Abstract
Central blood pressure (cBP) is highly associated with cardiovascular risk. Although reduction of salt intake leads to lower peripheral blood pressure (BP), the studies on cBP provided inconsistent results. Therefore, we performed a systematic review and a meta‐analysis of the available intervention trials of salt reduction on cBP values to reach definitive conclusions. A systematic search of the online databases available (up to December 2018) was conducted including the intervention trials that reported non‐invasively assessed cBP changes after two different salt intake regimens. For each study, the mean difference and 95% confidence intervals were pooled using a random‐effect model. Sensitivity, heterogeneity, publication bias, subgroup, and meta‐regression analyses were performed. Fourteen studies met the pre‐defined inclusion criteria and provided 17 cohorts with 457 participants with 1‐13 weeks of intervention time. In the pooled analysis, salt restriction was associated with a significant reduction in augmentation index (9.3%) as well as central systolic BP and central pulse pressure. There was a significant heterogeneity among studies (I2 = 70%), but no evidence of publication bias. Peripheral BP changes seemed to partially interfere on the relationship between salt restriction and cBP. The results of this meta‐analysis indicate that dietary salt restriction reduces cBP. This effect seems to be, at least in part, independent of the changes in peripheral BP.
Keywords: augmentation index, central blood pressure, meta‐analysis, pulse wave analysis, salt, sodium intake
1. INTRODUCTION
Central blood pressure (cBP) indices and its derivatives are independent predictors of organ damage, 1 , 2 cardiovascular events, and all‐cause mortality, 3 and several works indicated that they are more strongly related to cardiovascular risk than peripheral blood pressure (BP). 4 , 5 , 6 cBP can be non‐invasively assessed by several devices using methods that evaluate central aortic pressure waveform. 7 This wave is composed by a forward traveling wave generated by left ventricular ejection and a backward traveling wave reflected from the periphery. 7 Clinical trials found that antihypertensive drugs may exert different effects on cBP compared with brachial BP. 8 , 9 In addition, some studies tested the changes in cBP during lifestyle modifications with special emphasis for dietary intervention. 10 , 11
Dietary salt (ie, sodium chloride) intake is an important determinant of BP, with clear evidence that a high‐salt intake is associated with increased BP. 12 In addition, high‐salt intake is related to cardiovascular events and cardiovascular organ damage both directly and through its BP effects. 13 , 14 , 15 Epidemiological and clinical studies indicated a direct association between habitual salt consumption and cBP. 16 , 17 This association is supported by experimental evidence of structural and functional alterations induced by high‐salt intake on the arterial wall and also by the effect of higher BP. Indeed, high‐salt intake is associated with increased oxidative stress with a reduction of nitric oxide bioavailability 18 , 19 , 20 , 21 and increment in smooth muscle cell tone. 22 , 23 Several intervention studies in humans evaluated the effect of dietary salt changes on peripheral BP as well as on cBP: however, the evidence with respect to the effect on cBP is inconsistent 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 mainly because of the low statistical power of most studies, the heterogeneity of the participants' features, the short length of intervention, and the magnitude of salt restriction.
A number of intervention studies that assessed both pulse wave velocity and cBP 27 , 28 , 30 , 31 , 32 , 33 , 34 , 35 were the object of a recent meta‐analysis assessing the effect of salt intake on vascular damage [ie, carotid‐femoral pulse wave velocity]. 15 However, given that pulse wave velocity is purely expression of aortic stiffness whereas cBP is determined by aortic stiffness and peripheral resistance, we performed a further systematic review and meta‐analysis of the available intervention trials testing the effect of dietary salt intake reduction on cBP using additional data. 24 , 25 , 26 , 29 , 36 , 37
2. METHODS
2.1. Data sources and search strategy
This meta‐analysis was planned, conducted, and reported according to the PRISMA statement 38 (Table S1). We performed a systematic search of the available publications using MEDLINE, Scopus, WOS, and the Cochrane Library, up to December 2018. The search strategy, without restrictions, used the expressions "sodium intake/consumption" OR "salt intake/consumption" OR "dietary salt/sodium" AND "pulse wave analysis" OR "PWA" OR "central haemodynamic" OR "central blood pressure" OR “augmentation index,” or combinations thereof, either in medical subject headings or in the title/abstract. Further information was retrieved through a manual search of references from recent reviews and relevant published original studies.
2.2. Study selection and data extraction
The data selection and extraction was independently conducted and reported by two reviewers (LD, ELF). Discrepancies about inclusion of studies and interpretation of data were resolved in conference, and consensus was reached after discussion. To be included in the meta‐analysis, a published study had to meet the following criteria: (a) original article, (b) adult population study, (c) intervention study, (d) indication of a difference in cBP parameters between two different salt intake regimens in one or more patients’ cohorts, and (e) indication of the number of participants included in the exposed and control group for each cohort. The risk of bias of the studies included in the meta‐analysis was assessed according to established criteria 39 and reported in Table S2.
2.3. Statistical analysis
Weighted mean differences (MD)—and standard error of the mean (SEM)—of the defined outcomes were extracted from the selected publications. If these were not available, MD and SEM were calculated from the comparison of the outcomes at low and high‐salt regimens. The conversion to percentages was used in the analysis to calculate the between‐regimen changes in the outcomes. The pooled MD and 95% confidence interval (CI) were estimated using a random‐effect model. 40 The influence of the individual cohorts or of a particular study was estimated by sensitivity analysis. The Cochrane Q test and the I2 statistic were used to evaluate statistical heterogeneity across the studies. Funnel plots were constructed and visually assessed for possible publication bias. 41 Egger's and Begg's tests were also used to explore potential publication bias. Subgroup and meta‐regression analyses were used to identify associations between changes in central hemodynamic parameters and relevant study's or patients' characteristics as possible sources of heterogeneity. The analyses were carried out for augmentation index (Aix), AIx adjusted for heart rate, central systolic BP (cSBP), and central pulse pressure (cPP), since adequate data were available for these outcomes. The statistical analyses were performed using the Stata Corp. software (version11.2).
3. RESULTS
3.1. Characteristics of the studies included in the meta‐analysis
Of a total of 131 publications retrieved, 15 studies were identified that met the inclusion criteria (Figure 1). However, one of them was excluded because it was based on mixed intervention. 42 Thus, eventually 14 studies were used for the analysis. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 The main characteristics of the identified studies and of the respective study populations were recorded and reported in Table 1. Overall, the meta‐analysis involved 457 participants from six countries. Three studies provided multiple cohorts including different categories of patients, one study recruited patients retrospectively stratified by BP salt sensitivity, another one included healthy normotensive participants stratified by age, and another one a group of healthy women stratified by history of pre‐eclampsia. With respect to the comparison of the effects of higher vs lower salt intake, all but three studies were randomized controlled trials with a crossover design. Almost all studies used 24 hours urinary sodium excretion as a proxy for sodium intake during intervention, while two studies utilized 8 hours overnight urine specimens and one both 24 hours and 8 hours overnight collections. cBP was assessed by different methods: in the majority of studies by applanation tonometry, in two studies by a pressure transducer, and in two other studies by Doppler transducer.
FIGURE 1.
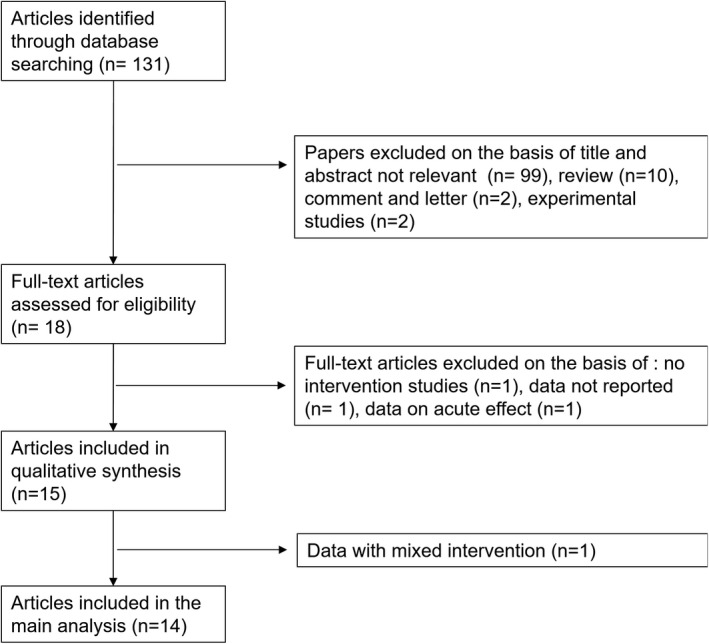
Stepwise procedure for selection of the studies. Flowchart indicating the results of the systematic review with inclusions and exclusions
TABLE 1.
Characteristics of the studies included in the meta‐analysis
| First Author, year (ref) | Country | Cohort (n. of participants) | Selected features of the study participants |
Mean Age (yrs) |
Mean BMI (Kg/m2) | Duration of intervention (days) | Assessment Method | Low vs High Sodium Comparison (mmoL/24 h) | Changes in SBP/DBP (mm Hg) | Study Design |
|---|---|---|---|---|---|---|---|---|---|---|
| Seals, 2001 24 | USA | Post‐menopausal female participants (W17) | Post‐menopausal status, SBP 130‐159, DBP ≤99 mm Hg, no treatment, not smoking, absence of chronic diseases | 65 | 28.1 | 90 |
Pressure trasducer (TCB‐500, Millar Instruments) |
86 vs 124 | −16/−7 |
Intervention study single‐blind (High‐salt diet vs low‐salt diet) |
| Gates, 2004 25 | USA | White hypertensive older participants (6M, 6W) | Untreated stage 1 systolic hypertension | 64 | 24.7 | 28 |
Pressure transducer (TCB‐500, Millar Instruments) |
54 vs 135 | −7/−1.6 |
Crossover Double‐blind (slow‐sodium suppl. vs placebo) |
| Al‐Solaiman, 2009 26 | USA | SR participants (3M, 7W) | Lean normotensive without metabolic syndrome and obese hypertensive subjects with metabolic syndrome | 34.3 | 30.1 | 21 | Applanation tonometry (Sphygmocor) | 34 vs 97 | 2.8/3.2 |
Intervention study (DASH vs LS‐DASH) |
| SS participants (2M, 7W) | 44.1 | 26.5 | 51 vs 104 | −7.1/−3.2 | ||||||
| Dickinson, 2009 27 | Australia | Overweight/obese participants (7M, 22W) | SBP < 160 mm Hg, No CVD, no antihypertensive therapy, BMI > 27 and <40 Kg/m2 | 52.7 | 31.6 | 14 | Doppler transducer | 64 vs 156 | −5/−1 | Crossover (sodium restriction vs regular sodium) |
| Pimenta, 2009 28 | USA | Hypertensive White and Black patients (4M, 8W) | Resistent Hypertension (with HCT and RAAS‐blocking treatment) | 55.5 | 32.9 | 7 | Applanation tonometry (Sphygmocor) | 46 vs 252 | −22.8/−9.1 |
Crossover (slow‐sodium suppl. vs low‐salt diet) |
| Starmans‐Kool, 2011 29 | UK | Healthy young subjects (10M) | SBP/DBP < 140/90 mm Hg, no treatment; no CVD, no diabetes | 32 | ‐ | 14 | Applanation tonometry (SPT 301; Millar Instruments, Houston, TX) | 94 vs 191 | −2/0 |
Crossover Double‐blind (slow‐sodium suppl. vs placebo) |
| Todd, 2010 30 | New Zealand | (Pre)Hypertensive or hypertensive participants (13M, 21W) | SBP/DBP > 130/85 mm Hg or treatment | 51.8 | 25.7 | 28 | Applanation tonometry (Sphygmocor) | 60 vs 200 a | −5.8/−3.4 |
Crossover Single‐blind (sodium suppl. vs low‐salt diet) |
| Todd, 2012 31 | New Zealand | Healthy Caucasian subjects (5M, 18W) | SBP/DBP < 130/85 mm Hg, no treatment; no CVD, BMI <30 Kg/m2 | 43.7 | 25.3 | 28 | Applanation tonometry (Sphygmocor) | 60 vs 200 a | 0.1/0.4 |
Crossover Single‐blind (sodium suppl. vs low‐salt diet |
| McMahon, 2013 32 | Australia | CKD patients (15M, 5W) | Hypertension (SBP 130‐169, DBP ≥ 70 mm Hg), CKD stage 3 or 4 (not transplanted). | 68.5 | 29.3 | 42 | Applanation tonometry (Sphygmocor) | 75 vs 168 | −9.7/−3.9 b |
Crossover Double‐blind (slow‐sodium suppl. vs placebo) |
| Dickinson, 2014 33 | Australia | Overweight/obese normotensive participants (8M, 17W) | BMI: 27‐40 Kg/m2, no CVD, SBP/DBP < 140/90 mm Hg, no treatment | ‐ | ‐ | 42 | Doppler transducer | 113 vs 155 | −3/−1 |
Crossover Single‐blind (slow‐sodium suppl. vs placebo) |
| Gijsbers, 2015 34 | Netherlands | (Pre)Hypertensive Caucasian participants (24M, 12W) | No smoking, SBP 130‐159 mm Hg, no treatment, no CVD, no diabetes | 65.8 | 27.2 | 28 | Applanation tonometry (Sphygmocor) | 105 vs 203 | −7.5/−3.3 |
Crossover Double‐blind (slow‐sodium suppl. vs placebo) |
| Van der Graaf, 2016 35 | Netherlands | Subjects with history of NP (18W) | SBP/DBP < 140/90 mm Hg, non‐smokers | 36 | 22.6 | 7 | Applanation tonometry (Sphygmocor) | 39 vs 221 | ‐/‐ | Crossover (sodium restriction vs high sodium) |
| Subjects with history of PP (18W) | 36 | 25.3 | 7 | 45 vs 258 | ‐/‐ | |||||
| Muth, 2017 36 | USA |
Healthy normotensive participants—Young (30M, 19W) |
SBP/DBP < 140/90 mm Hg, no CVD, no diabetes, non‐smokers, non‐obese | 27 | 23.6 | 7 | Applanation tonometry (Sphygmocor) | 32 vs 243 | −3/−3 |
Crossover (slow‐sodium suppl. vs placebo) |
|
Healthy normotensive participants—Middle‐aged (13M, 23W) |
52 | 25.1 | 28 vs 243 | −8/−1 | ||||||
| Xing, 2018 37 | China | (Pre)Hypertensive participants (60M, 39W) | SBP 130‐159, DBP 85‐100 mm Hg, no treatment, absence of chronic diseases | 53.4 | 25.1 | 7 | Applanation tonometry (Sphygmocor) | 234 vs 55 a | −10.1/−5.6 |
Intervention study (High‐salt diet vs low‐salt diet) |
Abbreviations: CKD, chronic kidney disease; CVD, cardiovascular disease; DASH, dietary approaches to stop hypertension; DBP, diastolic blood pressure; HCT, hydrochlorothiazide; LS, low salt; M, men; MBP, mean blood pressure; NP, normotensive pregnancy; PP, pre‐eclamptic pregnancy; PWV, pulse wave velocity; RAAS, renin‐angiotensin‐aldosterone system; SBP, systolic blood pressure; SR, salt‐resistant participants; SS, salt‐sensitive participants; W, women.
8‐h overnight urine.
Assessed by ABPM (ambulatory blood pressure monitoring).
Most of the studies assessed peripheral BP by an automated or semi‐automated device, three by a mercury sphygmomanometer, 26 , 28 , 37 and only one by ambulatory blood pressure monitoring procedure. 32 All but two studies 35 , 36 described a careful standardization of the peripheral BP assessment method (ie, average of measurements and total number of measurements). The length of intervention ranged from 1 to 13 weeks. The evaluation of the “risk of bias” indicated that all but four studies were at low risk (Table S2).
3.2. Effect of salt reduction on augmentation index
Detailed features of 14 studies (17 cohorts, 457 total participants) included in this analysis are given in Table 1. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 In the pooled analysis, lower salt intake (average reduction of 24‐hour urinary sodium excretion = 64%, ranged from 27% to 88%) was associated with significantly lower average AIx compared with the higher salt regimen (MD = −9.3%, 95% CI: −15.5 to −3.0, P = .003). There was significant between‐study heterogeneity (P < .01; I2 = 70%) (Figure 2A). Visual analysis of the funnel plot indicated little asymmetry (Figure S1), whereas Egger's and Begg's tests did not find significant evidence of publication bias (Egger: P = .8, Begg: P = .4). A trend toward a direct association between lower salt intake and reduction in AIx was detected in almost all of the cohorts included in the analysis, and it was statistically significant in four of them, while there was a non‐significant opposite trend in only two cohorts (Figure 2A). Sensitivity analysis showed that the average change in AIx did not vary substantially with the exclusion of any individual study (Table S3).
FIGURE 2.
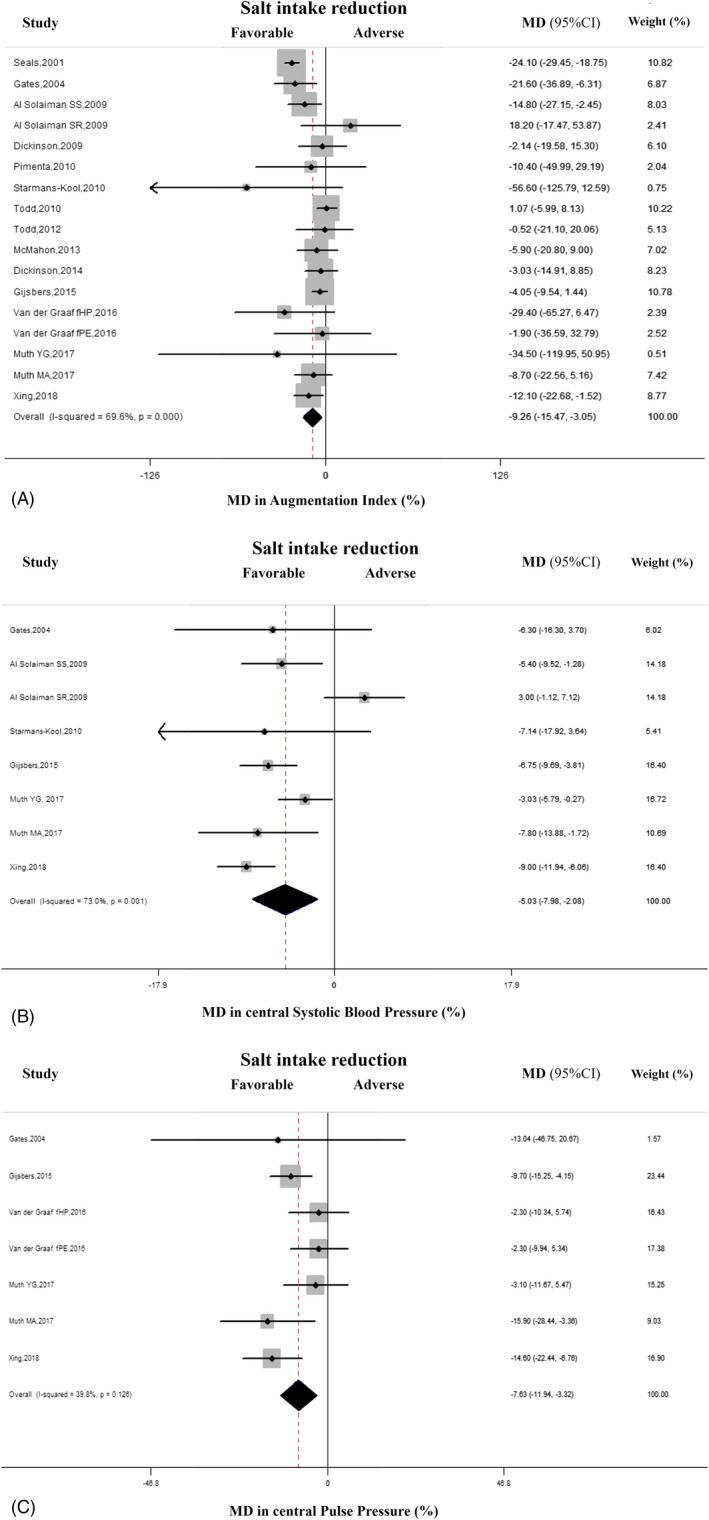
A, Effect of lower sodium intake on augmentation index (AIx). Forest plot of the effect of lower dietary sodium intake on AIx in 17 population cohorts from 14 published studies. B, Effect of lower sodium intake on central systolic blood pressure (cSBP). Forest plot of the effect of lower dietary salt intake on cSBP in eight population cohorts from six published studies. C, Effect of lower sodium intake on central pulse pressure (cPP). Forest plot of the effect of lower dietary sodium intake on cPP in seven population cohorts from five published studies. Results are expressed as mean difference (MD) and 95% confidence intervals (95% CI). Squares indicate study‐specific relative risk estimates (size of the square reflects the study‐specific statistical weight); horizontal lines indicate 95% CI; and diamond indicates the overall relative risk with its 95% CI. fHP, formerly healthy pregnant women; fPE, formerly pre‐eclamptic women; SS, salt‐sensitive participants; SR, salt‐resistant participants; YG, young participants; MA, middle‐aged participants
Separate analysis after exclusion of the cohorts at high risk of bias 24 , 26 , 35 , 37 confirmed a beneficial effect of salt restriction (MD = −4.3%; 95% CI: −8.1 to −0.4). The analysis stratified by countries suggested a stronger effect of salt restriction in studies performed in United States compared to those carried out in Oceania, Europe, or China (P for interaction = .001) (Table 2).
TABLE 2.
Subgroup and meta‐regression analysis of the effect of salt restriction on augmentation index
| Subgroup analysis |
Variables (n. of cohorts) |
Pooled mean (%) reduction AIx |
95% CI | P for interaction |
|---|---|---|---|---|
| Age | <60 (13) | −5.8 | −10.7 to −0.9 | 0.001 |
| >60 y(4) | −13.9 | −26.8 to −1.0 | ||
| Length of intervention | 1 wk (6) | −11.4 | −19.2 to −3.7 | 0.9 |
| >1 wk (11) | −8.3 | −16.2 to −0.4 | ||
| Country of origin | USA (7) | −16.6 | −24.6 to −8.6 | 0.001 |
| Oceania (5) | −0.9 | −6.1 to 4.2 | ||
| Europe (4) | −9.6 | −24.5 to 5.3 | ||
| Asia (1) | −12.1 | −22.7 to 1.5 | ||
| (Pre)Hypertension status | Yes (7) | −10.9 | −20.3 to −1.4 | 0.3 |
| No (10) | −7.3 | −13.4 to −1.2 | ||
| Antihypertensive treatment a | Yes (2) | −6.5 | −20.4 to 7.5 | 0.4 |
| No (14) | −10.8 | −17.7 to −4.0 | ||
| Assessment device of central BP | Sphygmocor (13) | −5.6 | −9.8 to −1.5 | 0.001 |
| Pressure transducer (2) | −23.8 | −28.9 to −18.8 | ||
| Doppler transducer (2) | −2.7 | −12.6 to 7.1 | ||
| Assessment device of peripheral BP b | Automated/semi‐automated sphygmomanometer (12) | −9.7 | −17.8 to −1.5 | 0.7 |
| Mercury sphygmomanometer (4) | −11.7 | −19.4 to −4.0 | ||
| Study design | Randomized controlled trials (13) | −4.5 | −8.2 to −0.7 | 0.06 |
| Non‐randomized controlled trials (4) | −15.2 | −25.6 to −4.8 |
| Meta‐regression analysis |
Reduction in AIx (%) (coefficient) |
95% CI |
|---|---|---|
| Variables (n. of cohorts) | ||
| Age (y)(16) | −0.18 | −0.81 to 0.45 |
| BMI (Kg/m2)(15) | 1.08 | −1.94 to 4.11 |
| Length of intervention (week) | −0.17 | −0.37 to 0.04 |
| Number of participants (n) (17) | 0.05 | −0.21 to 0.31 |
| Gender (% men) (17) | 0.11 | −0.12 to 0.35 |
| SBP at low‐salt intake (mm Hg) (15) | −0.19 | −0.86 to 0.48 |
| SBP at high‐salt intake (mm Hg) (15) | −0.27 | −0.77 to 0.23 |
| DBP at low‐salt intake (mm Hg) (15) | 0.36 | −1.26 to 1.98 |
| DBP at high‐salt intake (mm Hg) (15) | −0.09 | −1.30 to 1.13 |
| SBP difference (reduction in %) (15) | 2.47 | 1.21 to 3.72 |
| DBP difference (reduction in %) (15) | 1.80 | 0.04 to 3.56 |
| AIx at low‐salt intake (%) (17) | 0.51 | −0.54 to 1.56 |
| AIx at high‐salt intake (%) (17) | −0.13 | −1.00 to 0.74 |
| Urinary Na at low‐salt intake (mmol/24 h) (17) | 0.04 | −0.19 to 0.27 |
| Urinary Na at high‐salt intake (mmol/24 h) (17) | 0.03 | −0.01 to 0.13 |
| Urinary Na difference (reduction in %) (17) | −0.06 | −0.38 to 0.25 |
Abbreviations: AIx, augmentation index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Analysis did not include the study by Todd et al (ref. 30), because participants with and without antihypertensive therapy were included.
Analysis did not include the study by McMahon et al (ref. 32) because only ambulatory blood pressure monitoring values were reported.
The change in peripheral BP during the intervention was a significant source of heterogeneity (1% lower systolic or diastolic BP being associated with a decrease in AIx of 2.5% and 1.8%, respectively) (Table 2). The percentage of residual variation due to heterogeneity was reduced from 70% to 20% (systolic BP) and to 47% (diastolic BP). However, the influence of changes in BP disappeared when the cohorts of relatively younger and older people were separately analyzed. Indeed, in the analysis of 11 cohorts of relatively younger participants the reduction in systolic BP and diastolic BP did not affect the changes in AIx (reduction in systolic BP(%): β = 1.8, −0.3‐3.9; reduction in diastolic BP(%): β = 1.4, 1.0‐3.8). Likewise, also in the cohorts of older participants changes in peripheral BP were not sources of heterogeneity (reduction in systolic BP (%): β = 2.3, −6.0‐10.6; reduction in diastolic BP (%): β = 1.0, −8.6‐10.6). BP during high‐ and low‐salt regimen did not affect the relationship between salt intake and AIx (Table 2). Also, AIx values during high‐ and low‐salt intake were not a significant source of heterogeneity (Table 2).
Although meta‐regression analysis did not identify the urinary sodium excretion at high‐ and low‐salt intake as a significant source of heterogeneity (Table 2), the stratification by a cutoff of 90 mmoL/24 hours (median of excretion changes) in urinary sodium excretion changes indicated a significantly greater reduction of the AIx in cohorts with lower change (<90 mmoL/24 hours: MD = −13.7%, −24.7 to −2.8, vs > 90 mmoL/24 hours: MD = −4.3%, −7.9 to −0.8; P for interaction < .01). Of note, these cohorts also had a higher average length of intervention.
A significant reduction in AIx after low‐salt diet was more pronounced in older subjects (more than 60 years) as compared to younger participants (Table 2). Also, the instrumental method was a source of heterogeneity, a significantly higher effect of salt restriction being found in studies that assessed cBP by pressure transducers (P for interaction = .001) (Table 2). On the contrary, the different methods utilized to measure peripheral BP were not a significant source of heterogeneity (Table 2). As well as, the different description of its assessment did not affect the effect of the salt restriction on cBP (P for interaction = .97).
Meta‐regression analysis indicated no influence of BMI, total number of participants, and gender on the association between dietary salt restriction and AIx (Table 2). Subgroup analysis did not detect the hypertensive status, antihypertensive treatment, and study design as significant sources of heterogeneity (Table 2). A similar result was obtained by subgroup and meta‐regression analysis in relation to length of intervention (Table 2).
3.3. Effect of salt reduction on augmentation index adjusted for heart rate
The separate analysis of 6 cohorts including AIx adjusted for heart rate 34 , 35 , 36 , 37 also showed a significant reduction of AIx on dietary salt restriction (MD = −6.3%; −10.9 to −1.8%; P < .01) (Figure S2). There was no significant heterogeneity among the studies (P = .6, I2 = 0%). The funnel plot for the effect of salt restriction on AIx suggested no significant publication bias, which was confirmed by Egger's and Begg's tests (Egger: P = .2, Begg: P = 1.0). A trend toward a direct association between lower salt intake and reduction in AIx was detected in all but one cohort included in the analysis. Sensitivity analysis showed that the average change in AIx did not vary substantially with the exclusion of any individual study. As expected, no features affected the effect of salt restriction.
3.4. Effects of salt reduction on central systolic blood pressure
In total, six studies with eight cohorts and 261 total participants were included in the assessment of the association between salt intake reduction and changes in cSBP. 25 , 26 , 29 , 34 , 36 , 37 In the pooled analysis of eight cohorts, reduced salt intake (average difference in 24‐hour urinary sodium excretion = 67%, ranged from 48% to 88%) was associated with significantly lower cSBP (MD = −5.0%; −8.0 to −2.1%, P = .001), with significant heterogeneity between studies (P = .001, I2 = 73%) (Figure 2B). The funnel plot for the effect of salt restriction on cSBP suggested no significant publication bias, which was confirmed by Egger's and Begg's tests (Egger: P = .97, Begg: P = 1.0) (Figure S3).The evaluation of individual studies showed a trend toward a favorable association between salt reduction and the changes in cSBP in all but one cohort, with significantly lower MD in five of them, whereas a non‐significant opposite trend was observed only in one small cohort. Sensitivity analysis indicated that the decrease in cSBP during salt reduction did not differ after the exclusion of any individual study.
A non‐significant more pronounced reduction in cSBP was detected in the cohorts of old‐aged subjects (MD=−6.7%, −9.5 to −3.9, vs −4.6%, −8.4 to −0.8, P for interaction .2), while meta‐regression analysis indicated the changes in BP as significant sources of heterogeneity (SBP: β = 1.14, 0.57‐1.70; DBP: β = 0.86, 0.18‐1.53), but only in younger participants (P < .05).
3.5. Effects of salt reduction on central pulse pressure
In the pooled analysis of five studies (seven cohorts, 268 total participants), 25 , 34 , 35 , 36 , 37 there was a direct relationship between reduction in salt intake (average difference in 24 hours urinary sodium excretion = 76%, ranged from 48% to 88%) and changes in cPP (MD = −7.6%; 95% CI: −11.9 to −3%‐3%, P = .001) (Figure 2C). There was low heterogeneity among studies (P = .13, I2 = 40%) and no evidence of publication bias by inspection of funnel plot and by Egger's (P = .8) and Begg's tests (P = .7) (Figure S4). The evaluation of individual studies showed a trend toward an association between reduction in salt intake and cPP in all cohorts, with a significant change in three of them. Sensitivity analysis showed that the average change in cPP did not vary substantially with the exclusion of any individual study. Meta‐regression analysis did not detect any significant sources of heterogeneity (P > .05).
3.6. Effects of salt reduction on peripheral blood pressure
A meta‐analysis of the effects of salt restriction on brachial BP in the same cohorts was performed. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 36 , 37 Pooled analyses showed a significant reduction of both systolic (MD = −4.9%, −6.6 to −3.2) and diastolic BP (MD=−3.3%, −5.2 to −1.4) upon reduction of salt intake. There was high heterogeneity among studies (systolic BP: I2 = 73%, P < .01; diastolic BP: I2 = 80%, P < .01), whereas no evidence of publication bias was detected (Egger's test, systolic BP:P = .8, diastolic BP:P = .3; Begg's tests, systolic BP:P = .6, diastolic BP:P = .5).
The reduction in BP after salt restriction was detected both in younger and older participants, but it was more pronounced in old‐aged subjects (systolic BP, MD=−6.9%, −9.6 to −4.3 vs −4.2%, −6.4 to −2.1; p for interaction = 0.01; diastolic BP, MD=−5.2%, −8.0 to −2.3 vs −2.8%, −5.3 to −0.2, p for interaction = 0.02).
Additional analyses did not detect significant difference between changes in brachial BP and cBP (p for interaction > 0.1).
4. DISCUSSION
The results of this meta‐analysis indicate that reduction of dietary salt intake is associated with lowering cBP. The effect of lower salt intake was more pronounced after prolonged salt reduction, and in fact, greater reduction in cBP was achieved in the cohorts with relatively lower levels of salt reduction but higher length of intervention.
The effect of low‐salt intake on cBP was not limited to hypertensive patients as indeed a significant reduction in cBP was detected both in the cohorts including pre‐hypertensive and/or hypertensive participants and in the cohorts that enrolled only normotensive individuals.
By contrast, age was an important cause of heterogeneity in the response of cBP to salt restriction in as much as the effect of the latter was greater in the cohorts including older participants. This result may be at least partly explained by the greater salt sensitivity of older compared with younger subjects. 7 , 43 On the other hand, gender, BMI, and heart rate did not seem to play a role in the response of cBP to reduction in salt intake. With regard to the study design, there was not a significant difference between results of randomized controlled trials and not.
4.1. Potential mechanisms involved
Although our study had no specific potential to address the mechanisms of the effect of salt intake restriction on cBP, a few considerations can be made as to the possible explanations of our study results. In the first place, because of the calibration method of cBP assessment, that includes brachial BP values, 7 it might be hypothesized that the decrease in cBP upon reduction in salt intake is determined by the decrease in peripheral BP. Our analyses indicate that the peripheral BP at high and at low‐salt intake did not affect the relationship between salt intake and cBP. However, our results suggest that changes in peripheral BP did not fully explain the reduction in cBP. Of note, although the effect of salt restriction on changes in cBP seems greater than those on peripheral BP, this difference is not statistically significant.
There is experimental evidence in support of the relationship between salt intake and cBP. In particular, a large body of evidence indicated a reduced nitric oxide bioavailability after sodium loading due to increase in reactive oxygen species and reduction of endothelial nitric oxide synthase activity. 18 , 19 , 20 , 21 , 44 , 45 , 46 , 47 The effects of salt loading were especially seen in smooth muscle cells, which featured decreased availability of and reduced responsiveness to nitric oxide, 22 , 23 increased sympathetic nerve activity, 48 and increased renin‐angiotensin‐aldosterone system (RAAS) activity. 49 , 50 , 51 , 52 , 53 Also, salt sensitivity of BP might play a role, 43 but only one study with a weak methodology among those included in our meta‐analysis evaluated this condition. 26
4.2. Study strengths and limitations
Major strengths of our meta‐analysis are the following: (a) the finding of a trend to reduction of cBP upon dietary salt restriction in the majority of the cohorts examined; (b) the “low risk” of bias in the majority of the studies; (c) the lack of detectable publication bias; (d) the inclusion of only intervention trials with exclusive evaluation of the salt effect; (e) the inclusion as outcomes of only non‐invasively assessed cBP parameters; and (f) the measurement of 24 hours urinary sodium excretion, a recognized gold standard for monitoring salt intake, 54 in all but three studies.
Nonetheless, this meta‐analysis has some limitations: the first one is the inability to rationally assess a dose‐effect relationship between salt intake reduction and decrease in cBP. Second, this study does not allow to draw definitive conclusions about the long‐term effects of dietary salt restriction on cBP, given that only one trial included in the meta‐analysis had an intervention period longer than 6 weeks. Third, our meta‐analysis was conducted based on aggregated data and not on individual data, so limiting the possibility to carry out additional potential analyses. Fourth, some characteristics of the studies included in the meta‐analysis represent inherent limitations of the study. In particular, the potential influence of the concomitant antihypertensive drug treatment cannot be definitely ruled out also because of the small number of studies with antihypertensive treatment included and the mixed therapy considered. Although there was a greater reduction of cBP in the participants without antihypertensive therapy, the subgroup analysis did not detect significant difference. On the other hand, previous meta‐analyses showed a greater effect of salt restriction on subclinical organ damage during concomitant administration of renin‐angiotensin‐aldosterone blockers. 14 , 15 However, they included a greater number of studies with antihypertensive treatment.
Likewise, the heterogeneity of method used in included studies may be a limitation. Although there are no studies directly comparing the methods, there may be differences between the output of the methods, especially cPP and AIx, because of high‐frequency signals. Indeed, carotid applanation tonometry does not use transfer function, while radial tonometry uses a transfer function with calibration from brachial BP. Therefore, use of brachial BP may introduce some errors. However, the potential influence of the measurement device used in the different studies cannot be correctly assessed since the majority of the studies utilized applanation tonometry in respect to small number of studies that used other devices. On the other hand, the different methods utilized to evaluate peripheral BP did not affect the results. Likewise, also a careful description of the peripheral BP assessment method was not a significant source of heterogeneity.
Also, the evaluation of race differences cannot be assessed, although subgroup analysis indicated country of origin as potential source of heterogeneity.
Another possible limitation was given by the small sample size of most of the available studies, high heterogeneity of studies' characteristics, for example, length of intervention, magnitude of salt restriction, and participants' features.
Finally, although the cBP has been debated in its usefulness in the risk prediction compared to brachial BP, some evidence suggested that cBP is more closely associated with cardiovascular organ damage and a better predictor of future cardiovascular events than brachial BP. 4 , 5 , 6 , 7
4.3. Implications for public health
In keeping with previous demonstrations of a favorable effect of salt restriction on cardiovascular damage, 14 , 15 the results of this meta‐analysis support the concept of a protective role of lower dietary salt in individuals both with (pre)hypertension and not. This concept is in line with the recognized beneficial effect of moderate dietary salt restriction on BP.
The results of the present study have important implications for public health: cBP (expressed as AIx or cSBP or cPP) is a recognized predictor of cardiovascular events. 3 Based on previous studies indicating increments of 38% in all‐cause mortality and of 32% in cardiovascular events for 10% increase in AIx, 3 a decrease in AIx upon reduction of salt intake is expected to translate into a substantial reduction in cardiovascular risk. Likewise, in consideration of the increase in cardiovascular events of 13% for an increase of cSBP or cPP by 10 mm Hg, 3 a substantial reduction of this risk is expected as a result of dietary salt restriction.
Based on our results, benefit from salt intake restriction may be expected in younger and older people, during moderate long‐term salt restriction, and independently of baseline risk.
As the habitual salt intake in most countries in the world is close to 10 g per day, 55 , 56 , 57 , 58 an average reduction of 60% per day, as in our meta‐analysis, would lead to the achievement of the recommended target of 5 g or lower per day for the population 55 : this observation suggests that the results of our study could be applicable to real life conditions and are relevant to population‐based strategies for reduction of salt intake. 59
5. CONCLUSIONS AND PERSPECTIVES
The results of this study show that dietary salt restriction reduces cBP parameters, at least in part independently from the concomitant changes in peripheral BP. In consideration of the importance of cBP as a predictor of cardiovascular morbidity and mortality and that the non‐invasive assessment underestimates the actual cBP values, 60 this effect of salt restriction significantly adds to its recognized value in cardiovascular disease prevention. In addition, these findings indicate cBP as a possible additional parameter to clinically evaluate the response to salt reduction. Our results support the recommendations in favor of moderate and long‐term reduction in dietary salt intake to decrease the risk of cardiovascular diseases. Future powered randomized controlled trials should be carried out to focus on the effect of long‐term moderate dietary salt reduction on central hemodynamics, to further support the conclusions of our review and extend current knowledge in this field.
CONFLICT OF INTEREST
LD was a technical advisor to the World Health Organization and is a member of the scientific committee of the Italian Society of Human Nutrition. PS is an unpaid member of WASH, scientific coordinator of the Interdisciplinary Working Group for Reduction of Salt Intake in Italy (GIRCSI), and member of the committee for the preparation of the Italian Nutritional Guidelines. The remaining authors do not disclose any conflict of interest.
AUTHORS' CONTRIBUTIONS
Lanfranco D'Elia, Ersilia La Fata, and Ferruccio Galletti designed the study; Lanfranco D'Elia, Ersilia La Fata, and Alfonso Giaquinto conducted the systematic literature review and extracted the data; Lanfranco D'Elia conducted the statistical analysis; Lanfranco D'Elia and Ferruccio Galletti wrote the manuscript; Ersilia La Fata, Alfonso Giaquinto, and Pasquale Strazzullo contributed to the manuscript.
Disclosures
Part of the preliminary study data was previously presented at the British and Irish Hypertension Society 2019 Meeting.
Supporting information
Figure S1
Figure S2
Figure S3
Figure S4
Table S1‐S3
D'Elia L, La Fata E, Giaquinto A, Strazzullo P, Galletti F. Effect of dietary salt restriction on central blood pressure: A systematic review and meta‐analysis of the intervention studies. J Clin Hypertens. 2020;22:814–825. 10.1111/jch.13852
REFERENCES
- 1. Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the strong heart study. J Hypertens. 2010;28:384‐388. [DOI] [PubMed] [Google Scholar]
- 2. Jankowski P, Kawecka‐Jaszcz K, Czarnecka D, et al. Ascending aortic, but not brachial blood pressure‐derived indices are related to coronary atherosclerosis. Atherosclerosis. 2004;176:151‐155. [DOI] [PubMed] [Google Scholar]
- 3. Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with central haemodynamics: a systematic review and meta‐analysis. Eur Heart J. 2010;31(15):1865‐1871. [DOI] [PubMed] [Google Scholar]
- 4. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension. 2007;50:197‐203. [DOI] [PubMed] [Google Scholar]
- 5. Safar ME, Blacher J, Pannier B, et al. Central pulse pressure and mortality in end‐stage renal disease. Hypertension. 2002;39:735‐738. [DOI] [PubMed] [Google Scholar]
- 6. Pini R, Cavallini MC, Palmieri V, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe dicomano study. J Am Coll Cardiol. 2008;51:2432‐2439. [DOI] [PubMed] [Google Scholar]
- 7. McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35(26):1719‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asmar RG, London GM, O'Rourke ME, Safar ME. Improvement in blood pressure, arterial stiffness and wave reflections with a very‐low‐dose perindopril/indapamide combination in hypertensive patient: a comparison with atenolol. Hypertension. 2001;38:922‐926. [DOI] [PubMed] [Google Scholar]
- 9. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the conduit artery function evaluation (CAFE) study. Circulation. 2006;113:1213‐1225. [DOI] [PubMed] [Google Scholar]
- 10. Jennings A, Berendsen AM, de Groot LCPGM, et al. Mediterranean‐style diet improves systolic blood pressure and arterial stiffness in older adults. Hypertension. 2019;73(3):578‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumagai H, Zempo‐Miyaki A, Yoshikawa T, Tsujimoto T, Tanaka K, Maeda S. Lifestyle modification increases serum testosterone level and decrease central blood pressure in overweight and obese men. Endocr J. 2015;62(5):423‐430. [DOI] [PubMed] [Google Scholar]
- 12. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 13. Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D'Elia L, Rossi G, Schiano di Cola M, Savino I, Galletti F, Strazzullo P. Meta‐analysis of the effect of dietary sodium restriction with or without concomitant renin–angiotensin–aldosterone system‐inhibiting treatment on albuminuria. Clin J Am Soc Nephrol. 2015;10:1542‐1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D'Elia L, Galletti F, La Fata E, Sabino P, Strazzullo P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta‐analysis of the randomized controlled trials. J. Hypertens. 2018;36:734‐743. [DOI] [PubMed] [Google Scholar]
- 16. Han W, Han X, Sun N, Chen Y, Jiang S, Li M. Relationships between urinary electrolytes excretion and central hemodynamics, and arterial stiffness in hypertensive patients. Hypertens Res. 2017;40(8):746‐751. [DOI] [PubMed] [Google Scholar]
- 17. Polonia JJ, Magalhaes MT, Senra D, Barbosa L, Silva JA, Ribeiro SM. Association of 24‐h urinary salt excretion with central haemodynamics and assessment of food categories contributing to salt consumption in Portuguese patients with hypertension. Blood Press Monit. 2013;18:303‐310. [DOI] [PubMed] [Google Scholar]
- 18. Bragulat E, de la Sierra A, Antonio MT, Coca A. Endothelial dysfunction in salt‐sensitive essential hypertension. Hypertension. 2001;37:444‐448. [DOI] [PubMed] [Google Scholar]
- 19. Hermann M, Camici G, Fratton A, et al. Differential effects of selective cyclooxygenase‐2 inhibitors on endothelial function in salt‐induced hypertension. Circulation. 2003;108:2308‐2311. [DOI] [PubMed] [Google Scholar]
- 20. Nickenig G, Strehlow K, Roeling J, Zolk O, Knorr A, Bo¨hm M. Salt induces vascular AT1 receptor overexpression in vitro and in vivo. Hypertension. 1998;31:1272‐1277. [DOI] [PubMed] [Google Scholar]
- 21. Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens. 2001;14:155‐163. [DOI] [PubMed] [Google Scholar]
- 22. Matrougui K, Levy BI, Schiavi P, Guez D, Henrion D. Indapamide improves flow‐induced dilation in hypertensive rats with a high salt intake. J Hypertens. 1998;16:1485‐1490. [DOI] [PubMed] [Google Scholar]
- 23. Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagin, is a novel mediator of sodium chloride‐dependent hypertension. Circulation. 2002;105:1122‐1127. [DOI] [PubMed] [Google Scholar]
- 24. Seals DR, Tanaka H, Clevenger CM, et al. Blood pressure reductions with exercise and sodium restriction in postmenopausal women with elevated systolic pressure: role of arterial stiffness. J Am Coll Cardiol. 2001;38(2):506‐513. [DOI] [PubMed] [Google Scholar]
- 25. Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35‐41. [DOI] [PubMed] [Google Scholar]
- 26. Al‐Solaiman Y, Jesri A, Zhao Y, Morrow JD, Egan BM. Low‐sodium DASH reduces oxidative stress and improves vascular function in salt‐sensitive humans. J Hum Hypertens. 2009;23(12):826‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dickinson KM, Keogh JB, Clifton PM. Effects of a low‐salt diet on flow‐mediated dilatation in humans. Am J Clin Nutr. 2009;89(2):485‐490. [DOI] [PubMed] [Google Scholar]
- 28. Pimenta E, Gaddam KK, Oparil S, et al. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;54(3):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Starmans‐Kool MJ, Stanton AV, Xu YY, McG Thom SA, Parker KH, Hughes AD. High dietary salt intake increases carotid blood pressure and wave reflection in normotensive healthy young men. J Appl Physiol (1985). 2011;110(2):468‐471. [DOI] [PubMed] [Google Scholar]
- 30. Todd AS, Macginley RJ, Schollum JB, et al. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr. 2010;91(3):557‐564. [DOI] [PubMed] [Google Scholar]
- 31. Todd AS, Macginley RJ, Schollum JB, et al. Dietary sodium loading in normotensive healthy volunteers does not increase arterial vascular reactivity or blood pressure. Nephrology. 2012;17(3):249‐256. [DOI] [PubMed] [Google Scholar]
- 32. McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol. 2013;24(12):2096‐2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dickinson KM, Clifton PM, Keogh JB. A reduction of 3 g/day from a usual 9 g/day salt diet improves endothelial function and decreases endothelin‐1 in a randomised cross_over study in normotensive overweight and obese subjects. Atherosclerosis. 2014;233(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 34. Gijsbers L, Dower JI, Mensink M, Siebelink E, Bakker SJ, Geleijnse JM. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. J Hum Hypertens. 2015;29(10):592‐598. [DOI] [PubMed] [Google Scholar]
- 35. van der Graaf AM, Paauw ND, Toering TJ, et al. Impaired sodium‐dependent adaptation of arterial stiffness in formerly preeclamptic women: the RETAP‐vascular study. Am J Physiol Heart Circ Physiol. 2016;310(11):H1827‐H1833. [DOI] [PubMed] [Google Scholar]
- 36. Muth BJ, Brian MS, Chirinos JA, Lennon SL, Farquhar WB, Edwards DG. Central systolic blood pressure and aortic stiffness response to dietary sodium in young and middle‐aged adults. J Am Soc Hypertens. 2017;11(10):627‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xing X, Liu F, Yang X, et al. Central blood pressure responses to dietary sodium and potassium interventions. Am J Hypertens. 2018;31(5):582‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA group: preferred reporting items for systematic review and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC.Chapter 8: assessing risk of bias in a randomized trial. cochrane handbook for systematic reviews of interventions. https://training.cochrane.org/handbook/current/chapter‐08. (Accessed August 1, 2019).
- 40. Dersimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177‐188. [DOI] [PubMed] [Google Scholar]
- 41. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomized controlled trials. BMJ. 2011;342:d4002. [DOI] [PubMed] [Google Scholar]
- 42. Hu J, Jiang X, Li N, et al. Effects of salt substitute on pulse wave analysis among individuals at high cardiovascular risk in rural China: a randomized controlled trial. Hypertens Res. 2009;32(4):282‐288. [DOI] [PubMed] [Google Scholar]
- 43. Strazzullo P, Galletti F, D'Elia L. Salt‐sensitivity of blood pressure. In Huhtaniemi I, Martini L, eds. Encyclopedia of Endocrine Diseases 2nd Edn. Cambridge, MA:Academic Press. 2019:558‐563. 10.1016/B978-0-12-801238-3.64332-5 [DOI] [Google Scholar]
- 44. Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium‐dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol. 2000;279(1):H14. [DOI] [PubMed] [Google Scholar]
- 45. Greaney JL, DuPont JJ, Lennon‐Edwards SL, Sanders PW, Edwards DG, Farquhar WB. Dietary sodium loading impairs microvascular function independent of blood pressure in humans: role of oxidative stress. J Physiol. 2012;590(21):5519‐5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dishy V, Sofowora GG, Imamura H, et al. Nitric oxide production decreases after salt loading but is not related to blood pressure changes or nitric oxide‐mediated vascular responses. J Hypertens. 2003;21:153‐157. [DOI] [PubMed] [Google Scholar]
- 47. Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation. 2000;101:856‐861. [DOI] [PubMed] [Google Scholar]
- 48. Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha‐adrenergic vasoconstriction. J Physiol. 2001;536:977‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Izzo JL Jr. Systolic hypertension, arterial stiffness, and vascular damage: role of the renin‐angiotensin system. Blood Press Monit. 2000;5:S7‐S11. [DOI] [PubMed] [Google Scholar]
- 50. McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin‐1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975‐1981. [DOI] [PubMed] [Google Scholar]
- 51. Matavelli LC, Zhou X, Varagic J, Susic D, Frohlich ED. Salt loading produces severe renal hemodynamic dysfunction independent of arterial pressure in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H814‐H819. [DOI] [PubMed] [Google Scholar]
- 52. Frohlich ED. The salt conundrum: a hypothesis. Hypertension. 2007;50:161‐166. [DOI] [PubMed] [Google Scholar]
- 53. Kocks MJ, Buikema H, Gschwend S, Boomsma F, de Zeeuw D, Navis G. High dietary sodium blunts affects of angiotensin‐converting enzyme inhibition on vascular angiotensin I‐to‐angiotensin II conversion in rats. J Cardiovasc Pharmacol. 2003;42:601‐606. [DOI] [PubMed] [Google Scholar]
- 54. World Health Organization . How to obtain measures of population‐level sodium intake in 24‐hour urine samples; WHO‐EM/NUT/279/E; world health organization/regional office of the eastern Mediterranean. Cairo, Egypt: World Health Organization; 2018;1‐51. [Google Scholar]
- 55. World Health Organization . Guideline. Sodium Intake for Adults and Children; World Health Organization (WHO). Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 56. D'Elia L, Brajovic M, Klisic A, et al. Sodium and potassium intake, knowledge attitudes and behaviour towards salt consumption amongst adults in Podgorica, Montenegro. Nutrients. 2019;11(1):160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Venezia A, Barba G, Russo O, et al. Dietary sodium intake in a sample of adult male population in southern Italy: results of the olivetti heart study. Eur J Clin Nutr. 2010;64(5):518‐524. [DOI] [PubMed] [Google Scholar]
- 58. Vasara E, Marakis G, Breda J, et al. Sodium and potassium intake in healthy adults in thessaloniki greater metropolitan area‐the salt intake in Northern Greece (SING) Study. Nutrients. 2017;22:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cappuccio FP, Beer M, Strazzullo P, European Salt Action Network . Population dietary salt reduction and the risk of cardiovascular disease. A scientific statement from the European salt action network. Nutr Metab Cardiovasc Dis. 2018;29(2):107‐114. [DOI] [PubMed] [Google Scholar]
- 60. Papaioannou TG, Karageorgopoulou TD, Sergentanis TN, et al. Accuracy of commercial devices and methods for non invasive estimation of aortic systolic blood pressure a systematic review and meta‐analysis of invasive validation studies. J Hypertens. 2016;34(7):1237‐1248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Figure S3
Figure S4
Table S1‐S3


