uncertainty about assessing the role of these conditions to predispose for suffering infection. 19
In the adjusted analyses, receiving ARBs was the unique covariable that appeared associated (marginally significant) with a decreased risk for COVID‐19 infection, as in the total study cohort as well as in community‐dwelling individuals and nursing home residents. No other medication, neither anti‐hypertensive nor other cardiovascular medication, was associated with an increased or decreased risk of COVID‐19 in the multivariable analysis assessing the total study cohort.
If we consider the 3 performed multivariable‐adjusted analyses (overall cohort, community‐dwelling, and nursing home residents), we found that receiving diuretics (HRs ranging from 1.12 to 1.34) or calcium channel blockers (HRs ranging from 1.20 to 1.29) were related with a non‐significant increased risk. In contrast, receiving ACEIs (HRs ranging between 0.72 and 0.90) and especially ARBs (HRs ranging between 0.53‐0.71) were associated with non‐significant or nearly significant decreased risk, respectively. We note, however, that the number of cases was small and confidence intervals were wide, which makes possible interpretations difficult. There is some evidence suggesting that inhibiting the formation or blocking the action of angiotensin II could prevent acute respiratory distress syndrome and this finding would support a beneficial effect in patients with severe COVID‐19 20 ; however, a biological explanation for a reduced risk of infection is uncertain.
Concerns have been raised about the possible association between the use of RAAS inhibitors (mainly ACEIs and/or ARBs) with the risk of SARS‐CoV‐2 infection, the risk of severe COVID‐19 among those infected, or the risk of in‐hospital death among those with a positive test. 4 , 5 , 6 Several studies have concluded that there is no evidence of poor outcomes (increased severity or in‐hospital death) in patients infected with SARS‐CoV‐2 taking RAAS inhibitors. 8 , 9 , 10 A recent review even concluded that ACEIs and ARBs may be associated with lower incidence and/or improved outcome in patients with lower respiratory tract infection, 20 But there are not data assessing specifically whether the previous use of RAAS inhibitors affects the likelihood of COVID‐19 infection in ambulatory patients. While our results did not reach statistical significance (marginally significative for ARBs) and study interpretation needs to consider the potential for residual confounders, our data support that ambulatory use of RAAS inhibitors does not increase the susceptibility for COVID‐19 infection.
Our results are in accordance with data reported in two recent large population‐based case‐control studies conducted in Lombardy (Italy) and Madrid (Spain), where neither ACEIs nor ARBs were associated with the likelihood of SARS‐CoV‐2 infection in hospitalized patients. 8 , 9 . As main difference, we included all PCR positive cases (hospitalized or outpatients). We also note that our study outcome is the occurrence of laboratory‐confirmed COVID‐19 infection and not COVID‐19 severity, which difficult comparison between studies.
A large study conducted in New York (USA) aimed to estimate the association between the use of anti‐hypertensive medications (concretely, tiacidic diuretics, ACEIs, ARBs, beta‐blockers, and calcium channel blockers) and the likelihood of a positive test for COVID‐19 did not find a significant association between the previous use of any of the analyzed anti‐hypertensive drugs with a higher likelihood to tested positive for COVID‐19. 10 Another study has reported lower risk of all‐cause mortality among COVID‐19‐hospitalized patients receiving RAAS inhibitors. 21 Other studies have concluded that there is no clinical or experimental evidence supporting that ARBs and ACEIs either augment the susceptibility to SARS‐CoV‐2or aggravate the severity and outcomes of COVID‐19 at present. 22 Indeed, a recent review has concluded that ACEIs and ARBs may be associated with lower incidence and/or improved outcome in patients with lower respiratory tract infections. 20 Our data agree with the above mentioned studies and support the use of ACEIs/ARBs in hypertensive ambulatory persons in the current COVID‐19 pandemic.
Of note, in the present study, other cardiovascular medications (ie, statins, antiplatelet, warfarin, or other new oral anticoagulant drugs) used by hypertensive patients before exposure did not significantly alter the risk of COVID‐19 infection. Studies analyzing the influence of the use of these drugs and the COVID‐19 infection are scarce and mostly are focused on interactions with antiviral therapy. 23 , 24
Major strengths of this study were its population‐based design and the use of multivariable analysis methods (Cox regression) to accurately estimate possible relationships between PCR‐confirmed COVID‐19 and common cardiovascular medications. Main limitations are related with its observational nature and retrospective design. Unfortunately, the availability of PCR testing was scarce in the study area across the study period. Obviously, selection bias may not be excluded considering that PCR testing was not routinely performed on all hypertensive patients. We made subgroup analysis (community‐dwelling/nursing home) and multivariable adjustments but, as all observational studies, a residual confounding in risk estimates may not be completely excluded. Although we adjusted for many baseline potential confounding factors in our multivariable analyses, other potentially important confounders (eg, epidemiological, socio‐economical, or work‐related factors) have not been considered because they were unavailable for us at present. Of note, COVID‐19 cases presented in this report are those occurring in patients with positive for SARS‐CoV‐2 RT by PCR, independently from pre‐existing symptoms. We do not have data about clinical course (hospitalization/ICU admission or death), and consequently, the study was not able to assess the degree of severity of the cases. Despite the large size of the study cohort, the present study includes relatively few events (n = 205) which limits statistical power, especially in subgroup analysis. The study was conducted in a single geographical setting and logically specific incidence data may not be directly extrapolated to other geographical regions with distinct epidemic conditions. Nevertheless, adjusted risk estimates may be helpful to better characterize risk profile of suffering COVID‐19 infection (independently of the severity) in hypertensive ambulatory patients in relation with common anti‐hypertensive medications (ie, diuretics, beta‐blockers, ACEIs, ARBs, calcium channel blockers) adjusted for major concomitant comorbidities/underlying conditions.
In conclusion, increasing age and nursing home residence appear as major conditions related to a higher risk of suffering COVID‐19 infection among hypertensive people. Our data support the fact that receiving RAAS inhibitors does not increase the risk of suffering SARS‐COV‐2 infection among middle‐aged and older adults with hypertension. Receiving ARBs was related with a reduced risk, although it must be emphasized that statistical significance for benefit of ARBs was not achieved and caution is needed to interpret this result. Other anti‐hypertensive (diuretics, beta‐blockers, and calcium channel blockers) or cardiovascular medications (oral anticoagulant, antiplatelet, and statins) did not significantly alter COVID‐19 infection risk in community‐dwelling and/or nursing home residents with hypertension. Data support maintaining RAAS inhibitors (even prioritizing them) to treat hypertensive patients in the current era of COVID‐19 global pandemic, as experts and scientific societies have recommended. 25 , 26 , 27 , 28
CONFLICT OF INTEREST
All authors declared that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
A.Vila‐Corcoles designed the study and wrote the manuscript; C. Torrente‐Fraga and F. Gomez‐Bertomeu obtained data; E. Satue‐Gracia, I. Hospital‐Guardiola, C. de Diego‐Cabanes, and D. Rovira‐Veciana assessed outcomes; O. Ochoa‐Gondar and A. Vila‐Rovira did statistical analyses; F. Gomez‐Bertomeu revised pharmacological data; A.Vila‐Corcoles and J. Basora‐Gallisa coordinated the study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the ethical committee of the Institution (ethic committee IDIAP Jordi Gol, Barcelona, file 20/065‐PCV) and was conducted in accordance with the general principles for observational studies. Given this is a non‐interventional study, an informed consent for all 2 025 730 study participants was not required. Data were anonymized, and risk of identification was null.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Timothy Bowring who helped write the article in English and Joan Fort who helped to improve graphic’s quality.
Vila‐Corcoles A, Satue‐Gracia E, Ochoa‐Gondar O, et al. Use of distinct anti‐hypertensive drugs and risk for COVID‐19 among hypertensive people: A population‐based cohort study in Southern Catalonia, Spain. J Clin Hypertens. 2020;22:1379–1388. 10.1111/jch.13948
Data Availability Statement
The datasets used and analyzed during the current study will be available from the corresponding author on reasonable request.
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID‐19): situation report‐87, https://www.who.int/docs/default‐source/coronaviruse/situation‐reports/20200416‐sitrep‐87‐covid‐19.pdf?sfvrsn=9523115a_2 (2020 [accessed 10 May 2020].
- 2. Yang J, Zheng Y, Gou X, et al, Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopes RD, Macedo AVS, de Barros E, et al. Continuing versus suspending angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) [published online ahead of print, 2020 May 13]. Am Heart J. 2020.49‐59 10.1016/j.ahj.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esler M, Esler D. Can angiotensin receptor‐blockingdrugsperhaps be harmful in the COVID‐19 pandemic? J Hypertens. 2020;38(5):781‐782. [DOI] [PubMed] [Google Scholar]
- 6. Versmissen J, Verdonk K, Lafeber M, et al. Angiotensin‐converting enzyme‐2 in SARS‐CoV‐2 infection: goodorbad? J Hypertens. 2020;38:1196‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wan Y, Shang J, Graham R, et al. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade‐long structural studies of SARS coronavirus. J Virol. 2020;94:e00127‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancia G, Rea F, Ludergnani M, et al. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Abajo FJ, Rodríguez‐Martín S, Lerma V, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study [published online ahead of print, 2020 May 14]. Lancet. 2020;395(10238):1705‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐Angiotensin‐aldosterone system inhibitors and risk of Covid‐19 [published online ahead of print, 2020 May 1]. N Engl J Med. 2020;382(25):2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Medical Association . WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. Available at: https://www.wma.net/policies‐post/wma‐declaration‐of‐helsinki‐ethical‐principles‐for‐medical‐research‐involving‐human‐subjects/. [Accessed May 14, 2020].
- 12. Vila‐Corcoles A, Hospital‐Guardiola I, Ochoa‐Gondar O, et al. Rationale and design of the CAPAMIS study: effectiveness of pneumococcal vaccination against community‐acquired pneumonia, acute myocardial infarction and stroke. BMC Public Health. 2010;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Generalitat de Catalunya . Sub‐direcció General de Vigilància i Resposta a Emergències de Salut Pública. Procediment d’actuació enfront de casos d’infecció pel nou coronavirus SARS‐CoV‐2. Available at: https://canalsalut.gencat.cat/web/.content/_A‐Z/C/coronavirus‐2019‐ncov/material‐divulgatiu/procediment‐actuacio‐coronavirus.pdf [Accessed May 16, 2020].
- 14. Lieberman JA, Pepper G, Naccache SN, et al. Comparison of commercially available and laboratory developed assays for in vitro detection of sars‐cov‐2 in clinical laboratories. J Clin Microbiol. 2020; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Who Collaborating Centre for Drug Statistics Methodology . ATC/DDD Index 2020, Available at: https://www.whocc.no/atc_ddd_index/ [Accessed May 12, 2020].
- 16. Hosmer DW, Lemeshow S. Applied Survival Analysis. Regression Modeling of Time to Event Data. New York, NY: John Wiley & Sons; 1999. [Google Scholar]
- 17. Consumo. Dirección General de Salud Pública, Calidad e innovación. Centro de Coordinación de Alertas y Emergencias Sanitarias . Información científica‐técnica. Enfermedad por coronavirus, COVID19. Actualización 17 de abril, Available at: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov‐China/documentos/20200417_ITCoronavirus.pdf [Accessed May 5, 2020].
- 18. Deng G, Yin M, Chen X, Zeng F. Clinical determinants for fatality of 44,672 patients with COVID‐19. Crit Care. 2020;24:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS‐CoV‐ 2: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kreutz R, Algharably EAE, Azizi M, et al. Hypertension, the renin–angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res. 2020:cvaa097. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and Angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19 [published online ahead of print, 2020 Apr 17]. Circ Res. 2020.126(12):1671‐1681 10.1161/CIRCRESAHA.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID‐19. Hypertens Res. 2020;43(7):648‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reiner Ž, Hatamipour M, Banach M, et al. Statins and the COVID‐19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16(3):490‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Testa S, Prandoni P, Paoletti O, et al. Direct oral anticoagulant plasma levels’ striking increase in severe COVID‐19 respiratory syndrome patients treated with antiviral agents: the Cremona experience. J Thromb Haemost. 2020:18: 1320–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB, Harrington DP. Inhibitors of the Renin‐Angiotensin‐Aldosterone system and Covid‐19 [published online ahead of print, 2020 May 1]. N Engl J Med. 2020;382(25):2462‐2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park S, Lee HY, Cho EJ, et al. Is the use of RAS inhibitors safe in the current era of COVID‐19 pandemic? Clin Hypertens. 2020;26:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. European Society of Cardiology . Position Statement of the ESC Council on Hypertension on ACE‐Inhibitors and Angiotensin Receptor Blockers, Available at: https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang [Accessed May 15, 2020].
- 28. American Heart Association . HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID‐19, Available at: https://professional.heart.org/professional/ScienceNews/UCM_505836_HFSAACCAHA‐statement‐addresses‐concerns‐re‐using‐RAAS‐antagonists‐in‐COVID‐19.jsp [Accessed May 15, 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The datasets used and analyzed during the current study will be available from the corresponding author on reasonable request.


