Abstract
Clinical practice guidelines recommend several routine laboratory tests in patients diagnosed with hypertension. However, the rates of clinically relevant laboratory abnormalities are unknown. Therefore, we conducted a retrospective cohort study using administrative and laboratory data of patients diagnosed with hypertension between April 2010 and March 2015 in Alberta, Canada. Laboratory investigations for renal function, serum electrolytes (sodium and potassium), low‐density lipoprotein (LDL) cholesterol, and diabetes (fasting blood glucose and hemoglobin A1c), measured within 1 year of diagnosis, were examined, and the frequency of abnormalities determined. A total of 225 296 cases of incident hypertension were identified. Of these, 74.3% received at least one of the four guideline‐recommended laboratory tests, but only 42.3% received all four tests. Patients who received any testing, compared to subjects who did not, were on average older (median age 55.9 vs 51.2 years, P < .001) and had more comorbidity (14.5% vs 2.8% with a Charlson comorbidity index ≥ 3, P < .001). Laboratory abnormalities with the potential to affect clinical decision‐making were more common among multi‐comorbid patients. Patients with renal dysfunction (6.7% vs 11.6%, 26.3%, P < .001), electrolyte abnormalities (9.8% vs 12.6%, 20.5%, P < .001), and diabetes (13.4% vs 25.1% vs 38.8%, P < .001) were found in patients with Charlson scores of 0 vs 1‐2 vs ≥3, respectively. Our study found most patients diagnosed with hypertension received some laboratory testing, but rates of laboratory testing and frequency of abnormalities varied by clinical context. Testing and abnormalities detected were both more common among older patients and patients with comorbidities.
Keywords: abnormalities, clinical practice guidelines, diagnosis, high blood pressure, laboratory testing
1. INTRODUCTION
Hypertension is one of the most common chronic medical conditions globally, and is the leading risk factor for cardiovascular disease. 1 , 2 Laboratory investigations performed during the initial evaluation of hypertension complement the history and physical examination, and may help to inform prognosis, diagnosis, and treatment. 3
The principal goals of laboratory testing for hypertension are to assist in global cardiovascular risk assessment (eg, detection of end organ damage and other cardiovascular risk factors); to identify remediable forms of hypertension (eg, consideration of hyperaldosteronism with hypokalemia); and to help guide drug selection and monitoring (eg, avoidance of thiazide diuretics in a patient with hyponatremia). 4 , 5 , 6 Recommendations for laboratory testing in patients newly diagnosed with hypertension are largely based on expert opinion. 4 , 5 , 6 Previous studies examining rates of laboratory testing in hypertension did not report how often abnormalities were detected. 7 , 8 , 9 , 10 Therefore, it is unknown how often laboratory investigations reveal abnormalities, which is an important initial step in judging whether testing may improve clinical outcomes.
Defining the role of laboratory tests has significant implications for health care resource utilization and expenditures owing to the high prevalence of hypertension. 11 To address this, we conducted a cohort study examining the use of common laboratory tests among newly diagnosed cases of hypertension within the province of Alberta in Canada. We intentionally focused on specific laboratory tests that were commonly recommended by multiple major hypertension practice guidelines. 4 , 5 , 6 We examined the proportion of laboratory tests with clinically relevant abnormalities.
2. MATERIALS AND METHODS
2.1. Data sources
We conducted a population‐based cohort study of patients with newly diagnosed hypertension. We analyzed data obtained from linked administrative databases of Alberta Health, a provincial government ministry providing universal health coverage to >99% of the approximately 4 million people in Alberta, Canada. 12 These databases included population registries with details for demographics and vital statistics, physician billing codes, diagnostic codes from hospital administrative discharge abstract data (DAD), and laboratory services. Medication data however were not available. Physician encounters were coded using the International Classification of Disease, 9th revision, clinical modification version (ICD‐9‐CM), and the DAD were coded using the ICD‐9‐CM (prior to 2010) or ICD‐10‐CA, Canadian version, (beginning in 2010). These data have been used in numerous studies, and are considered to be both high quality and comprehensive. 11 , 13 , 14
2.2. Population
Using a validated case definition, we identified individuals with hypertension from physician and hospital diagnosis codes (2 physician claims within 2 years or 1 hospitalization) 13 from April 1, 1993 to March 31, 2015 (Table S1). As laboratory results were only available after 2010, we excluded individuals who were identified with new hypertension prior to April 1, 2010. All subjects with incident hypertension (ie, no diagnosis in the prior 17 fiscal years of April 1, 1993 to March 31, 2010) were followed from their index diagnosis date for 1 year, or until termination of Alberta Health coverage, death, or March 31, 2015 (whichever came first). Baseline demographics, including sex and age at the time of hypertension diagnosis, were retrieved from the health insurance registry. The Charlson comorbidity index was calculated using outpatient physician claims and the hospital DAD up to 2 years prior to the diagnosis of hypertension. 15 , 16 These diagnostic codes were also used to identify relevant comorbidities that may have affected the frequency of laboratory testing (eg, coronary artery disease, stroke, congestive heart failure [CHF], chronic kidney disease [CKD], and diabetes mellitus).
2.3. Laboratory testing
Measurements of serum creatinine, serum electrolytes, low‐density lipoprotein (LDL) cholesterol, fasting blood glucose, and glycated hemoglobin (HbA1c) collected within the first year after hypertension diagnosis were obtained through laboratory information systems. These were selected because they are the most commonly recommended tests for the initial evaluation of newly diagnosed hypertension by major clinical practice guidelines. 3 , 4 , 5 , 6 If not already automatically reported, the estimated glomerular filtration rates (eGFR) were calculated using the four variable Modification of Diet in Renal Disease equation to align with the methodology used by the major laboratories in Alberta. 17
Some investigations were grouped together into laboratory panels if they were routinely ordered together or were ordered for a similar purpose. For instance, serum sodium and potassium were grouped together as “electrolytes.” Likewise, investigations used to diagnose diabetes (ie, fasting plasma glucose and HbA1c) were considered to be part of a single “diabetes” panel for this study. In total, we defined four laboratory panels: renal function (eGFR), electrolytes (serum sodium and potassium), LDL cholesterol, and tests for diabetes (fasting glucose and HbA1c). Individuals were considered to have received testing if any component within a laboratory panel was ordered (eg, measurement of either fasting plasma glucose or HbA1c was sufficient to conclude a person received testing for diabetes).
2.4. Laboratory outcomes
For the laboratory investigations of interest, we determined the frequency of testing within 365 days after the diagnosis date for hypertension, in order to allow for adequate time for testing to be arranged following the recognition of hypertension. Abnormal test results were defined by values outside the standardized reference intervals as set by the laboratory. Among the abnormal test results, we further defined those that were most likely to affect clinical decision‐making (using cutoffs informed by clinical practice guidelines and clinical reasoning; Table S2). 18 , 19 Examples of laboratory abnormalities with the potential of affecting clinical decision‐making included the presence of an elevated serum potassium > 5.0 mmol/L (ie, prompting caution with the use of renin‐angiotensin system [RAS]‐blockers), hyponatremia < 133 mmol/L (ie, precluding the use of a thiazide or thiazide‐like diuretic), renal impairment with an eGFR < 60 mL/min/1.73 m2 (ie, a scenario where a RAS‐blocker may be considered), and LDL cholesterol ≥ 3.5 mmol/L (ie, a scenario where a statin may be considered). 19
2.5. Subgroup analyses
For our subgroup analyses, we stratified our cohort according to age (<65 vs ≥65), whether or not they were hospitalized for any reason during the first year of hypertension diagnosis, and comorbidities (ie, coronary artery disease, stroke, CHF, CKD, diabetes mellitus, and by the Charlson comorbidity index score; Table S1).
2.6. Statistical analysis
Descriptive statistics were reported for baseline demographic and comorbid variables. The frequency of laboratory testing was calculated for the entire study population. If a particular test was performed multiple times for the same individual during the time of ascertainment, only the first occurrence was included for the analysis. The proportions of abnormal laboratory tests (of any degree) and abnormal tests with the potential to affect clinical decisions were determined (Table S2). Chi‐square tests were used to compare groups. Multivariate logistic regression was used to examine which patient characteristics were associated with receiving laboratory testing. Statistical analysis was performed using SAS version 9.4 (SAS Institute, Inc, Cary, NC). This study was approved by the Conjoint Health Research Ethics Board at the University of Calgary. A waiver of consent was granted for access to personal identifiable health information consistent with the conditions under section 50, Health Information Act of Alberta.
3. RESULTS
A total of 225 296 cases of incident hypertension were identified between April 1, 2010 and March 31, 2015. Slightly more than half of patients were male. Patients who received any testing, compared to subjects who received none, were more likely elderly (19.6% vs 15.0% were ≥65 years), had a greater burden of comorbidity (14.5% vs 2.8% with a Charlson comorbidity index ≥ 3), and were more likely to have a history of coronary artery disease (6.9% vs 1.6%), stroke (6.5% vs 2.2%), CHF (6.1% vs 1.6%), CKD (4.1% vs 0.7%), and diabetes (13.1% vs 3.6%) at baseline (P < .001 for all comparisons; Table 1). After multivariable adjustment, patients who were female, aged 65 years or older, or had cardiovascular comorbidities had a higher odds of receiving at least one of the guideline‐recommended laboratory tests (P < .00001 for all comparisons; Table S3).
Table 1.
Baseline characteristics of hypertensive patients, stratified by number of laboratory tests received within first year of diagnosis
| Variables a | No testing | Testing (1 panel) | Testing (2 panels) | Testing (3 or 4 panels) | Any testing (≥1 panel) |
|---|---|---|---|---|---|
| Total | 57 974 | 8240 | 36 100 | 122 982 | 167 322 |
| Male — no. (%) | 34 430 (59.4) | 4536 (55.0) | 18 634 (51.6) | 68 804 (55.9) | 91 974 (55.0) |
| Age ≥ 65 — no. (%) | 8711 (15.0) | 1611 (19.6) | 10 005 (27.7) | 31 140 (25.3) | 42 756 (25.6) |
| Age — median (25‐75th percentiles) | 51.2 (42.5‐60.0) | 53.8 (45.4‐62.5) | 55.4 (45.6‐66.6) | 56.1 (47.5‐65.1) | 55.9 (47.0‐65.2) |
| Comorbidities—no. (%) | |||||
| Diabetes mellitus | 2076 (3.6) | 693 (8.4) | 3119 (8.6) | 18 041 (14.7) | 21 853 (13.1) |
| Chronic kidney disease | 385 (0.7) | 86 (1.0) | 1783 (4.9) | 4981 (4.1) | 6850 (4.1) |
| Congestive heart failure | 929 (1.6) | 177 (2.1) | 2854 (7.9) | 7166 (5.8) | 10 197 (6.1) |
| Coronary artery disease | 934 (1.6) | 219 (2.7) | 2112 (5.9) | 9155 (7.4) | 11 486 (6.9) |
| Stroke | 1295 (2.2) | 331 (4.0) | 2750 (7.6) | 7837 (6.4) | 10 918 (6.5) |
| Charlson index of 1 or 2 | 13 288 (22.9) | 2668 (32.4) | 12 421 (34.4) | 42 400 (34.5) | 57 489 (34.4) |
| Charlson index ≥ 3 | 1652 (2.8) | 535 (6.5) | 6550 (18.1) | 17 199 (14.0) | 24 284 (14.5) |
| Hospitalized for any reason within 1st year of diagnosis | 2904 (5.0) | 888 (10.8) | 13 086 (36.2) | 27 224 (22.1) | 41 198 (24.6) |
Comorbidities are based on physician and hospital billing codes.
A quarter of patients did not receive any pertinent laboratory testing in the first year after hypertension diagnosis. The remaining 167 322 patients (74.3%) had at least one of the four guideline‐recommended laboratory tests; 3.7%, 16.0%, 12.3%, and 42.3% of people received one, two, three, and four of the laboratory panels, respectively (Figure 1). All four different laboratory panels were ordered with similar frequency: renal function (69.5%), serum electrolytes (64.3%), LDL cholesterol (54.3%), and diabetes (53.7%).
FIGURE 1.
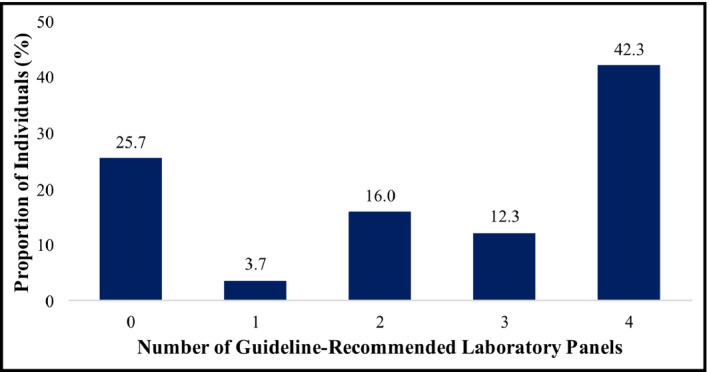
Proportion of hypertensive patients who received any of the guideline‐recommended laboratory panels within 1 y of diagnosis
3.1. Investigations for renal failure and electrolyte abnormalities
Approximately 1 in 10 patients (n = 17 846 of 156 652; 11.4%) had abnormalities in renal function and 1 in 7 patients (n = 21 392 of 144 826; 14.8%) had abnormal electrolytes with the potential of affecting clinical care (Figure 2). Serum potassium was abnormal (9.7%) more often than sodium (6.2%). When stratified by comorbid subgroups, abnormal eGFR and electrolytes were more commonly identified among patients with pre‐existing CKD, CHF, stroke, and diabetes (P < .001). Patients who were hospitalized within the first year of hypertension diagnosis (n = 44 102) were also commonly found to have renal failure (18.5% vs 9.9%, P < .001) and electrolyte abnormalities (18.5% vs 10.2%, P < .001) when tested compared to those who were not hospitalized. Among older patients, renal function was more often abnormal (25.5% vs 6.4% for ages ≥ 65 vs <65 years, P < .001). Electrolyte abnormalities for hyponatremia, hypokalemia, and hyperkalemia were more common among females, elderly (age ≥ 65 years), having additional comorbidities, or hospitalized for any reason within their first year of hypertension diagnosis (P‐value < .0001 for all comparisons; Table S4C).
FIGURE 2.
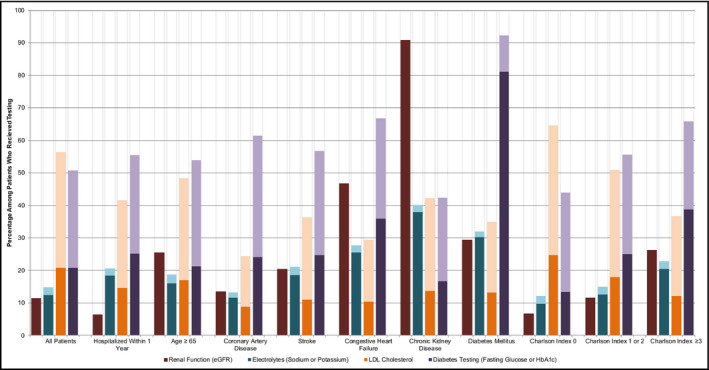
Laboratory panels with identified abnormalities, stratified according to hospitalization and comorbidities. Darker colors represent abnormalities that affect clinical decision‐making and lighter colors represent abnormalities that do not affect clinical decision‐making. For electrolytes and diabetes panels, if any component was abnormal, the entire panel was considered to be abnormal. LDL Cholesterol = low‐density lipoprotein cholesterol, HbA1C = glycated hemoglobin A1c
3.2. Detection of elevated LDL cholesterol
Elevated LDL cholesterol (LDL cholesterol ≥ 3.5 mmol/L) was present in 1 in 5 patients (n = 25 457 of 122 269; 20.8%). In contrast to other laboratory abnormalities, abnormal LDL cholesterol was less frequent in patients with multiple comorbidities (24.8% vs 17.9% vs 12.1% for Charlson comorbidity index scores of 0, 1‐2, vs ≥3, respectively, P < .001). Elevated LDL cholesterol was more common in younger patients (22.0% vs 17.0% for ages < 65 vs ≥65 years, P < .001) and those who were not hospitalized (22.4% vs 14.7% for non‐hospitalized vs hospitalized, P < .001).
3.3. Investigations for diabetes
When measured, half of patients had abnormalities in HbA1c and/or fasting plasma glucose (n = 61 413 of 120 919; 50.8%). Among patients who were not classified as having diabetes at baseline (based on diagnostic codes), 24 484 of 120 191 (20.4%) patients had a HbA1c level or fasting glucose that was within the diagnostic range for diabetes within the first year of hypertension diagnosis. Abnormalities in diabetes testing were more common among patients who were hospitalized within their first year of hypertension diagnosis (25.3%), those with coronary artery disease (24.1%), stroke (24.8%), congestive heart failure (36.0%), and complex multimorbidity (38.9% for Charlson comorbidity index scores of ≥3) compared to those without the associated conditions (P < .001 for all comparisons).
4. DISCUSSION
In this study, we evaluated the frequency of laboratory testing and associated clinically relevant abnormalities in patients with hypertension. More than half of patients received at least three of the four commonly recommended laboratory panels evaluated in this study. Investigations used to screen for end organ damage or secondary causes of hypertension (ie, serum creatinine and electrolytes) were frequently normal, but those principally used for risk stratification (ie, plasma lipids, fasting plasma glucose, and HbA1c) were associated with high rates of abnormalities. Not surprisingly, laboratory testing was more commonly performed in patients who were older, with comorbid conditions, and those who were hospitalized within the first year of hypertension diagnosis. Elderly patients and people with multiple comorbidities had the highest proportion of detected laboratory abnormalities.
Large variations in ambulatory testing for patients with hypertension or taking anti‐hypertensive classes of medications have been reported across different health systems and jurisdictions. 7 , 8 , 9 , 10 We have found similar rates of testing to a previous Canadian study where patients with hypertension from 1993 to 1995 were evaluated for frequency of testing (but not rates of abnormalities). 8 This study found hypertensive patients were commonly evaluated for renal function (~70%), serum electrolytes (~55%), and lipid levels (~60%), but we observed a twofold higher rate of testing for diabetes. This may be due to a greater awareness of diabetes and increased screening in general over the last two decades. 8 , 20 Furthermore, as the uptake of Hypertension Canada's clinical practice guidelines have made a demonstrable impact on national hypertension diagnosis, treatment, and control rates since inception, 21 these may have also contributed to more widespread laboratory testing over time. One study in the United States reported that between 1999 and 2000, 68% patients on RAS‐blockers had both their creatinine and potassium monitored. 10 However, a separate study in the United States, of a similar time span of 1999 to 2001, found a lower frequency of laboratory testing for creatinine (~35%) and electrolytes (~40%) for patients on RAS‐blockers, and similar rates for patients on diuretics. 7 However, neither of these studies specifically examined patients with hypertension, but broadly included all patients who may have received these drug classes irrespective of indication.
It is reassuring that clinicians are more inclined to test individuals known (or perceived) to be at higher cardiovascular risk. As is the case, laboratory testing appears to be more commonly ordered in the elderly, those with multiple comorbidities, and patients prescribed concomitant chronic medications. 7 , 9 , 10 Clinicians may also be following guideline standards for management of other coexisting conditions, such as CKD, dyslipidemia, and diabetes, which recommend routine laboratory testing as well. The high frequency of laboratory abnormalities observed in this study may be related to the fact that many patients who were investigated also tended to be older with more comorbid conditions.
Although laboratory abnormalities were relatively common overall, some groups had especially high rates of detected abnormalities (eg, renal dysfunction was commonly present in patients with congestive heart failure), whereas the rates of detection were much lower in other groups (eg, renal function and serum electrolytes were often normal in patients without significant comorbidity). Selected testing may be reasonable in certain cases, acknowledging that the associated costs of laboratory testing can be substantial. 11 Beyond the direct costs of testing, there are indirect costs arising from laboratory abnormalities, including those associated with additional investigations, physician follow‐up visits, and specialty consultations. There are costs to patients related to lost productivity from work. Abnormal results may also add to unnecessary patient anxiety as many of these may pose little risk to their health. In many cases, clinicians in our study may have appropriately chosen to defer laboratory testing in selective low‐risk individuals where abnormalities would be less common. Accordingly, judicious clinical judgment may have led to less frequent testing among younger patients and those without cardiovascular comorbidities. Furthermore, little is known about the implications of routine laboratory testing in patients with hypertension, and even when abnormalities are detected, results may not necessarily affect clinical decision‐making. It also remains uncertain whether selective testing in targeted high‐risk groups, compared to universal screening for all patients with hypertension, is cost‐effective and can be implemented without adversely affecting clinical outcomes or quality of care. 22 , 23 Future research should explore better ways to personalize testing by considering the benefits of systematic screening for highly prevalent conditions, while potentially limiting certain tests to selected high‐risk groups.
Even with the many strengths of our study (ie, a large cohort with longitudinal follow‐up drawn from a well‐defined population; complete capture of all physician encounters and laboratory tests performed; and the presence of a universal health insurance system such that access to physician and laboratory services were not limited by cost barriers), there were some limitations. First, we did not have clinical information on why certain tests were ordered and we were only able to report on the frequency of laboratory abnormalities when testing was actually performed. Although an intervention study with systematic laboratory testing would be methodologically ideal, it would be impractical to conduct on a provincial level. As such, an observational study provides the best possible evidence given the nature of the subject. Secondly, we did not have prescription data and it is possible that some people with normal laboratory results were already treated (eg, a normal LDL cholesterol while receiving a statin or a normal HbA1C on glucose lowering agents) such that the frequency of laboratory‐based comorbid conditions may well be underestimated. Thirdly, we were limited to these four laboratory panels and did not have the data to analyze other investigations commonly performed for hypertension, such as electrocardiogram or urine analysis. However, the four panels investigated were universally recommended in major hypertension practice guidelines. 4 , 5 , 6 Finally, we used laboratory cutoffs that often, but not always, impact clinical care. We also could not evaluate any clinical outcomes in this study. Therefore, it cannot be assumed that routine testing, or even the presence of abnormal laboratory results, led to changes in patient care or differences in clinical outcomes.
5. CONCLUSIONS
There is a significant variation in laboratory test ordering and frequency of detected abnormalities in patients newly diagnosed with hypertension. Higher rates of testing and abnormalities were detected among elderly and comorbid patients. Personalization of the work‐up for hypertension may be an area where patient care and practice can be further improved. 24 Efforts to improve resource sustainability might be prioritized by focusing on individuals who are most likely to benefit from testing, especially patients who are known or suspected to be at higher risk of complications, or in patients more likely to have clinically significant abnormalities. 24 Instead of a “one‐size‐fits‐all” panel for all patients with hypertension, resources may be more efficiently utilized if laboratory testing is guided by patient factors and clinical context.
CONFLICT OF INTEREST
AAL is supported by the Hypertension Canada New Investigator Award. NRCC was a paid consultant to the Novartis Foundation (2016‐2017) to support their program to improve hypertension control in low to middle income countries, which included travel support for site visits and a contract to develop a survey. NRCC provided paid consultative advice on accurate blood pressure assessment to Midway Corporation (2017) and is an unpaid member of World Action on Salt and Health (WASH). SQ, being resident trainee, was paid by Hypertension Canada and the University of Saskatchewan in support of travel costs for attending the 2017 and the 2019 Canadian Hypertension Congresses.
AUTHOR CONTRIBUTIONS
All authors had contributed important intellectual content to the research project and have actively participated the writing of this manuscript. AA is the principal investigator responsible for the study conception, design, acquisition of data, analysis, interpretation of data, drafting, and revising the manuscript. SQ participated in the study design, analysis, interpretation of data, drafting, and revising the manuscript with AA. GC, ZL, and YF contributed to the data cleaning, statistical analysis, interpretation of data, and revisions of the manuscript. RP, FM, KT, NC, and DR contributed important intellectual content to the study design, interpretation of data, and revisions of the manuscript. All authors have participated in the work of this project, have reviewed, and agreed with the content of the article of publication.
Supporting information
Table S1‐S4
ACKNOWLEDGMENTS
Members of Hypertension Canada's Research and Evaluation Committee include Karen C. Tran, Ross T. Tsuyuki, Finlay A. McAlister, Norman R.C. Campbell, Nadia A. Khan, Raj S. Padwal, Hude Quan, and Alexander A. Leung.
Quan S, Chen G, Padwal RS, et al; for Hypertension Canada’s Research, Evaluation Committee . Frequency of laboratory testing and associated abnormalities in patients with hypertension. J Clin Hypertens. 2020;22:2077–2083. 10.1111/jch.14040
REFERENCES
- 1. Padwal RS, Bienek A, McAlister FA, Campbell NR, Outcomes Research Task Force of the Canadian Hypertension Education P . Epidemiology of hypertension in Canada: an update. Can J Cardiol. 2016;32:687‐694. [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;317:165‐182. [DOI] [PubMed] [Google Scholar]
- 3. Schwartz GL, Krakoff LR. Diagnostic evaluation: initial evaluation: laboratory testing. J Am Soc Hypertens. 2014;8:677‐679. [DOI] [PubMed] [Google Scholar]
- 4. Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada's 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;2018(34):506‐525. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127‐e248. [DOI] [PubMed] [Google Scholar]
- 7. Hurley JS, Roberts M, Solberg LI, et al. Laboratory safety monitoring of chronic medications in ambulatory care settings. J Gen Intern Med. 2005;20:331‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McAlister FA, Teo KK, Lewanczuk RZ, Wells G, Montague TJ. Contemporary practice patterns in the management of newly diagnosed hypertension. CMAJ. 1997;157:23‐30. [PMC free article] [PubMed] [Google Scholar]
- 9. McAlister FA, Tu K, Majumdar SR, Padwal R, Chen Z, Campbell NR. Laboratory testing in newly treated elderly hypertensive patients without co‐morbidities: a population‐based cohort study. Open Med. 2007;1:e60‐e67. [PMC free article] [PubMed] [Google Scholar]
- 10. Raebel MA, McClure DL, Simon SR, et al. Laboratory monitoring of potassium and creatinine in ambulatory patients receiving angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Pharmacoepidemiol Drug Saf. 2007;16:55‐64. [DOI] [PubMed] [Google Scholar]
- 11. Weaver CG, Clement FM, Campbell NR, et al. Healthcare costs attributable to hypertension: Canadian population‐based cohort study. Hypertension. 2015;66:502‐508. [DOI] [PubMed] [Google Scholar]
- 12. Statistics Canada . Census profile, 2016 census Alberta [province] and Canada [country] (table). Statistics Canada Catalogue no. 98‐316‐X2016001. https://www12.statcan.gc.ca/census‐recensement/2016/dp‐pd/prof/details/Page.cfm?Lang=E&Geo1=PR&Code1=48&Geo2=&Code2=&Data=Count&SearchText=Alberta&SearchType=Begins&SearchPR=01&B1=All&GeoLevel=PR&GeoCode=48. Updated: August 9, 2019. Accessed January 17, 2020
- 13. Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423‐1428. [DOI] [PubMed] [Google Scholar]
- 14. Quan H, Chen G, Walker RL, et al. Incidence, cardiovascular complications and mortality of hypertension by sex and ethnicity. Heart. 2013;99:715‐721. [DOI] [PubMed] [Google Scholar]
- 15. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130‐1139. [DOI] [PubMed] [Google Scholar]
- 16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373‐383. [DOI] [PubMed] [Google Scholar]
- 17. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038‐2047. [DOI] [PubMed] [Google Scholar]
- 18. Diabetes Canada Clinical Practice Guidelines Expert Committee , Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10‐S15. [DOI] [PubMed] [Google Scholar]
- 19. Anderson TJ, Gregoire J, Pearson GJ, et al. 2016 Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2016;32:1263‐1282. [DOI] [PubMed] [Google Scholar]
- 20. Diabetes Canada Clinical Practice Guidelines Expert Committee . Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2018;42:S1‐S325. [DOI] [PubMed] [Google Scholar]
- 21. McAlister FA, Feldman RD, Wyard K, Brant R, Campbell NR, CHEP Outcomes Research Task Force . The impact of the Canadian hypertension education programme in its first decade. Eur Heart J. 2009;30:1434‐1439. [DOI] [PubMed] [Google Scholar]
- 22. Epstein AM, Hartley RM, Charlton JR, Harris CM, Jarman B, McNeil BJ. A comparison of ambulatory test ordering for hypertensive patients in the United States and England. JAMA. 1984;252:1723‐1726. [PubMed] [Google Scholar]
- 23. Epstein AM, McNeil BJ. The effects of patient characteristics on ambulatory test ordering. Soc Sci Med. 1985;21:1071‐1075. [DOI] [PubMed] [Google Scholar]
- 24. McAlister FA, Laupacis A, Armstrong PW. Finding the right balance between precision medicine and personalized care. CMAJ. 2017;189:e1065‐e1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S4


