Abstract
Increased arterial stiffness is independently associated with renal function decline in patients with diabetes mellitus (DM). Whether DM has additional deleterious effects on central hemodynamics and arterial stiffness in chronic kidney disease (CKD) patients is yet unknown. This study aimed to compare ambulatory central BP, arterial stiffness parameters, and trajectories between patients with diabetic and non‐diabetic CKD. This study examined 48 diabetic and 48 non‐diabetic adult patients (>18 years) with CKD (eGFR: <90 and ≥15 ml/min/1.73 m2), matched in a 1:1 ratio for age, sex, and eGFR within CKD stages (2, 3a, 3b and 4). All patients underwent 24‐h ABPM with the Mobil‐O‐Graph device. Parameters of central hemodynamics [central systolic (cSBP) and diastolic blood pressure (cDBP), pulse pressure (PP)], wave reflection [augmentation index (AIx), and pressure (AP)] and pulse wave velocity (PWV) were estimated from the 24‐h recordings. Diabetic CKD patients had higher 24‐h cSBP (118.57 ± 10.05 vs. 111.59 ± 9.46, P = .001) and 24‐h cPP (41.48 ± 6.80 vs. 35.25 ± 6.98, P < .001) but similar 24‐h cDBP (77.09 ± 8.14 vs. 76.34 ± 6.75 mmHg, P = .625) levels compared to patients with non‐diabetic CKD. During day‐ and nighttime periods, cSBP and cPP levels were higher in diabetics compared to non‐diabetics. 24‐h PWV (10.10 ± 1.62 vs. 9.61 ± 1.80 m/s, P = .165) was numerically higher in patients with DM, but no between‐group differences were noted in augmentation pressure and index. In multivariate analysis, DM, female gender, and peripheral SBP were independently associated with higher cPP levels. Patients with diabetic CKD have higher ambulatory cSBP and increased arterial stiffness, as indicated by higher ambulatory cPP. These finding suggest that DM is a factor independently contributing to the adverse macrocirculatory profile of CKD patients.
Keywords: ambulatory blood pressure monitoring, arterial stiffness, central blood pressure, diabetes mellitus, pulse pressure
1. INTRODUCTION
Diabetes mellitus (DM) is a major cardiovascular risk factor and over the past decades presents a gradually increasing prevalence estimated at 9%‐10% of adults. 1 One of the most common complications in patients with DM is diabetic kidney disease, presenting in about 40% of these individuals. 2 Cardiovascular events, including sudden death, myocardial infarction, stroke, or decompensated heart failure, are the main causes of mortality in DM, accounting for 50% of deaths. 3 On the other hand, the risk of cardiovascular events rises exponentially as the glomerular filtration rate (GFR) declines. 4 Hypertension is another common complication accompanying both DM and CKD, being present in >90% of type‐2 diabetics, as well as in 80% of the patients with CKD stage 1 increasing to >90% in CKD Stages 4‐5. 5 , 6
Observational data from the general population suggest that central blood pressure (BP) may not reliably inferred from peripheral BP levels. 7 Central BP levels present stronger associations with cardiovascular complications and target organ damage, including LV mass, intima–media thickness, glomerular filtration rate, and proteinuria, compared with brachial BP levels in several populations. 8 In patients with DM, longitudinal studies indicate that central BP is of higher prognostic significance compared to brachial BP. 9 This can be mainly attributed to increased arterial stiffness, that is, the combined result of structural and functional changes in the viscoelastic properties of the arterial wall, which impair the large artery cushioning function leading to increased central systolic BP (cSBP) and pulse pressure (PP). 10 The underlying mechanisms of these changes are poorly understood, but accelerated collagen cross‐linking from advanced glycation end‐products, aortic wall calcification, endothelial dysfunction chronic inflammation, increased oxidative stress, and sympathetic nervous system are considered to have a key role in this process. 11 All these phenomena are profound in patients with impaired glucose tolerance or DM. 12 In addition, impaired cushioning function is the predominant vascular abnormality in CKD 13 and, thus, arterial stiffness may increase even further in diabetic patients who develop diabetic kidney disease. 14
Previous evidence on the effect of DM on central hemodynamics and arterial stiffness derives mainly from unmatched studies, focusing on brachial BP and arterial stiffness measured in office conditions and with results being contradictory. 15 A prospective study including 211 individuals with type‐2 DM with eGFR ≥ 45 ml/min suggested that increased arterial stiffness was an independent factor of renal function decline in younger (<60 years) individuals. 16 To the best of our knowledge, no study so far has examined another important question, whether the presence of DM per se has an additional effect on central hemodynamics and arterial stiffness in patients with CKD using ambulatory BP monitoring. Thus, the aim of this study was to examine the effect of DM on ambulatory central BP and arterial stiffness parameters and trajectories by comparing matched patients with diabetic and non‐diabetic CKD.
2. MATERIALS AND METHODS
2.1. Population
This is a prespecified secondary analysis of a previous work of our group including ambulatory peripheral SBP as the primary end point. 17 The study population consisted of adult (>18 years) patients with diabetic and non‐diabetic chronic kidney disease (CKD) at stages 2‐4 (CKD‐EPI eGFR: <90 and ≥15 ml/min/1.73 m2), followed in the outpatient clinics of our Nephrology Department. We excluded patients with: (a) biopsy‐proven primary glomerulonephritis treated with immunosuppressive agents currently or in the past; (b) biopsy‐proven secondary glomerulonephritis treated with immunosuppressive agents currently or in the past; (c) hereditary nephropathies (autosomal dominant polycystic kidney disease, Alport syndrome etc); (d) myocardial infarction or unstable angina episode within the past 3 months or congestive heart failure class III‐IV according to New York Heart Association criteria; (e) chronic atrial fibrillation or other arrhythmia possibly interfering with the ABPM; and (f) modification of antihypertensive treatment during one month prior to study enrollment. Diabetes was defined as previous diagnosis of type‐1 or type‐2 DM, according to the American Diabetes Association criteria. 18 All study participants provided informed written consent to participate in the study.
For the purposes of the study, a blinded investigator performed matching of patients with diabetic CKD that consented to participate in the study (cases) with potential controls in a 1:1 ratio for age, sex, and eGFR within each CKD stage (2, 3a, 3b and 4). Potential controls were patients with non‐diabetic CKD actively followed in our outpatient clinics and having scheduled appointments within the next 3 months. After the matching process, controls were invited to participate in the study during their upcoming appointment. The protocol was approved by the ethical committee of the School of Medicine, Aristotle University of Thessaloniki, and all investigations were performed according to the Declaration of Helsinki (2013 amendment).
2.2. Protocol procedures and assessments
Participants visited the study center in a scheduled morning (between 7 and 8 am) after a 12‐h period of fasting and refraining from smoking, caffeine, and alcohol from awakening. All patients were also instructed to perform a 24‐h urine collection ending at the morning of the baseline evaluation to measure urine albumin, creatinine, sodium, and potassium levels. A study investigator recorded baseline demographics, anthropometric characteristics, medical history, and concomitant medications were recorded. A detailed physical examination of the participants was also performed. Office BP was recorded with a validated oscillometric device and a cuff of appropriate size with the patient sitting for at least 10 min and with three measurements per occasion taken 2 min apart. Venous blood sampling for routine laboratory tests also took place.
After blood sampling, all participants underwent a 24‐h ABPM with the Mobil‐O‐Graph NG (IEM). During ABPM, participants were instructed to continue their regular medication and carry out their usual activities. The device was monitoring BP every 20 min during the daytime (7:00 to 23:00) and every 30 min during the nighttime (23:00 to 7:00). Measurements were used for the analysis if >80% of recordings were valid with ≤2 non‐consecutive day‐hours with <2 valid measurements and ≤1 night‐hour without valid recording. 19 The following day, patients returned to the study center for the Mobil‐O‐Graph device to be removed. Patients with invalid measurements were invited to undertake the ABPM within the next day or the next available working day in case of upcoming weekend. In this analysis, only measurements recorded at the prespecified time intervals at which the device was set to take measurements were used.
2.3. Ambulatory monitoring of BP and central hemodynamics
The Mobil‐O‐Graph NG (IEM) is an oscillometric device, whose brachial BP detection unit was validated according to standard protocols and was shown to provide practically identical values with a widely used ABPM monitor. 20 The device calculates various indexes, such as augmentation pressure (AP), AIx, central SBP (cSBP), DBP (cDBP), pulse pressure (PP), and PWV. In brief, after recording brachial BP, the cuff is automatically re‐inflated above DBP for approximately 10 s and records brachial pulse waves with a high‐fidelity pressure sensor (MPX5050, Freescale). Brachial BP is then used for the calibration of the pulse waveform. Thereafter, the aortic pulse waveform is reconstructed by the software (HMS version 5.1) using a ARCSolver algorithm with a generalized transfer function, as previously described. 21 , 22 Wave separation analysis is performed by decomposing the aortic pulse waveform into forward‐traveling (incident) and backward‐traveling (reflected) pulse waves with a triangular aortic flow waveform. The generated aortic pulse wave is used for the pulse wave analysis, while the PWV is estimated via mathematical models taking into account the characteristic impedance and age and assuming a 3‐element Windkessel model. 21 , 22 Previous validation studies in healthy volunteers and diseased populations showed acceptable agreement between Mobil‐O‐Graph‐derived parameters and invasive and non‐invasive measurements. 22 , 23 , 24
Calibration of pulse waves was performed with the brachial SBP and DBP (C1) method. The raw Mobil‐O‐Graph dataset obtained for each patient was exported in a Microsoft Excel file. Separate Excel files were built for each of the parameters of interest, that is, cSBP, cDBP, cPP, AP, AIx(75), and PWV. In these, each column represented a Mobil‐O‐Graph recording at a prespecified time‐point (ie 07:00 am, 07:20 am, etc), and each line represented data for each patient. Average values for every time‐point were calculated for diabetic and non‐diabetic CKD groups and were transformed in graphic depictions for the compared groups with Excel.
2.4. Statistical analysis
Statistical analysis was performed with Statistical Package for Social Sciences 22 (SPSS Inc). Continuous variables are expressed as mean values ± standard deviation (SD) or median [interquartile range] according to the normality of the distribution Categorical variables are presented as absolute frequencies and percentages (n, %). Comparisons for continuous variables were performed with the Student t test or the Mann–Whitney U test, according to the normality of the distribution. Chi‐square or Fishers exact test was used for comparisons of categorical variables. Probability values of P < .05 (two‐tailed) were considered statistically significant. To evaluate possible factors associated with 24‐h cSBP levels, we performed uni‐ and multivariate analyses (enter method). Variables were included in the multivariate model if P < .2 in univariate analysis. The β‐coefficients are reported with 95% confidence intervals (CI). A study sample of 96 patients (48 per group) was calculated to have > 80% power to detect a between‐group difference in the primary end point (24‐h peripheral SBP) of 6 mmHg, with α = .05 (two‐sided) and SD of 10 mmHg, as described previously. 17
3. RESULTS
3.1. Demographic and clinical characteristics of study participants
Table 1 presents baseline demographic, clinical, and biochemical characteristics in CKD patients with and without DM. Age (69.44 ± 9.73 vs. 68.35 ± 10.52; P = .602) and BMI (82.73 ± 17.33 vs. 84.21 ± 16.92 P = .673) were similar in the two groups. No differences were evident in existing risk factors and comorbidities between diabetics and non‐diabetics, except for dyslipidemia, which was more prevalent in the former (81.3% vs. 62.5%; P = .041). With the exception of RAS blockers, use of antihypertensive drugs, statin, and erythropoietin was similar in the two groups. This was the case for 24‐h urine albumin and parathyroid hormone. As expected, glucose and HbA1c levels were higher in patients with DM.
Table 1.
Demographic, clinical, and routine biochemical characteristics in patients with diabetic and non‐diabetic CKD
| Parameter | Group | p | |
|---|---|---|---|
| Diabetic CKD | Non‐diabetic CKD | ||
| Ν | 48 | 48 | ‐ |
| Age (years) | 69.44 ± 9.73 | 68.35 ± 10.52 | .602 |
| Male (n, %) | 29 (60.4%) | 29 (60,4%) | 1.000 |
| Weight (kg) | 82.73 ± 17.33 | 84.21 ± 16.92 | .673 |
| BMI (m2) | 29.89 ± 6.49 | 29.45 ± 6.14 | .733 |
| Active smoking (n, %) | 12 (25.0%) | 9 (18.8%) | .459 |
| Hypertension (n, %) | 47 (97.9%) | 47 (97.9%) | 1.000 |
| Coronary heart disease (n, %) | 10 (20.8%) | 7 (14.9%) | .450 |
| Stroke (n, %) | 4 (8.3%) | 2 (4.2%) | .677 |
| Peripheral vascular disease (n, %) | 5 (10.4%) | 8 (16.7%) | .371 |
| Heart failure (n, %) | 1 (2.1%) | 3 (6.3%) | .617 |
| Dyslipidemia (n, %) | 39 (81.3%) | 30 (62.5%) | .041 |
| Antihypertensive (any) (n, %) | 47 (97.9%) | 47 (97.9%) | 1.000 |
| ACEI or ARB (n, %) | 36 (75.0%) | 26 (54.2%) | .033 |
| Diuretics (n, %) | 33 (68.8%) | 26 (54.2%) | .142 |
| CCB (n, %) | 38 (79.2%) | 33 (68.8%) | .352 |
| Β–blocker (n, %) | 28 (58.3%) | 24 (50.0%) | .413 |
| Statin (n, %) | 37 (77.1%) | 29 (60.4%) | .078 |
| Erythropoietin (n, %) | 2 (4.2%) | 3 (6.3%) | .646 |
| Insulin (n, %) | 22 (45.8%) | 0 (0%) | <.001 |
| Oral hypoglycemic agents (n, %) | 40 (83.3%) | 0 (0%) | <.001 |
| Hemoglobin (gr/dl) | 13.25 ± 1.46 | 13.32 ± 1.50 | .810 |
| HbA1c (%) | 7.02 ± 0.68 | 5.56 ± 0.43 | <.001 |
| Glucose (mg/dl) | 140.88 ± 40.46 | 89.65 ± 11.11 | <.001 |
| eGFR (ml/min/1.73 m2) | 46.69 ± 18.63 | 46.78 ± 17.85 | .981 |
| 24‐h Urine albumin (mg/24 h) | 185.75 [1203.12] | 178.95 [554.73] | .235 |
| 24‐h Urine sodium (mmol/24 h) | 144.17 ± 54.19 | 128.50 ± 57.49 | .173 |
| Uric acid (mg %) | 6.58 ± 1.72 | 6.34 ± 1.35 | .450 |
| Potassium (mEq/L) | 4.72 ± 0.45 | 4.56 ± 0.37 | .071 |
| Sodium (mEq/L) | 138.58 ± 2.81 | 138.79 ± 2.55 | .705 |
| Calcium (mg/dl) | 9.29 ± 0.75 | 9.48 ± 0.43 | .126 |
| Parathormone (pg/ml) | 57.70 [57.70] | 62.45 [34.85] | .892 |
| 24‐h brachial SBP | 132.13 ± 10.71 | 124.16 ± 11.45 | .001 |
| 24‐h brachial DBP | 75.00 ± 8.43 | 74.62 ± 6.86 | .809 |
| 24‐h heart rate | 69 ± 9 | 64 ± 9 | .024 |
| Antihypertensive (any) (n, %) | 47 (97.9%) | 47 (97.9%) | 1.000 |
| ACEI or ARB (n, %) | 36 (75.0%) | 26 (54.2%) | .033 |
| Diuretics (n, %) | 33 (68.8%) | 26 (54.2%) | .142 |
| CCB (n, %) | 38 (79.2%) | 33 (68.8%) | .352 |
| Β‐blocker (n, %) | 28 (58.3%) | 24 (50.0%) | .413 |
| Statin (n, %) | 37 (77.1%) | 29 (60.4%) | .078 |
| Erythropoietin (n, %) | 2 (4.2%) | 3 (6.3%) | .646 |
| Insulin (n, %) | 22 (45.8%) | 0 (0%) | <.001 |
| Oral hypoglycemic agents (n, %) | 40 (83.3%) | 0 (0%) | <.001 |
Statistically significant P values are marked with bold.
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, Body mass index; COPD, Chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
3.2. Between‐group comparison of 24‐h central hemodynamic and arterial stiffness parameters
Comparisons of the 24‐h central BP levels and the arterial stiffness parameters between patients with and without DM are presented in Table 2. As shown in the table, patients with DM had higher 24‐h cSBP (118.57 ± 10.05 vs. 111.59 ± 9.46 mmHg, P = .001) but similar 24‐h cDBP (77.09 ± 8.14 vs. 76.34 ± 6.75 mmHg, P = .625) levels compared to patients without DM. Moreover, levels of 24‐h cPP (41.48 ± 6.80 vs. 35.25 ± 6.98 mmHg, P < .001) were higher in diabetic patients compared to their non‐diabetic counterparts. With regard to wave reflections, 24‐h AP (13.05 ± 4.46 vs. 11.89 ± 5.20 mmHg, P = .243), AIx, and AIx(75) (24.73 ± 6.73 vs. 23.95 ± 6.97%, P = .578) were not different between the two study groups. Levels of 24‐h PWV (10.10 ± 1.62 vs. 9.61 ± 1.80 m/s, P = .165) were numerically higher in patients with DM, but the difference was not statistically significant.
Table 2.
Ambulatory central blood pressure, wave reflection, and arterial stiffness parameters in patients with diabetic and non‐diabetic CKD
| Parameter | Diabetic CKD | Non‐diabetic CKD | p |
|---|---|---|---|
| 24‐h cSBP (mmHg) | 118.57 ± 10.05 | 111.59 ± 9.46 | .001 |
| 24‐h cDBP (mmHg) | 77.09 ± 8.14 | 76.34 ± 6.75 | .625 |
| 24‐h cPP (mmHg) | 41.48 ± 6.80 | 35.25 ± 6.98 | <.001 |
| 24‐h AP (mmHg) | 13.05 ± 4.46 | 11.89 ± 5.20 | .243 |
| 24‐h AIx (%) | 28.34 ± 7.01 | 29.74 ± 8.38 | .376 |
| 24‐h AIx(75) (%) | 24.73 ± 6.73 | 23.95 ± 6.97 | .578 |
| 24‐h PWV (m/s) | 10.10 ± 1.62 | 9.61 ± 1.80 | .165 |
Statistically significant P values are marked with bold.
3.3. Between‐group comparison and relevant trajectories of central hemodynamic and arterial stiffness parameters during the day and nighttime periods
Table 3 presents the comparisons of the central hemodynamic and arterial stiffness parameters during the day‐ and the nighttime periods between the two study groups. Figure 1 presents the trajectories of cSBP, cDBP, and cPP levels in the two patient groups, averaged from 10:00 am of the Day 1 to 09:00 am the Day 2 during the 24‐h ABPM. Figure 2 presents the relevant trajectories of and the AIx, AIx(75), and PWV. Patients with DM had significantly higher cSBP levels, during both the day‐ (120.15 ± 10.86 vs. 112.68 ± 9.20 mmHg, P < .001) and nighttime periods (114.85 ± 12.62 vs. 108.38 ± 12.50 mmHg, P = .013), but day‐ and nighttime cDBP levels were not different between the two groups. Central PP levels were higher in diabetics during both periods, with the difference being greater during the nighttime (daytime: 41.06 ± 7.13 vs. 35.08 ± 6.94, P < .001; nighttime: 42.84 ± 8.35 vs. 35.83 ± 8.13 mmHg, P < .001) period.
Table 3.
Day‐ and nighttime central blood pressure, wave reflection, and arterial stiffness parameters in patients with diabetic and non‐diabetic CKD
| Parameter | Diabetic CKD | Non‐diabetic CKD | p |
|---|---|---|---|
| Daytime cSBP (mmHg) | 120.15 ± 10.86 | 112.68 ± 9.20 | <.001 |
| Daytime cDBP (mmHg) | 79.09 ± 8.86 | 77.59 ± 7.07 | .365 |
| Daytime cPP (mmHg) | 41.06 ± 7.13 | 35.08 ± 6.94 | <.001 |
| Daytime AP (mmHg) | 12.43 ± 4.59 | 11.47 ± 5.27 | .343 |
| Daytime AIx (%) | 27.19 ± 6.91 | 28.86 ± 8.64 | .300 |
| Daytime AIx(75) (%) | 24.27 ± 6.72 | 23.87 ± 7.33 | .777 |
| Daytime PWV (m/s) | 10.15 ± 1.62 | 9.64 ± 1.78 | .143 |
| Nighttime cSBP (mmHg) | 114.85 ± 12.62 | 108.38 ± 12.50 | .013 |
| Nighttime cDBP (mmHg) | 72.02 ± 8.73 | 72.55 ± 8.58 | .761 |
| Nighttime cPP (mmHg) | 42.84 ± 8.35 | 35.83 ± 8.13 | <.001 |
| Nighttime AP (mmHg) | 15.10 ± 5.68 | 13.26 ± 5.54 | .111 |
| Nighttime AIx (%) | 32.23 ± 8.95 | 32.63 ± 9.60 | .832 |
| Nighttime AIx(75) (%) | 26.06 ± 8.59 | 24.32 ± 7.91 | .303 |
| Nighttime PWV (m/s) | 9.97 ± 1.62 | 9.52 ± 1.88 | .215 |
Statistically significant P values are marked with bold.
Figure 1.
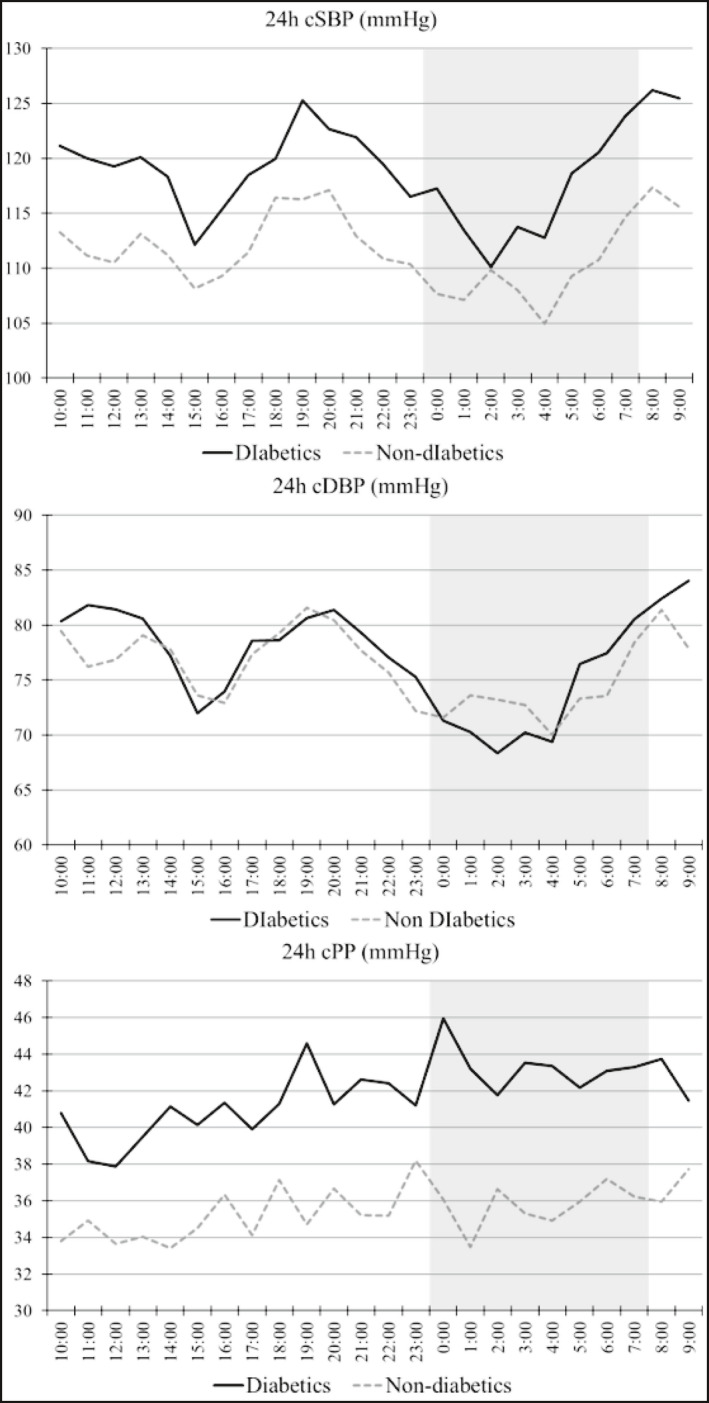
Trajectories of (A) central SBP, (B) central DBP, and (C) central pulse pressure in patients with and without diabetes. Graph presents averaged values averaged between 10:00 am of the Day 1 and 09:00 am the Day 2. The grayed zone indicates the nighttime period
Figure 2.
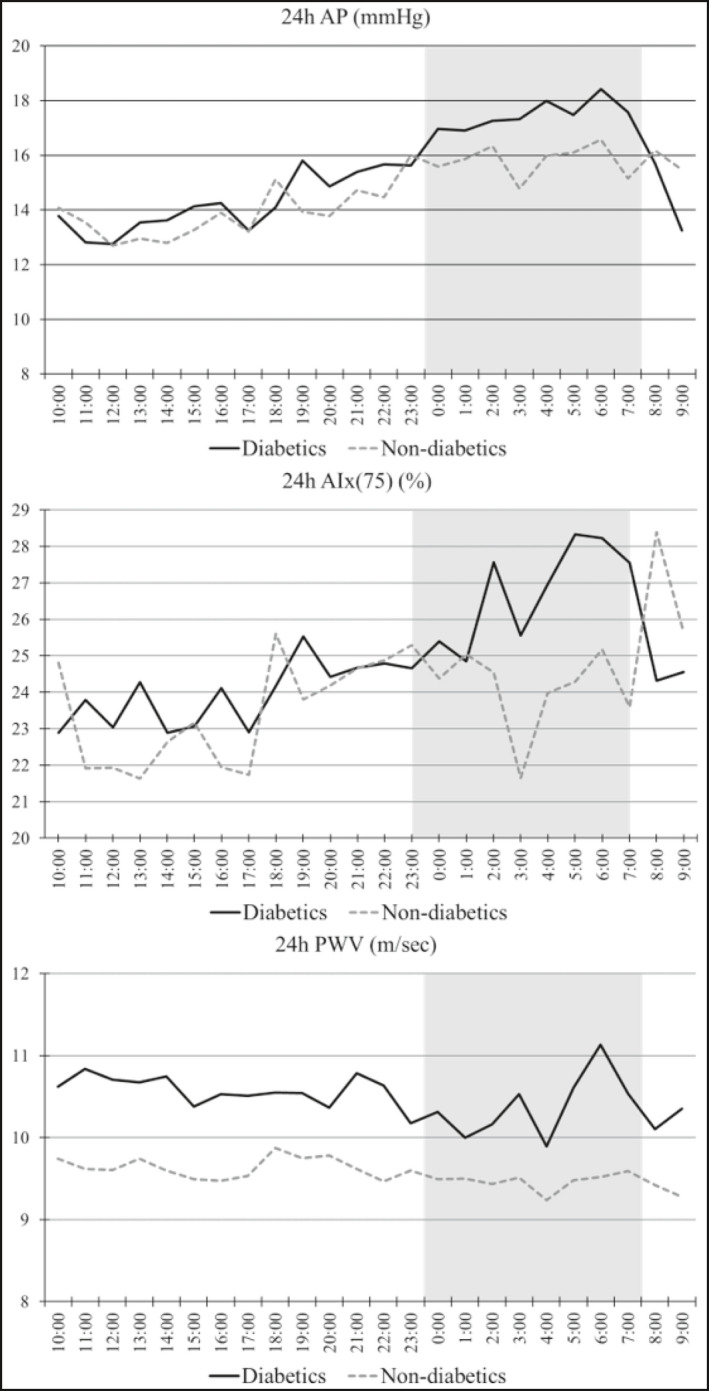
Trajectories of (A) augmentation index, (B) heart rate adjusted augmentation index, and (C) pulse wave velocity in patients with and without diabetes. The grayed zone indicates the nighttime period
Daytime and nighttime AP levels were similar in the two groups following almost identical trajectories, while AIx(75) was again similar in the two groups during both periods, but presented gradually increasing levels overnight in patients with DM. Levels of PWV were insignificantly higher during the day (10.15 ± 1.62 vs. 9.64 ± 1.78 m/s, P = .143)‐ and nighttime (9.97 ± 1.62 vs 9.52 ± 1.88 m/s, P = .215) periods in patients with compared to those without DM and presented a steady pattern during the ambulatory recording.
3.4. Factors associated with 24‐h cPP levels
In Table 4, we present the univariate and multivariable regression analyses in the total population studied, including 24‐h cPP levels as the dependent variable and a set of demographic, clinical, and laboratory factors as independent variables. As shown in the table, female gender (2.489, 95% CI: 0.244‐4.734; P = .030), DM (2.427, 95% CI: 0.196‐4.657; P = .033), and higher peripheral SBP (0.404, 95% CI: 0.304‐0.505; P < .001) were the only factors associated with higher cPP levels in the multivariate analysis.
Table 4.
Linear regression analysis for factors possibly associated with 24‐h cPP levels in the total population
| Parameter | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Age (per year increase) | 0.142 | −0.009 to 0.292 | .064 | 0.061 | −0.052 to 0.175 | .287 |
| Female | 2.723 | −0.370 to 5.815 | .084 | 2.489 | 0.244 to 4.734 | .030 |
| BMI (per kg/m2 increase) | 0.182 | −0.061 to 0.425 | .139 | 0.139 | −0.040 to 0.318 | .126 |
| Active smoking | −0.129 | −3.845 to 3.588 | .945 | |||
| Diabetes | 6.233 | 3.437 to 9.028 | <.001 | 2.427 | 0.196 to 4.657 | .033 |
| Coronary heart disease | 0.869 | −3.138 to 4.875 | .668 | |||
| Stroke | 4.109 | −2.183 to 10.401 | .198 | 4.226 | −0.037 to 8.489 | .052 |
| Peripheral vascular disease | −1.561 | −6.041 to 2.918 | .491 | |||
| Heart failure | 0.233 | −7.456 to 7.923 | .952 | |||
| Dyslipidemia | 4.082 | 0.768 to 7.396 | .016 | 2.306 | −0.135 to 4.746 | .064 |
| Antihypertensive treatment | 5.685 | −5.011 to 16.380 | .294 | |||
| eGFR (per ml/min/1.73 m2 increase) | −0.014 | −0.099 to 0.072 | .753 | |||
| 24‐h Urine sodium (per mEq/L increase) | 0.001 | −0.0001 to 0.002 | .088 | −0.0001 | −0.001 to 0.001 | .537 |
| 24‐h SBP (mmHg) | 0.435 | 0.338 to 0.532 | <.001 | 0.404 | 0.304 to 0.505 | <.001 |
| 24‐h DBP (mmHg) | −0.065 | −0.266 to 0.137 | .527 | |||
Statistically significant P values are marked with bold.
4. DISCUSSION
This study was designed to examine in comparison parameters of central hemodynamics and arterial stiffness in CKD patients with and without DM, in order to examine whether DM has additional deleterious effects the adverse macrocirculatory profile of CKD. The main finding was that patients with diabetic CKD had significantly higher 24‐h cSBP and cPP, as well as numerically higher PWV, but similar cDBP, AP, AIx, and AIx(75) levels compared to those without DM. Ambulatory cSBP presented similar variations with time in both groups, cPP displayed a similar gradually increasing trend, and PWV was relatively stable in the two groups. These patterns over time resulted in significantly higher day‐ and nighttime cSBP, cPP, and insignificantly higher PWV levels in diabetics compared to their non‐diabetic counterparts. The trajectories of day and nighttime cDBP, AP, and daytime AIx(75) were almost identical in the two groups, whereas nighttime AIx(75) increased in the DM group and slightly decreased in controls but the difference was not significant.
DM is affecting central hemodynamics in several ways and is currently associated with arterial stiffness, adverse pulsatile hemodynamics, and abnormal arterial–ventricular interactions, as well as left ventricular remodeling, fibrosis, and dysfunction. 25 Patients with CKD also present accelerated arteriosclerosis and increased arterial stiffness, which is their predominant arterial abnormality. 14 The consequences of reduced arterial compliance are the reduction of central diastolic BP (cDBP) and the development of isolated cSBP elevation. 26 From a pathophysiological point of view, such changes lead to earlier reflections of the forward pressure waves back to the aorta which result in a steeper pressure‐flow relation in early systole; this opposes left ventricular ejection leading to ventricular hypertrophy and dysfunction, increased myocardial oxygen consumption, and reduced coronary perfusion gradient during diastole. 27 These reflections do not derive from a single dominant reflection site across the arterial tree, but they constitute an amalgamation of multiple proximal small reflections. 28 Inevitably, the adverse cardiac remodeling and dysfunction predispose patients with DM and/or CKD to higher cardiovascular events and mortality. 14 , 29
Previous evidence on the distinct effect of DM on central BP and arterial stiffness mainly derives from studies evaluating levels in office conditions and studies using ABPM are scarce. In as secondary analysis in 508 (52 with DM and 456 without DM) community‐dwelling participants from the Maine‐Syracuse Longitudinal Study, diabetics presented significantly higher office PWV (12.5 ± 0.36 vs. 10.4 ± 0.12 m/s), while the risk of PWV levels ≥ 12 m/s was 9‐fold higher for those with uncontrolled DM compared to non‐diabetics (OR 9.14, 95% CI 3.23‐25.9, P < .001). 30 A post hoc analysis of the French cohort DESIR (Data from an Epidemiologic Study on the Insulin Resistance syndrome) study, which compared BP and arterial stiffness measured in office conditions between 126 diabetic and 203 non‐diabetic patients found higher PWV in patients with DM [13.9 (11.6‐16.4) vs. 11.5 (9.9‐13.0) m/s, P < .0001] but similar cSBP and cDBP levels compared to their non‐diabetic counterparts. 31 An earlier study by Laugersen and colleagues, including 89 patients with type‐2 DM diagnosis for < 5 years and 89 sex‐ and age‐matched controls, showed that patients with DM had higher 24‐h PP (52 ± 68 vs. 49 ± 9, P = .03) levels, a difference evidenced also during nighttime but not daytime, and higher office PWV levels (9.3 ± 6 2.0 vs. 8.0 ± 6 1.6 m/s; P < .0001) compared to patients without DM. 32 In contrast to the above, a study including 23 twin pairs from the Australian Twin Registry, which were discordant for type‐2 DM presence, showed that patients with DM had reduced nocturnal cSBP dipping (β = −3.79, P = .027), but similar cSBP, cDBP, AIx, and PWV levels measured in office. 33 In the only large study utilizing ambulatory BP monitoring (ABPM) to compare brachial, central BP, and arterial stiffness parameters in several sub‐groups from a total population of 1200 patients, diabetic patients had higher day‐ and nighttime cSBP (123.1 ± 12.4 vs. 120.1 ± 13.1, P = .049 and 114.2 ± 16.7 vs. 109.0 ± 14.5, P = .003) and PWV (11.8 ± 2.0 vs. 10.6 ± 2.6, P = .0001 and 11.6 ± 1.9 vs. 10.2 ± 2.7 mmHg, P = .0001, for day and night), but similar cDBP and AIx levels compared to patients without DM. 34
In all of the above studies, possible between‐group differences in renal function or CKD Staging were not assessed in the various groups examined. Only a recent study in which 111 type‐2 diabetics and 54 controls were included reports similar mean eGFR, but again this was around normal levels (91.7 ± 9.9 vs. 95.9 ± 17.3 accordingly, P = .10); this study used ABPM to evaluate brachial BP and applanation tonometry for central hemodynamic parameters and showed that although 24‐SBP and 24‐DBP levels were similar in the two groups, patients with DM had higher office cPP levels (41.8 ± 11.7 vs. 34.8 ± 10.6 mmHg, P < .001). 35 Therefore, to the best of our knowledge, no study so far has evaluated whether DM has an additional effect on central circulation hemodynamics and stiffness in patients with CKD. In a previous cross‐sectional study including 434 patients type‐2 DM patients and 192 unmatched healthy controls, office heart‐femoral PWV was increased in diabetic patients without CKD and was further stepwise increased with the advancing of CKD stages compared to controls (Control: 979 ± 19, Non‐CKD diabetics: 1081 ± 19b, Stage 1 CKD: 1105 ± 33, Stage 2 CKD: 1226 ± 48, Stage 3 CKD: 1189 ± 52, Stage 4‐5 CKD: 1224 ± 68, P < .05 for pair‐wise comparisons and P < .0001 for trend), 36 but again no comparison between patients with and without diabetic CKD was made. Our study expands the existing knowledge by showing that in patients with CKD, the presence of DM is associated with higher ambulatory cSBP and cPP levels, which is persistent during both day‐ and nighttime periods.
In our study, wave reflection parameters were not different between the two groups, suggesting a different pattern of findings for augmentation pressure and central pressure indices. Elegant studies using invasive techniques have shown that reflections from more distant reflection sites (eg femoral arteries) are markedly attenuated by the time they reach the proximal aorta due to the large impedance mismatches of bifurcations traversed in the backward direction. 37 Furthermore, results from a metanalysis of 64 studies including 13 770 patients of different ages suggest that wave reflections from the periphery can result in even smaller pressure augmentations in older patients such as those included in our study. 38 Such observations may partially explain our findings on wave reflection parameters. Another relevant issue can be the fact that AP, AIx, and AIx(75) may not be the best available methods to evaluate wave reflections. Previous evidence in normotensives suggest that AIx is correlated positively with age (r = .39; P = .01), inversely with height (r = −.43; P = .001), and is higher in women [13.0 (6.3, 19.6)% vs. 0.2 (24.5, 4.8]%; p = .001], while its values can be significantly affected when type C waveforms are included in analyses (ie when AIx is negative). 39 Results from earlier studies indicate that AIx calculations from transformed waveforms are significantly different compared to measured levels obtained from invasively measured aortic waveforms. 40 , 41 Furthermore, recent studies using simulation models have shown that decreases in LV myocardial shortening velocity can also result in a significant increase of AIx from 21% to 42%. 42
With regard to treatment perspectives, existing evidence suggests that antihypertensive agents with vasodilating properties (ie, ACEIs, ARBs, and CCBs) improve arterial stiffness, possibly beyond the degree expected from BP lowering 43 ; however, in these studies patients with DM were a minority. Moreover, diuretic drugs, such as thiazides, may improve arterial stiffness by causing vascular smooth muscle relaxation after induction of a negative sodium balance. 9 Importantly, sodium‐glucose co‐transporter 2 (SGLT‐2) inhibitors which both can reduce BP levels and lead to negative sodium balance in patients with DM have shown to improve PWV in preliminary studies. 44 In addition, in a recent randomized clinical trial in diabetic patients with eGFR > 60 ml/min/1.73 m2, we observed significant reductions in 24‐h cSBP and 24‐h PWV with dapagliflozin versus placebo. 45 Whether similar effects can be achieved in patients with diabetic CKD of different stages is yet to be answered.
This study is the first designed to evaluate differences in ambulatory central BP levels and arterial stiffness in matched patients with and without diabetic CKD. We employed a careful design, including blinded matching of diabetic and non‐diabetic individuals resulting in two study groups without differences for a large set of baseline characteristics. Using ABPM, we were also able to depict the trajectories of the parameters under study in addition to comparing the mean values during independent periods or the recording. The main limitation of the study is its observational nature with a single evaluation, not enabling to prove cause and effect associations. The Mobil‐O‐graph device provides estimates and not direct recording of central BP, wave reflections, and arterial stiffness parameters, which can be considered a study limitation. However, with the exception of a slight underestimation of carotid‐femoral PWV, previous validation studies showed generally acceptable agreement between Mobil‐o‐graph derived estimates and values obtained from invasive measurements or direct measurements with applanation tonometry. 24 , 46 Importantly, this study aimed to record these variables in ambulatory conditions, where invasive or direct recordings were not feasible. Finally, our sample was perhaps not adequate to detect significant differences in the secondary outcomes of PWV levels.
In conclusion, this study showed that patients with diabetic CKD have higher 24‐h, daytime, and nighttime cSBP levels but similar cDBP levels compared to those without DM. DM was also associated with increased arterial stiffness, as indicated by the higher 24‐h, daytime, and nighttime cPP levels, while ambulatory PWV levels were numerically higher in patients with DM. These finding suggest that the presence of DM is another factor further contributing to the adverse profile of the macrocirculation in CKD patients. Existing evidence suggests that antihypertensive agents with vasodilating properties and SGLT‐2 inhibitors are associated with improvement of arterial stiffness in DM. 44 , 45 Future studies should examine whether if such interventions could retard these changes in patients with diabetic CKD.
CONFLICT OF INTEREST
All authors disclose that they do not have any financial or other relationships, which might lead to a conflict of interest regarding this paper.
AUTHOR CONTRIBUTION
Each author contributed important intellectual content. PS conceptualized the research idea and designed the study. MS and TH acquired the data. CL, MS, MD, and PS analyzed and interpreted the data. CL and PS performed the statistical analysis. CL and PS performed the statistical analysis. AG, AK, and AP performed critical analysis of the paper. AG, AK, AP, and PS provided supervision and mentorship. All authors revised and approved the final manuscript.
Loutradis C, Schoina M, Dimitroulas T, et al. Comparison of ambulatory central hemodynamics and arterial stiffness in patients with diabetic and non‐diabetic CKD. J Clin Hypertens. 2020;22:2239–2249. 10.1111/jch.14089
REFERENCES
- 1. Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet. 2011;378:31‐40. [DOI] [PubMed] [Google Scholar]
- 3. Sharma A, Green JB, Dunning A, et al. Causes of death in a contemporary cohort of patients with Type 2 diabetes and atherosclerotic cardiovascular disease: insights from the TECOS trial. Diabetes Care. 2017;40:1763‐1770. [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296‐1305. [DOI] [PubMed] [Google Scholar]
- 5. Papadopoulou E, Angeloudi E, Karras S, Sarafidis P. The optimal blood pressure target in diabetes mellitus: a quest coming to an end? J Hum Hypertens. 2018;32:641‐650. [DOI] [PubMed] [Google Scholar]
- 6. Sarafidis PA, Sharpe CC, Wood E, et al. Prevalence, patterns of treatment, and control of hypertension in predialysis patients with chronic kidney disease. Nephron Clin Pract. 2012;120:c147‐c155. [DOI] [PubMed] [Google Scholar]
- 7. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197‐203. [DOI] [PubMed] [Google Scholar]
- 8. Smulyan H, Siddiqui DS, Carlson RJ, London GM, Safar ME. Clinical utility of aortic pulses and pressures calculated from applanated radial‐artery pulses. Hypertension. 2003;42:150‐155. [DOI] [PubMed] [Google Scholar]
- 9. McEniery CM, Yasmin MB, et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo‐Cardiff Collaborative Trial II. Hypertension. 2008;51:1476‐1482. [DOI] [PubMed] [Google Scholar]
- 10. Sarafidis PA. Metabolic syndrome and arterial stiffness: evidence for gender disparity and early effects of non‐traditional risk factors? J Hypertens. 2007;25:935‐938. [DOI] [PubMed] [Google Scholar]
- 11. London GM, Pannier B. Arterial functions: how to interpret the complex physiology. Nephrol Dial Transplant. 2010;25:3815‐3823. [DOI] [PubMed] [Google Scholar]
- 12. Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176‐181. [DOI] [PubMed] [Google Scholar]
- 13. Sarafidis PA, Loutradis C, Karpetas A, et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all‐cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension. 2017;70:148‐157. [DOI] [PubMed] [Google Scholar]
- 14. London GM. Arterial stiffness in chronic kidney disease and end‐stage renal disease. Blood Purif. 2018;45:154‐158. [DOI] [PubMed] [Google Scholar]
- 15. Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527‐539. [DOI] [PubMed] [Google Scholar]
- 16. Fountoulakis N, Thakrar C, Patel K, Viberti G, Gnudi L, Karalliedde J. Increased arterial stiffness is an independent predictor of renal function decline in patients with type 2 diabetes mellitus younger than 60 years. J Am Heart Assoc. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schoina M, Loutradis C, Minopoulou I, et al. Ambulatory blood pressure trajectories and blood pressure variability in diabetic and non‐diabetic chronic kidney disease. Am J Nephrol. 2020;51:411‐420. [DOI] [PubMed] [Google Scholar]
- 18. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Suppl 1):S81‐S90. [DOI] [PubMed] [Google Scholar]
- 19. Parati G, Stergiou G, O'Brien E, et al. European society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359‐1366. [DOI] [PubMed] [Google Scholar]
- 20. Sarafidis PA, Lazaridis AA, Imprialos KP, et al. A comparison study of brachial blood pressure recorded with Spacelabs 90217A and Mobil‐O‐Graph NG devices under static and ambulatory conditions. J Hum Hypertens. 2016;30:742‐749. [DOI] [PubMed] [Google Scholar]
- 21. Karpetas A, Sarafidis PA, Georgianos PI, et al. Ambulatory recording of wave reflections and arterial stiffness during intra‐ and interdialytic periods in patients treated with dialysis. Clin J Am Soc Nephrol. 2015;10:630‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff‐based method for estimating central systolic blood pressure. Hypertension. 2011;58:825‐832. [DOI] [PubMed] [Google Scholar]
- 23. Hametner B, Wassertheurer S, Kropf J, Mayer C, Eber B, Weber T. Oscillometric estimation of aortic pulse wave velocity: comparison with intra‐aortic catheter measurements. Blood Press Monit. 2013;18:173‐176. [DOI] [PubMed] [Google Scholar]
- 24. Sarafidis PA, Georgianos PI, Karpetas A, et al. Evaluation of a novel brachial cuff‐based oscillometric method for estimating central systolic pressure in hemodialysis patients. Am J Nephrol. 2014;40:242‐250. [DOI] [PubMed] [Google Scholar]
- 25. Chirinos JA, Bhattacharya P, Kumar A, et al. Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8:e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56:555‐562. [DOI] [PubMed] [Google Scholar]
- 27. Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015;238:370‐379. [DOI] [PubMed] [Google Scholar]
- 28. Davies JE, Alastruey J, Francis DP, et al. Attenuation of wave reflection by wave entrapment creates a "horizon effect" in the human aorta. Hypertension. 2012;60:778‐785. [DOI] [PubMed] [Google Scholar]
- 29. Cernes R, Zimlichman R, Shargorodsky M. Arterial elasticity in cardiovascular disease: focus on hypertension, metabolic syndrome and diabetes. Adv Cardiol. 2008;45:65‐81. [DOI] [PubMed] [Google Scholar]
- 30. Elias MF, Crichton GE, Dearborn PJ, Robbins MA, Abhayaratna WP. Associations between type 2 diabetes mellitus and arterial stiffness: a prospective analysis based on the Maine‐Syracuse study. Pulse. 2018;5:88‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Safar ME, Lange C, Blacher J, Eschwège E, Tichet J, Balkau B. Mean and yearly changes in blood pressure with age in the metabolic syndrome: the DESIR study. Hypertens Res. 2011;34:91‐97. [DOI] [PubMed] [Google Scholar]
- 32. Laugesen E, Høyem P, Stausbøl‐Grøn B, et al. Carotid‐femoral pulse wave velocity is associated with cerebral white matter lesions in type 2 diabetes. Diabetes Care. 2013;36:722‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karayiannis C, Moran C, Sharman JE, et al. Blood pressure, aortic stiffness, hemodynamics, and cognition in twin pairs discordant for Type 2 diabetes. J Alzheimers Dis. 2019;71:763‐773. [DOI] [PubMed] [Google Scholar]
- 34. Omboni S, Posokhov I, Parati G, et al. Ambulatory blood pressure and arterial stiffness web‐based telemonitoring in patients at cardiovascular risk. First results of the VASOTENS (Vascular health ASsessment Of The hypertENSive patients) registry. J Clin Hypertens (Greenwich). 2019;21:1155‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kannenkeril D, Bosch A, Harazny J, et al. Early vascular parameters in the micro‐ and macrocirculation in type 2 diabetes. Cardiovasc Diabetol. 2018;17:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kimoto E, Shoji T, Shinohara K, et al. Regional arterial stiffness in patients with type 2 diabetes and chronic kidney disease. J Am Soc Nephrol. 2006;17:2245‐2252. [DOI] [PubMed] [Google Scholar]
- 37. Baksi AJ, Davies JE, Hadjiloizou N, et al. Attenuation of reflected waves in man during retrograde propagation from femoral artery to proximal aorta. Int J Cardiol. 2016;202:441‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baksi AJ, Treibel TA, Davies JE, et al. A meta‐analysis of the mechanism of blood pressure change with aging. J Am Coll Cardiol. 2009;54:2087‐2092. [DOI] [PubMed] [Google Scholar]
- 39. Hughes AD, Park C, Davies J, et al. Limitations of augmentation index in the assessment of wave reflection in normotensive healthy individuals. PLoS One. 2013;8:e59371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hope SA, Tay DB, Meredith IT, Cameron JD. Comparison of generalized and gender‐specific transfer functions for the derivation of aortic waveforms. Am J Physiol Heart Circ Physiol. 2002;283:H1150‐H1156. [DOI] [PubMed] [Google Scholar]
- 41. Hope SA, Tay DB, Meredith IT, Cameron JD. Waveform dispersion, not reflection, may be the major determinant of aortic pressure wave morphology. Am J Physiol Heart Circ Physiol. 2005;289:H2497‐H2502. [DOI] [PubMed] [Google Scholar]
- 42. Heusinkveld MHG, Delhaas T, Lumens J, et al. Augmentation index is not a proxy for wave reflection magnitude: mechanistic analysis using a computational model. J Appl Physiol. 1985;2019(127):491‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ong KT, Delerme S, Pannier B, et al. Aortic stiffness is reduced beyond blood pressure lowering by short‐term and long‐term antihypertensive treatment: a meta‐analysis of individual data in 294 patients. J Hypertens. 2011;29:1034‐1042. [DOI] [PubMed] [Google Scholar]
- 44. Loutradis C, Papadopoulou E, Theodorakopoulou M, Karagiannis A, Sarafidis P. The effect of SGLT‐2 inhibitors on blood pressure: a pleiotropic action favoring cardio‐ and nephroprotection. Future Med Chem. 2019;11:1285‐1303. [DOI] [PubMed] [Google Scholar]
- 45. Papadopoulou E, Loutradis C, Tzatzagou G, et al. Dapagliflozin decreases ambulatory central blood pressure and pulse wave velocity in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled clinical trial. J Hypertens. 2020. (accepted). [DOI] [PubMed] [Google Scholar]
- 46. Salvi P, Scalise F, Rovina M, et al. Noninvasive estimation of aortic stiffness through different approaches. Hypertension. 2019;74:117‐129. [DOI] [PubMed] [Google Scholar]


