Abstract
Resistant hypertension was defined according to the 2008 scientific statement as office blood pressure ≥ 140/90 mm Hg and the 2018 scientific statement as office blood pressure ≥ 130/80 mm Hg. We investigated the prognostic significance of lowered blood pressure threshold for defining resistant hypertension in the 2018 American Heart Association scientific statement compared with that in the 2008 scientific statement. The participants of this prospective cohort were enrolled from December 2013 to November 2018. Major adverse cardiovascular events (MACEs) were defined as a composite of cardiovascular death, non‐fatal myocardial infarction, non‐fatal stroke, and heart failure hospitalization. Renal event was defined as a ≥ 50% decline in estimated glomerular filtration rate or progression to end‐stage renal disease. A total of 206 patients among 2018 (10.2%) were diagnosed with resistant hypertension by the previous definition (≥140/90 mm Hg), and 276 patients among 2011 (13.7%) were diagnosed with resistant hypertension by the updated definition (≥130/80 mm Hg). During a median follow‐up of 4.5 years, 33 MACEs (3.7 per 1000 patient‐years) and 164 renal events (19.9 per 1000 patient‐years) occurred in the study population. Treatment‐resistant hypertension groups had a higher incidence rate of MACEs and renal events than the control groups. In multivariate Cox proportional hazards regression analysis, resistant hypertension by both definitions was significantly associated with increased risk of MACE and renal event. Both the previous and updated definitions of resistant hypertension were significant predictors of MACEs and renal events. This finding supports the adoption of the updated criteria for resistant hypertension in clinical practice.
Keywords: major adverse cardiovascular event, office blood pressure, renal outcome, resistant hypertension
1. INTRODUCTION
Resistant hypertension is defined as blood pressure (BP) above treatment goals despite the concurrent use of 3 or more antihypertensive drugs, including diuretics, and also includes patients whose BP achieves target values on ≥4 antihypertensive drugs. 1 The prevalence of resistant hypertension is between 12% and 18% of the population. 1 , 2 , 3 It is more highly associated with high‐risk conditions such as diabetes mellitus (DM) and chronic kidney disease (CKD). 4 , 5 Resistant hypertension is associated with increased risk for end‐stage renal disease, cardiovascular events (CVEs), and mortality. 1 , 6 Therefore, correct identification and BP control in these high‐risk participants are imperative. Recently, the American College of Cardiology (ACC) and the American Heart Association (AHA) redefined hypertension as BP above 130/80 mm Hg. 7 Based on this new threshold, the 2018 scientific statement from the AHA lowered the BP threshold of resistant hypertension from above 140/90 mm Hg, based on the 2008 AHA definition, to above 130/80 mm Hg. 1 , 8 Since an important objective for diagnosing resistant hypertension is to identify a subset of high‐risk hypertensive participants, it is necessary to determine how much lowering of the BP threshold influences the risk profile of resistant hypertension. A previous study suggested that early and more intensive BP control contributes to better target organ protection and cardiovascular prevention in Asian populations. 9 However, it is unclear how lowering of the BP threshold changes the risks for CVEs and adverse renal outcomes, particularly in high‐risk hypertension patients. In a pooled analysis of patient‐level data of the Systolic Blood Pressure Intervention Trial and Action to Control Cardiovascular Risk in Diabetes trial, no significant difference was found in the risk for CVEs for treatment‐resistant hypertension based on the 2018 AHA definition versus the 2008 definition when the exposure time was ≥1.5 years. 10 However, these data were limited by not performing ambulatory blood pressure monitoring (ABPM) in the majority of the study participants. As white‐coat resistance is highly prevalent, the 2018 AHA statement and the 2018 European Society of Cardiology/European Society of Hypertension guidelines recommend the use of out‐of‐office BP measurements to rule out white‐coat hypertension before diagnosing resistant hypertension, and detection of masked uncontrolled hypertension, which is highly prevalent in Asian hypertensive populations, is also important. 1 , 11 , 12 , 13 Therefore, the objective of this study was to compare the prognostic significance between the 2018 and 2008 definitions of apparent treatment‐resistant hypertension (aTRH), confirmed by both office BP and ABPM, in a prospective cohort of high‐risk hypertensive patients without prior history of symptomatic cardiovascular disease (CVD) at baseline.
2. METHODS
2.1. Study population
The participants of this prospective cohort were recruited from a South Korean government‐sponsored prospective cohort study (Cardiovascular and Metabolic Disease Etiology Research Center‐High Risk Cohort [CMERC‐HI], clinicaltrials.gov: NCT02003781) that targeted those with high cardiovascular (CV) risk. The inclusion criteria of the CMERC‐HI have been published previously (Methods in Supporting Information). 14 , 15 According to the criteria, 3270 consecutive patients were enrolled in the cohort study from December 2013 to November 2018. We investigated the effect of resistant hypertension on future CV outcomes among patients treated with antihypertensive agents. We excluded patients with established symptomatic CVD (history of ischemic heart disease, ischemic or hemorrhagic stroke, heart failure [HF], and atrial fibrillation), CKD stage 5 (estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2), and end‐stage renal disease (ESRD) requiring regular dialysis therapy and those who received kidney transplant. ABPM was performed at baseline in 1666 (82.1%) of the remaining 2029 participants.
For participants classified according to previous diagnostic criteria, aTRH (hereafter, aTRHprior) was defined according to the 2008 AHA scientific statement as having elevated office systolic BP ≥ 140 or diastolic BP ≥ 90 mm Hg with elevated daytime average systolic BP ≥ 135 or diastolic BP ≥ 85 mm Hg on ABPM with use of 3 antihypertensive drugs, including a diuretic, at the time of enrollment; or use of ≥4 antihypertensive drugs at the time of enrollment regardless of uncontrolled or controlled office BP and/or ambulatory BP (ABP). The updated diagnostic criteria for aTRH according to the 2018 AHA scientific statement (hereafter, aTRHupdated) were defined as office systolic BP ≥ 130 or diastolic BP ≥ 80 mm Hg with daytime average systolic BP ≥ 130 or diastolic BP ≥ 80 mm Hg on ABPM with the same antihypertensive treatment criteria as aTRHprior. If the participants who used 3 antihypertensive drugs had uncontrolled office BP but did not have available ABPM data, they were excluded from the analysis because we could not determine whether it was white‐coat resistance (11 patients from the prior definition, 18 patients from the updated definition). The rest of the study participants according to each definition were categorized as control groups. Finally, we analyzed those who satisfied the diagnostic criteria for aTRH put forth by the prior AHA scientific statement (N = 2018; Figure 1) and the updated 2018 AHA scientific statement definition (N = 2011; Figure 1). 7 , 8 The CMERC‐HI study protocol was approved by the Institutional Review Boards of Yonsei University Health System (institutional review board number: 4‐2013‐0581). Written informed consent was provided by all participants.
Figure 1.
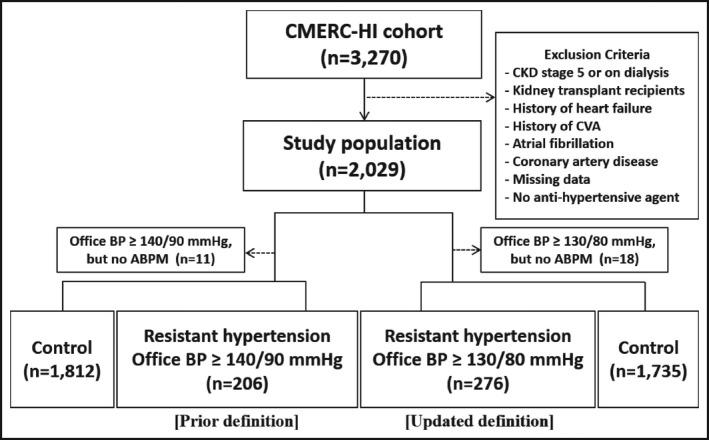
Flowchart of study participants
2.2. BP measurement
Office BP was obtained using a validated automatic device (HEM 7080‐IC; Omron), which was programmed to automatically measure the sitting BP of a person at 5, 7, and 9 minutes. 16 , 17 After positioning the subject in a sitting position with the right arm supported at heart level and setting the device, a trained nurse left the participants alone in the examination room. After a 5‐minute rest, automatic BP measurements at 2‐minute intervals were obtained. After three measurements, the trained nurse recorded the BP data. The mean of the three BP readings was used as the office BP. Twenty‐four‐hour ABP readings were obtained using the Takeda TM‐2430 instrument (A&D Medical, Tokyo, Japan), with readings taken every 30 minutes. We defined an adequate ABP recording as having at least 70% of the expected measurements, and at least 14 measurements during the day and 7 measurements at night. 18 Daytime and nighttime periods were defined according to the information provided in participants’ diaries. Ambulatory BP readings were averaged for 24‐hour, daytime, and nighttime values. 15 If the time difference between office BP and ABP measurements exceeded 3 months, only office BP measurements were included in the analyses. The mean time difference between office BP and ABP measurements was 5 (±40) days.
2.3. Outcomes
The primary outcome was first occurrence of major adverse cardiovascular events (MACEs), which were a composite of non‐fatal myocardial infarction, non‐fatal stroke, hospitalization for HF, and CV mortality. The secondary outcomes were non‐fatal CVE (non‐fatal myocardial infarction, non‐fatal stroke, and hospitalization for HF), each component event of a composite outcome, and renal event.
HF hospitalization was defined as an event that meets all of the following criteria: (a) requiring hospitalization due to clinical manifestations of HF (eg, dyspnea, orthopnea, paroxysmal nocturnal dyspnea, edema, pulmonary basilar crackles, jugular venous distension, third heart sound or gallop rhythm, radiological evidence of worsening HF) and additional therapy including oral or intravenous diuretic, inotrope, or vasodilator therapy, and (b) elevated serum biomarker (N‐terminal pro‐brain natriuretic peptide) level > 300 ng/L (if there was an evidence that left ventricular ejection fraction was <40% in any imaging modality including echocardiography, myocardial perfusion scan, or cardiac magnetic resonance imaging, a threshold of 600 ng/L was used).
Non‐fatal myocardial infarction was defined if there was evidence of myocardial necrosis in a clinical setting consistent with myocardial ischemia. It was defined if the patient was admitted for myocardial infarction with at least 2 of 3 criteria as follows: (a) symptom of ischemic chest pain, (b) elevation of cardiac enzyme level, and (c) significant luminal narrowing of coronary artery confirmed by any imaging modality including angiography or CT scan. Stroke was defined as a composite of hemorrhagic stroke and ischemic stroke requiring hospitalization due to new onset neurologic deficit and correlating lesions found in brain imaging studies.
The cause of death was determined by the principal condition that caused the death, not the immediate mode of death. The information was obtained from medical records and yearly telephone surveys. CV death was defined as death due to HF, stroke, or myocardial infarction. Non‐CV death was defined as any death not covered by CV death. Examples of non‐CV death are pulmonary causes, renal causes, infection (including sepsis), malignancy, accidental trauma, non‐CV organ failure (eg, hepatic failure), and non‐CV surgery.
Renal event was defined as follows: a decrease in eGFR of 50% or more compared with baseline to less than 60 mL/min/1.73 m2 in participants who had eGFR ≥ 60 mL/min/1.73 m2 at baseline, and a decrease in eGFR of 50% or more compared with baseline or progression to ESRD requiring either prolonged dialysis or kidney transplantation in participants who had eGFR < 60 mL/min/1.73 m2 at baseline. Serum creatinine was measured with an isotope‐dilution mass spectrometry traceable method, and the eGFR was calculated from the serum creatinine level by using the CKD Epidemiology Collaboration equation 19 Both clinical and renal events were analyzed by three independent investigators. Events that were agreed upon by all three investigators were deemed as clinical and renal outcomes.
2.4. Statistical analysis
All continuous data are presented as mean ± standard deviation, and categorical data are expressed as numbers and percentages for each group. In case of serious deviation from normal distribution, median and interquartile range and the Wilcoxon rank‐sum test were used. The effects of aTRH according to each definition on clinical events were analyzed with multivariate Cox proportional hazards models, which were adjusted for age, sex, current smoking, DM, and eGFR. To incorporate all events which comprise MACEs, we used the Prentice, Williams, and Peterson model analyses in Cox proportional hazards analysis for MACE. 20 Harrell's concordance index was used to assess the predictive accuracy of the prognostic models. To compare the values of Cox regression models in outcome prediction, comparisons of receiver operating characteristic (ROC) curves and pairwise comparisons were applied. The Cox proportional hazards models used to show the ROC curves were analyzed within the participants classified by the updated definition (N = 2011), and we used CKD (≥stage 3) as a binary covariate instead of eGFR level in these analyses. Areas under the ROC curves (AUCs) were calculated and compared using a method described by DeLong et al 21 Survival rates were estimated using the Kaplan‐Meier survival method, and differences were analyzed by a log‐rank test. All tests were two‐sided, and statistical significance was defined as P < .05. All statistical analyses were performed with R statistical software (version 3.6.3; R Foundation for Statistical Computing).
3. RESULTS
3.1. Baseline characteristics
Table 1 outlines the baseline characteristics of the study participants who were divided according to the prior/updated definition of resistant hypertension. A total of 206 patients among 2018 participants (10.2%) were diagnosed with aTRHprior, and a total of 276 patients among 2011 participants (13.7%) were diagnosed with aTRHupdated (Figure 2A). When defined only with office BP without ABPM, 230 (11.3%) and 318 (15.7%) among 2029 participants were diagnosed with resistant hypertension by using the prior and updated definitions, respectively. Patients with aTRH had higher body mass index (BMI) and waist and hip circumference, as well as higher proportion of CKD (≥stage 3) (Table 1). As expected, all BP measurements, except all‐day and daytime diastolic BP in aTRHprior, were higher, and eGFR levels were significantly lower in the aTRH groups than in the control groups. Patients used a median of 4 antihypertensive drugs in both aTRH groups, and the proportion of participants taking 4 or more drugs were 77.6% and 57.4% in aTRHprior and aTRHupdated, respectively (Table S1), whereas the rest of the participants in each aTRH group were uncontrolled hypertensive patients who were taking 3 antihypertensive drugs (17.5% in aTRHprior, 38.4% in aTRHupdated). All classes of antihypertensives were prescribed more frequently in the aTRH groups than in the control groups, except for angiotensin‐converting enzyme inhibitors (Table 1).
Table 1.
Baseline characteristics
| aTRHprior (≥140/90 mm Hg) | Control | P‐value | aTRHupdated (≥130/80 mm Hg) | Control | P‐value | |
|---|---|---|---|---|---|---|
| (N = 206) | (N = 1812) | (N = 276) | (N = 1735) | |||
| Clinical demographics | ||||||
| Age (y) | 61.5 ± 11.2 | 60.7 ± 11.1 | .353 | 61.3 ± 10.9 | 60.7 ± 11.2 | .439 |
| Male sex, n (%) | 88 (42.7%) | 836 (46.1%) | .390 | 116 (42.0%) | 806 (46.5%) | .192 |
| BMI (kg/m2) | 27.3 ± 4.1 | 25.5 ± 3.5 | <.001 | 27.2 ± 4.0 | 25.4 ± 3.5 | <.001 |
| Waist circumference (cm) | 92.0 ± 11.0 | 87.9 ± 9.4 | <.001 | 92.1 ± 11.0 | 87.7 ± 9.3 | <.001 |
| Hip circumference (cm) | 97.9 ± 10.1 | 94.9 ± 6.4 | <.001 | 97.8 ± 9.4 | 94.8 ± 6.3 | <.001 |
| Diabetes, n (%) | 104 (50.5%) | 795 (43.9%) | .084 | 132 (47.8%) | 763 (44.0%) | .262 |
| CKD (≥stage 3), n (%) | 76 (36.9%) | 489 (27.0%) | .004 | 93 (33.7%) | 472 (27.2%) | .031 |
| Office BP measurement | ||||||
| Office SBP (mm Hg) | 135.8 ± 19.6 | 125.8 ± 14.7 | <.001 | 134.4 ± 17.6 | 125.6 ± 14.8 | <.001 |
| Office DBP (mm Hg) | 76.8 ± 10.8 | 75.3 ± 9.5 | .057 | 77.5 ± 10.0 | 75.1 ± 9.6 | <.001 |
| Pulse rate (bpm) | 64.8 ± 11.1 | 69.5 ± 11.0 | <.001 | 66.3 ± 11.7 | 69.5 ± 10.9 | <.001 |
| ABPM | ||||||
| All‐day SBP (mm Hg) | 134.8 ± 15.2 | 128.5 ± 13.7 | <.001 | 133.8 ± 13.9 | 128.4 ± 13.9 | <.001 |
| All‐day DBP (mm Hg) | 78.1 ± 7.9 | 77.2 ± 8.2 | .179 | 78.6 ± 7.7 | 77.1 ± 8.2 | .009 |
| Day time SBP (mm Hg) | 139.1 ± 15.2 | 133.4 ± 14.1 | <.001 | 138.5 ± 13.9 | 133.2 ± 14.3 | <.001 |
| Day time DBP (mm Hg) | 81.1 ± 8.3 | 80.4 ± 8.3 | .323 | 81.8 ± 8.2 | 80.2 ± 8.3 | .008 |
| Night time SBP (mm Hg) | 127.2 ± 18.1 | 119.4 ± 16.0 | <.001 | 125.2 ± 16.9 | 119.4 ± 16.2 | <.001 |
| Night time DBP (mm Hg) | 72.9 ± 9.7 | 71.2 ± 8.6 | .024 | 72.8 ± 9.3 | 71.2 ± 8.6 | .007 |
| Antihypertensive drugs | ||||||
| No. of drugs | 4 [4‐4] | 2 [1‐2] | <.001 | 4 [3‐4] | 2 [1‐2] | <.001 |
| RAS inhibitors | 198 (96.1%) | 1440 (79.5%) | <.001 | 260 (94.2%) | 1371 (79.0%) | <.001 |
| ARBs | 185 (89.8%) | 1361 (75.1%) | <.001 | 244 (88.4%) | 1295 (74.6%) | <.001 |
| ACE inhibitors | 13 (6.3%) | 95 (5.2%) | .630 | 16 (5.8%) | 92 (5.3%) | .846 |
| CCBs | 195 (94.7%) | 983 (54.2%) | <.001 | 255 (92.4%) | 917 (52.9%) | <.001 |
| Beta‐blockers | 179 (86.9%) | 368 (20.3%) | <.001 | 195 (70.7%) | 351 (20.2%) | <.001 |
| Diuretics | 199 (96.6%) | 377 (20.8%) | <.001 | 269 (97.5%) | 300 (17.3%) | <.001 |
| Spironolactone | 11 (5.3%) | 27 (1.5%) | <.001 | 14 (5.1%) | 24 (1.4%) | <.001 |
| Thiazide a | 146 (70.9%) | 316 (17.4%) | <.001 | 202 (73.2%) | 253 (14.6%) | <.001 |
| Minoxidil | 8 (3.9%) | 0 (0.0%) | <.001 | 8 (2.9%) | 0 (0.0%) | <.001 |
| Alpha‐blockers | 31 (15.0%) | 29 (1.6%) | <.001 | 32 (11.6%) | 28 (1.6%) | <.001 |
| Statins | 116 (56.3%) | 1071 (59.1%) | .485 | 160 (58.0%) | 1022 (58.9%) | .820 |
| Aspirin | 69 (33.5%) | 500 (27.6%) | .089 | 92 (33.3%) | 475 (27.4%) | .049 |
| Laboratory findings | ||||||
| Hemoglobin, g/dL | 13.3 ± 1.9 | 13.7 ± 1.7 | .005 | 13.5 ± 1.9 | 13.7 ± 1.7 | .107 |
| BUN (mg/dL) | 22.9 ± 11.0 | 19.4 ± 9.1 | <.001 | 22.2 ± 10.9 | 19.4 ± 9.1 | <.001 |
| Creatinine (mg/dL) | 1.3 ± 0.8 | 1.1 ± 0.6 | <.001 | 1.3 ± 0.8 | 1.1 ± 0.6 | <.001 |
| eGFR (mL/min/1.73 m2) | 62.1 ± 23.7 | 69.5 ± 21.5 | <.001 | 63.7 ± 23.8 | 69.5 ± 21.5 | <.001 |
| Total cholesterol (mg/dL) | 176.0 ± 41.7 | 171.8 ± 36.6 | .173 | 174.6 ± 41.1 | 171.9 ± 36.6 | .301 |
| Triglyceride (mg/dL) | 156.9 ± 83.7 | 144.5 ± 86.2 | .061 | 155.8 ± 82.5 | 144.3 ± 86.6 | .048 |
| HDL cholesterol (mg/dL) | 47.0 ± 12.5 | 49.4 ± 12.9 | .015 | 47.3 ± 12.5 | 49.5 ± 12.9 | .012 |
| LDL cholesterol (mg/dL) | 94.2 ± 28.3 | 94.0 ± 31.3 | .910 | 93.8 ± 28.6 | 94.1 ± 31.3 | .905 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; aTRH, apparent treatment‐resistant hypertension; BMI, body mass index; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RAS, renin‐angiotensin system; SBP, systolic blood pressure.
Thiazide includes thiazides and thiazide‐like drugs (indapamide, chlorthalidone).
Figure 2.
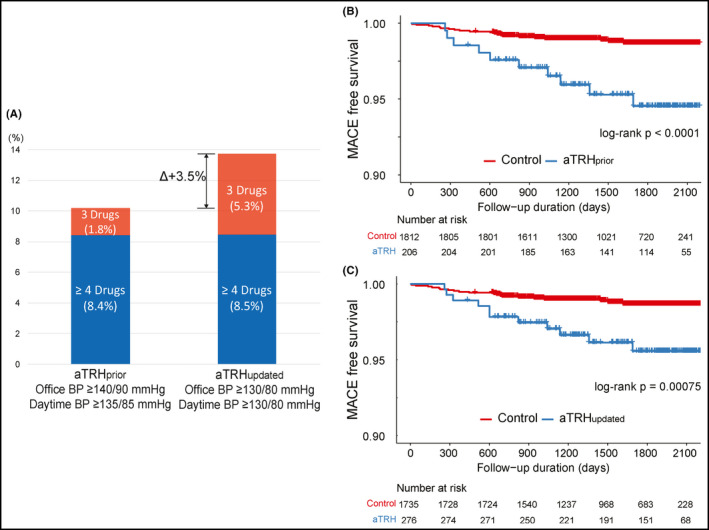
Proportion of apparent treatment‐resistant hypertension and cardiovascular outcomes. The proportion of participants with resistant hypertension was higher by the updated definition than by the prior definition in the total participants (A). The participants who were taking 3 antihypertensive drugs were divided into resistant hypertension by each definition, and those who were taking 4 or more antihypertensive drugs were classified as resistant hypertension regardless of their blood pressure. Kaplan‐Meier survival curves for major adverse cardiovascular events according to resistant hypertension defined by the prior (B) and updated criteria (C) showed that they all had a significantly higher risk for adverse cardiovascular events. BP, blood pressure; aTRH, apparent treatment‐resistant hypertension
3.2. CV and renal outcomes during follow‐up
After a median follow‐up of 4.5 years (interquartile range: 3.2‐5.4 years, maximum of 6.3 years), which corresponded to 8740 patient‐years (PY) of follow‐up, 33 MACEs occurred (crude event rate: 3.9 per 1000 PY) with 30 non‐fatal CVEs (crude event rate: 3.5 per 1000 PY, 14 HF hospitalization, 6 MI, and 11 strokes). Three patients died from CV causes (0.3 per 1000 PY). A total of 164 renal events occurred (crude event rate: 19.8 per 1000 PY, 84 patients started dialysis, 19 patients received kidney transplants). When compared with control participants, aTRHprior patients had a higher event rate of HF hospitalization, and aTRHupdated patients showed a trend toward higher event rate of HF hospitalization (Table 2). When compared with each control subject, aTRHprior and aTRHupdated patients had higher rates of renal event, non‐fatal CVE, and MACE (Table 2). This trend was also observed when we defined aTRH with office BP or ABPM alone (Tables S2 and S3). We evaluated the association of aTRH with MACE by Kaplan‐Meier survival analysis. Both aTRHprior and aTRHupdated had a higher risk for MACE (log‐rank P < .0001,.00075, respectively; Figure 2B,C) and renal event (both log‐rank P < .0001; Figure 3A,B). In multivariate Cox proportional hazards analysis after adjustment for age, sex, DM, eGFR, and current smoking (Table 3), both aTRHprior and aTRHupdated showed significantly higher risk for HF hospitalization, renal event, non‐fatal CVE, and MACE. When analyzed with office BP alone, a significantly higher risk for non‐fatal CVE and MACE was still found in the aTRH groups than in the control groups. However, the difference in the risk for renal event was not statistically significant for either definition of aTRH with office BP alone (Table S4). Moreover, when analyzed only with ABPM, a significantly higher risk for HF hospitalization, non‐fatal CVE, and MACE, but not for renal event, was still observed for either definition of aTRH (Table S5).
Table 2.
Clinical events and incidence rates according to the variable resistant hypertension definition
| aTRHprior (N = 206) | Control (N = 1812) | P‐value * | aTRHupdated (N = 276) | Control (N = 1735) | P‐value * | |
|---|---|---|---|---|---|---|
| HF hospitalization | 5 (5.2) | 9 (1.2) | .043 | 6 (4.6) | 8 (1.1) | .065 |
| Stroke (non‐fatal) | 3 (3.1) | 7 (0.9) | .229 | 3 (2.3) | 7 (0.9) | .325 |
| MI (non‐fatal) | 2 (2.1) | 4 (0.5) | .296 | 2 (1.5) | 4 (0.5) | .373 |
| Renal event | 34 (35.0) | 130 (16.8) | <.001 | 42 (32.3) | 122 (16.5) | .001 |
| Non‐fatal CVE | 10 (10.3) | 20 (2.6) | .020 | 11 (8.5) | 19 (2.6) | .010 |
| CV death | 1 (1.0) | 2 (0.3) | .461 | 1 (0.8) | 2 (0.3) | .530 |
| MACE | 11 (11.3) | 22 (2.8) | .007 | 12 (9.2) | 21 (2.8) | .019 |
Described as number of events (incidence rate, per 1000 patient‐years of follow‐up).
Abbreviations: CV, cardiovascular; CVE, cardiovascular event; HF, heart failure; MI, myocardial infarction. Other abbreviations are defined in Table 1.
Comparison was performed for incidence rate of the event.
Figure 3.
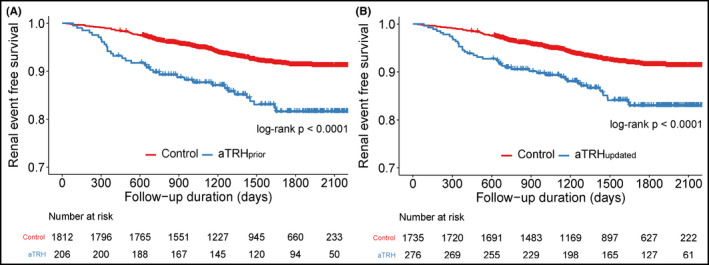
Survival curves for major adverse cardiovascular event. Kaplan‐Meier survival curves for renal event according to resistant hypertension defined by the prior (A) and updated scientific statements (B). aTRH, apparent treatment‐resistant hypertension; MACE, major adverse cardiovascular event
Table 3.
Multivariate Cox proportional hazards analysis for clinical outcomes according to the resistant hypertension definition
| aTRHprior HR [95% CI] | C‐index | P‐value | aTRHupdated HR [95% CI] | C‐index | P‐value | |
|---|---|---|---|---|---|---|
| HF hospitalization | 3.178 [1.040‐9.707] | 0.853 | .042 | 3.376 [1.134‐10.05] | 0.870 | .029 |
| Stroke (non‐fatal) | 2.393 [0.603‐9.505] | 0.839 | .215 | 1.767 [0.446‐7.009] | 0.836 | .418 |
| MI (non‐fatal) | 3.226 [0.567‐18.37] | 0.824 | .187 | 2.332 [0.410‐13.28] | 0.821 | .340 |
| Renal event | 1.537 [1.049‐2.252] | 0.880 | .028 | 1.493 [1.044‐2.135] | 0.879 | .028 |
| Non‐fatal CVE | 2.651 [1.189‐5.909] | 0.796 | .017 | 2.314 [1.058‐5.063] | 0.802 | .036 |
| CV death | 3.735 [0.284‐49.11] | 0.976 | .316 | 3.136 [0.233‐42.21] | 0.975 | .389 |
| MACE | 2.735 [1.355‐5.521] | 0.798 | .005 | 2.418 [1.182‐4.947] | 0.804 | .016 |
To investigate the difference of the predictive value for MACE between aTRHprior and aTRHupdated, we analyzed the ROC curves from each definition (Figure 4A). No significant difference was found in AUCs of the 2 multivariate Cox proportional hazards models with both aTRHprior and aTRHupdated for 4‐year MACE‐free survival. We also compared the ROC curves for renal event, and we obtained a similar result that no significant difference was observed in AUCs of the 2 models with both aTRH for 4‐year renal event‐free survival (Figure 4B). In addition, when we analyzed the predictive values of each aTRH for the outcomes across various subgroups, risk of both aTRH for MACE was not significantly different across various subgroups (Figure S1).
Figure 4.
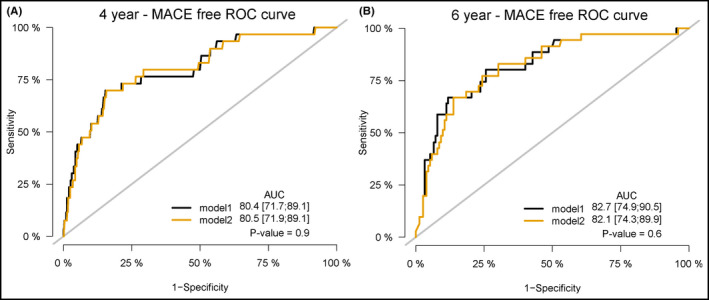
Comparison of receiver operating characteristic curves for the events. Comparison of receiver operating characteristic (ROC) curves for major adverse cardiovascular events (MACE) (A) and renal events (B) at 4 y according to resistant hypertension defined by the prior and updated scientific statements. Model 1 is Cox proportional hazards regression model with resistant hypertension by the prior definition, and model 2 is by the updated definition. All models were adjusted for age, sex, diabetes, chronic kidney disease (≥stage 3), and current smoking. AUC, area under the curve
4. DISCUSSION
The key findings from this study were as follows. First, despite the increased prevalence of resistant hypertension defined according to the updated statement (aTRHupdated), the increased risk for MACE was still significant compared with controlled hypertensive participants without any significant interaction for age, sex, BMI, presence of DM, and presence of CKD. Moreover, no significant difference was found in the accuracy for predicting MACE. Second, both aTRHprior and aTRHupdated were significant risk factors for predicting major renal end points without significant difference in accuracy. Recent changes in both the threshold for hypertension diagnosis and the target BP put forth by the 2017 ACC/AHA guideline have led to changes in the definition of resistant hypertension in 2018. However, whether or not a lower threshold for diagnosing resistant hypertension would change the degree of risk for resistant hypertension as a high‐risk factor for CVD was not clear. The present results showed that lowering the threshold could identify more participants with high‐risk resistant hypertension without sacrificing its predictive value for CV and renal end points.
To date, observational studies using the 2008 criteria showed that patients with resistant hypertension are at higher risk for poor outcomes compared with patients without resistant hypertension. 6 , 22 , 23 , 24 In a retrospective study of over 200 000 patients with hypertension, those with resistant hypertension have 47% higher risk for combined outcomes of death, HF, stroke, myocardial infarction, or CKD over a median follow‐up of 3.8 years. 22 In this study, the difference in the incidence of composite outcomes was driven largely by a higher risk for the development of CKD events. 22 In another study of over 400 000 patients, patients with resistant hypertension have 46% increased risk of HF, 32% increased risk of developing ESRD, 24% increased risk of an ischemic heart event, 14% increased risk of stroke, and 6% increased risk of death. 6 However, these studies applied the 2008 criteria for the diagnosis of resistant hypertension, and no studies have been conducted to analyze the prognosis of resistant hypertension based on the 2018 definition. The present study is the first to show that aTRH by both criteria had a higher risk for combined outcomes of HF, stroke, myocardial infarction, or CV death and renal outcomes of CKD progression or initiation of renal replacement therapy. In particular, the risk of HF hospitalization and renal event was significantly increased with both aTRH criteria. These results support the use of the updated criteria for resistant hypertension to better identify a subset of hypertensive participants who are at high risk. This change has important clinical implications as many participants with office BP between 130 and 139 mm Hg who would have been considered to have controlled hypertension according to the previous definition of resistant hypertension would be diagnosed with resistant hypertension and managed accordingly.
Various comorbidities including obesity, DM, and CKD are known to be associated with resistant hypertension. 25 , 26 , 27 In line with the previous reports, our cohort showed that patients with aTRH had higher BMI, waist/hip circumference, and higher CKD prevalence than controlled hypertensive participants. In subgroup analyses, we showed that the risk of both aTRH definitions for MACE was not significantly different according to older age, sex, DM, obesity, and CKD. This is interesting to note considering that aTRH is highly prevalent in DM and CKD and is associated with adverse prognosis. 4 , 5
The major CVE rate was relatively lower in the present study than in previous studies that analyzed aTRH in other populations. 28 , 29 The MACE incidence rate in our cohort was 11.3 per 1000 PY in patients with aTRH according to the previous definition and 2.8 per 1000 PY in patients with controlled hypertension, whereas the overall CVE (including stroke, coronary heart disease, and HF) incidence rate was 34.4 per 1000 PY in aTRH patients and 16.8 per 1000 PY in patients with controlled hypertension in a retrospective study involving a Western population. 29 The CVE rate may differ according to region, ethnicity, or socioeconomic status, even among Asian populations. In a large retrospective cohort study of 18 036 patients with resistant hypertension from the Cardiovascular Research Network hypertension registry, the non‐fatal CVE rates were approximately 3.36 events per 1000 PY and 28.34 renal events per 1000 PY. 22 Our results were consistent with these event rates. The difference in CVE rates may be attributed to several reasons. In our study cohort, the proportion of patients who were taking ≥ 4 antihypertensive drugs was relatively high (Table S1). Moreover, even among these patients taking ≥ 4 antihypertensive drugs, 54.4% classified by aTRHprior and 23.9% classified by aTRHupdated had controlled office BP. The proportion of patients with controlled BP was relatively higher in our study than in a recent study, which showed that patients with controlled BP have lower risks of adverse cardiovascular outcomes related to resistant hypertension. 28 This may be one of the reasons for the relatively lower CVE rate in our study than that in the previous study. Additionally, we excluded patients with atrial fibrillation to rule out the risk enhancement effect because atrial fibrillation is a major risk factor for embolic stroke. Furthermore, because of the relatively high prevalence of CKD (29% in the study cohort), renal events were 3 times more frequent than MACE in the study population. CKD is one of the major risk factors for aTRH, and patients with CKD are also prone to progression to ESRD compared with patients without CKD. 5 Despite these demographical differences in our study, significant differences were still found in the adverse event rate according to the updated resistant hypertension criteria.
The strength of our study is that we used office BP to diagnose resistant hypertension and ruled out white‐coat effect with ABPM data according to the criteria. Many of the previous studies conducted on resistant hypertension included resistant hypertension based on office BP measurement alone, which could lead to misclassification of white‐coat effect as resistant hypertension. When defining resistant hypertension, determining white‐coat effect is important due to the lower CV risk of participants with white‐coat effect compared with true sustained uncontrolled hypertension. 30 In resistant hypertension participants, clinically significant white‐coat effect has been shown to be present in up to 39% of the participants with aTRH by clinic BP measurement. 11 , 31 In the present study, we showed that resistant hypertension defined by the prior and updated criteria and by office BP plus ABPM had a significantly higher risk for adverse cardiovascular and poor renal outcomes. This comparative analysis is the first study to analyze the prognostic effect of each definition of resistant hypertension in Asian hypertension patients. Our findings have important clinical implications as Asian hypertension patients have unique features, including a high prevalence of masked hypertension and disrupted BP variability that differ from those of other ethnicities. 32 , 33
4.1. Study limitations
This study had several limitations. A major limitation of this study was that since drug adherence data were not available or evaluation was not performed for secondary hypertension, we could not verify that the aTRH participants enrolled in our study all had truly resistant hypertension. Second, 1666 out of 2029 participants underwent ABPM. As such, those participants who satisfied the clinical BP criteria for resistant hypertension but did not have available ABPM data were excluded from the analysis. However, as only a few participants (N = 11 for aTRHprior and N = 18 for aTRHupdated) were actually excluded from the analysis, we do not believe that this limitation significantly affected the results. Third, we recruited a relatively higher number of CKD participants (29% of the study cohort), which might have influenced the relatively high incidence rate of renal end point compared with other cardiovascular events. To eliminate this potential effect on outcome analysis, we excluded patients with CKD stage ≥ 5, those who were on dialysis, or those who had a history of kidney transplant.
5. CONCLUSIONS
In a cohort of more than 2000 high‐risk hypertension patients without a history of symptomatic atherosclerotic CVD, both the previous and updated definitions of resistant hypertension were significant predictors of MACEs and renal events. These results support the adoption of the updated criteria for resistant hypertension in clinical practice, particularly in Asian populations.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
KH Chun and CJ Lee drafted the manuscript. CJ Lee, J. Oh, and S. Park designed the prospective study. J. Oh, SH Lee, SM Kang, and S. Park recruited the study participants. KH Chun analyzed the data. J. Oh, SH Lee, SM Kang, K. Kario, and S. Park were involved in critical revision for important intellectual content and final approval of the submitted manuscript.
Supporting information
Fig S1
Supplementary Material
ACKNOWLEDGMENTS
None.
Chun K‐H, Lee CJ, Oh J, et al. Prevalence and prognosis of the 2018 vs 2008 AHA definitions of apparent treatment‐resistant hypertension in high‐risk hypertension patients. J Clin Hypertens. 2020;22:2093–2102. 10.1111/jch.14043
Kyeong‐Hyeon Chun and Chan Joo Lee contributed equally to this work.
Funding information
This research was supported by a grant from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI13C0715), and a research grant from the Korean Centers for Disease Control and Prevention (grant number: 2018ER630200). This study was also supported by a faculty research grant of Yonsei University College of Medicine (6‐2019‐0170).
REFERENCES
- 1. Carey RM, Calhoun DA, Bakris GL, et al. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53‐e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carey RM, Sakhuja S, Calhoun DA, Whelton PK, Muntner P. Prevalence of apparent treatment‐resistant hypertension In the United States. Hypertension. 2019;73(2):424‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Persell SD. Prevalence of resistant hypertension in the United States, 2003–2008. Hypertension. 2011;57(6):1076‐1080. [DOI] [PubMed] [Google Scholar]
- 4. Cardoso CRL, Leite NC, Bacan G, Ataide DS, Gorgonio LKC, Salles GF. Prognostic importance of resistant hypertension in patients with type 2 diabetes: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care. 2020;43(1):219‐227. [DOI] [PubMed] [Google Scholar]
- 5. Thomas G, Xie D, Chen HY, et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the chronic renal insufficiency cohort study. Hypertension. 2016;67(2):387‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sim JJ, Bhandari SK, Shi J, et al. Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int. 2015;88(3):622‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403‐1419. [DOI] [PubMed] [Google Scholar]
- 9. Kario K, Wang JG. Could 130/80 mm Hg be adopted as the diagnostic threshold and management goal of hypertension in consideration of the characteristics of Asian populations? Hypertension. 2018;71(6):979‐984. [DOI] [PubMed] [Google Scholar]
- 10. Smith SM, Gurka MJ, Winterstein AG, Handberg E, Pepine CJ, Cooper‐DeHoff RM. Redefining resistant hypertension: a comparison of cardiovascular risk associated with the 2018 versus 2008 American Heart Association definitions for resistant hypertension. Circ Cardiovasc Qual Outcomes. 2020;13(2):e005979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898‐902. [DOI] [PubMed] [Google Scholar]
- 12. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 13. Kario K, Park S, Chia YC, et al. 2020 Consensus summary on the management of hypertension in Asia from the HOPE Asia Network. J Clin Hypertens (Greenwich). 2020;22(3):351‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seo J, Lee CJ, Oh J, Lee SH, Kang SM, Park S. Large discrepancy between unobserved automated office blood pressure and ambulatory blood pressure in a high cardiovascular risk cohort. J Hypertens. 2019;37(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 15. Oh J, Lee CJ, Kim IC, et al. Association of morning hypertension subtype with vascular target organ damage and central hemodynamics. J Am Heart Assoc. 2017;6(2):e005424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Assaad MA, Topouchian JA, Asmar RG. Evaluation of two devices for self‐measurement of blood pressure according to the international protocol: the Omron M5‐I and the Omron 705IT. Blood Press Monit. 2003;8(3):127‐133. [DOI] [PubMed] [Google Scholar]
- 17. Kario K, Hoshide S, Okawara Y, et al. Effect of canagliflozin on nocturnal home blood pressure in Japanese patients with type 2 diabetes mellitus: The SHIFT‐J study. J Clin Hypertens (Greenwich). 2018;20(10):1527‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21(5):821‐848. [DOI] [PubMed] [Google Scholar]
- 19. Miller WG, Myers GL, Ashwood ER, et al. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Arch Pathol Lab Med. 2005;129(3):297‐304. [DOI] [PubMed] [Google Scholar]
- 20. Prentice RL, Williams BJ, Peterson AV. On the regression‐analysis of multivariate failure time data. Biometrika. 1981;68(2):373‐379. [Google Scholar]
- 21. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837‐845. [PubMed] [Google Scholar]
- 22. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsioufis C, Kasiakogias A, Kordalis A, et al. Dynamic resistant hypertension patterns as predictors of cardiovascular morbidity: a 4‐year prospective study. J Hypertens. 2014;32(2):415‐422. [DOI] [PubMed] [Google Scholar]
- 24. Irvin MR, Booth JN, Shimbo D, et al. Apparent treatment‐resistant hypertension and risk for stroke, coronary heart disease, and all‐cause mortality. J Am Soc Hypertens. 2014;8(6):405‐413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Acharya T, Tringali S, Singh M, Huang J. Resistant hypertension and associated comorbidities in a veterans affairs population. J Clin Hypertens (Greenwich). 2014;16(10):741‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sim JJ, Bhandari SK, Shi J, et al. Characteristics of resistant hypertension in a large, ethnically diverse hypertension population of an integrated health system. Mayo Clin Proc. 2013;88(10):1099‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oliveras A, de la Sierra A. Resistant hypertension: patient characteristics, risk factors, co‐morbidities and outcomes. J Hum Hypertens. 2013;28(4):213‐217. [DOI] [PubMed] [Google Scholar]
- 28. Cardoso CRL, Salles GC, Salles GF. Prognostic importance of on‐treatment clinic and ambulatory blood pressures in resistant hypertension: a cohort study. Hypertension. 2020;75(5):1184‐1194. [DOI] [PubMed] [Google Scholar]
- 29. Egan BM, Kai B, Wagner CS, Henderson JH, Chandler AH, Sinopoli A. Blood pressure control provides less cardiovascular protection in adults with than without apparent treatment‐resistant hypertension. J Clin Hypertens (Greenwich). 2016;18(8):817‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pierdomenico S, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18(11):1422‐1428. [DOI] [PubMed] [Google Scholar]
- 31. Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168(21):2340‐2346. [DOI] [PubMed] [Google Scholar]
- 32. Kario K, Chia Y‐C, Sukonthasarn A, et al. Diversity of and initiatives for hypertension management in Asia‐Why we need the HOPE Asia Network. J Clin Hypertens (Greenwich). 2020;22(3):331‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kario K, Shin J, Chen C‐H, et al. Expert panel consensus recommendations for ambulatory blood pressure monitoring in Asia: The HOPE Asia Network. J Clin Hypertens (Greenwich). 2019;21(9):1250‐1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


