Abstract
Reduced‐sodium, added‐potassium salt substitutes have favorable effects on blood pressure, but have not been tested in India. The Salt Substitute in India Study (SSiIS) is a double‐blinded, randomized‐controlled trial designed to investigate the effects of reduced‐sodium, added‐potassium salt substitution to replace usual cooking salt use and blood pressure (BP) among hypertensive patients in rural India. The primary objective is to assess effects on systolic blood pressure at 3 months. The secondary objectives are to determine effects on diastolic blood pressure, urinary sodium, and potassium levels, and to determine acceptability of the intervention. Eligible individuals received usual salt (100% sodium chloride) or salt substitute (70% sodium chloride and 30% potassium chloride) to replace all salt required for cooking and seasoning in the household. A total of 502 participants aged ≥18 years with a history of hypertension were successfully recruited and randomized in a 1:1 ratio to intervention or control, between November 2019 and January 2020. Mean blood pressure at baseline was 133.5/83.6 mm Hg and 96% were using one or more blood pressure‐lowering medications. The overall mean average 24‐hour urinary sodium excretion was 2825 (SD, 1166) mg/L, which corresponds to a urinary salt excretion of 10.4 g/d. Baseline findings suggest sodium intake in this population significantly exceeds World Health Organization recommendations. The SSiIS trial has successfully recruited participants and is well placed to determine whether salt substitution is an effective means of lowering blood pressure for rural Indian patients with hypertension.
Keywords: blood pressure, hypertension, India, salt substitute
Abbreviations
- BMI
body mass index
- BP
blood pressure
- CVD
cardiovascular disease
- DBP
diastolic blood pressure
- LMICs
low‐ and middle‐income countries
- NCDs
non‐communicable diseases
- SBP
systolic blood pressure
- SD
standard deviation
- SSiIS
Salt Substitute in India Study
- WHO
World Health Organization
1. INTRODUCTION
Hypertension is one of the leading causes of disease in India and causes huge health and economic burdens. 1 , 2 , 3 While blood pressure (BP) control has improved substantially in many high‐income countries, effective management of hypertension in low‐ and middle‐income settings remains challenging. 4 , 5 Joint interventions that utilize drug‐based strategies, dietary interventions, and other behavioral approaches are advocated by most national and international hypertension societies, including in India. 6 Excess sodium intake causes increased BP. 7 , 8 , 9 Mean dietary sodium intake in India is estimated to be 5 grams (g) per day (equivalent to about 12.5 g salt), 10 which is more than double the World Health Organization (WHO) recommendation of less than 2 g/day sodium (5 g/day of salt). 11 Scalable strategies to reduce dietary sodium intake and improve hypertension control, and prevent cardiovascular diseases (CVD) are required.
Sources of dietary salt are known to vary between countries and are highly associated with the level of economic development. 12 In low‐ and middle‐income countries, the predominant dietary source of sodium is often from homemade foods where salt (sodium chloride) is added during food preparation (discretionary salt use). 13 In India, more than 80 percent of dietary sodium is estimated to come from discretionary salt use. 14 Hence, strategies that reduce sodium from discretionary salt use are promising for lowering population‐level sodium consumption.
Effects of salt substitutes that replace a portion of the sodium contained in usual salt (100% sodium chloride) with other minerals such as potassium and magnesium have been assessed in multiple randomized controlled trials. 15 In such trials, salt substitutes that partially replace sodium chloride with potassium chloride (usually 25‐30%) have significant and clinically meaningful effects on systolic blood pressure (SBP, −7.81 mm Hg, 95% CI: −9.47 to −6.15 mm Hg) and diastolic blood pressure (DBP, −3.96 mm Hg, 95% CI −5.17 to −2.74 mm Hg), with no reported serious adverse side effects. 15 Potassium‐based salt substitutes are also relatively low in cost and are thus a promising intervention strategy for low‐ and middle‐income countries.
Although a systematic review and meta‐analysis of clinical studies have demonstrated blood pressure lowering effects of reduced‐sodium, added‐potassium salt substitutes, 16 such studies have to date mostly been conducted in China. 17 , 18 , 19 , 20 Given the high prevalence of excess sodium intake in India, the current study set out to test the effect of salt substitute on BP among hypertensive patients in India.
2. METHODS
2.1. Aims and hypotheses
The primary aim of SSiIS is to evaluate the effect of a 3‐month reduced‐sodium, added‐potassium salt substitute intervention on SBP among patients with hypertension in rural India. The primary hypothesis is that the salt substitute will effectively and safely lower SBP. The secondary aims are to determine the effects of the salt substitute on DBP, urinary sodium and potassium excretion, as well as to assess dietary sources of sodium and the acceptability of the salt substitute. The secondary hypotheses are that the salt substitute will effectively and safely lower DBP through reduced dietary sodium and increased dietary potassium consumption, discretionary salt will be the major dietary source of sodium, and that salt substitute will be generally acceptable to the study participants.
2.2. Study design
SSiIS is a randomized, double‐blind controlled trial of 3 months intervention duration conducted in rural India from November 2019 to April 2020. The trial is registered in the clinicaltrials.gov database on 10 April 2019 (NCT03909659).
2.3. Study sites
The study team recruited participants from 7 villages located in the Siddipet District, which is located in the northern region of the Indian state of Telangana. The population of each village ranged between 3000‐5000 people. Villages are purposively selected based on their willingness to be involved and their proximity to the infrastructure necessary for the study, including delivery of the intervention salts and being accessible to study personnel.
2.4. Study population
Adults aged 18 years or over with a history of hypertension diagnosed by a health professional were eligible for participation. In addition, individuals needed to eat most of their meals in their homes as assessed by self‐report, and written consent was required from both the main study participant and all household members (since due to the nature of the intervention, household members of the actual study participants will also consume the intervention salts during the study period).
Individuals were eligible for participation if they meet the following inclusion criteria:
Male or female aged 18 years or over.
History of hypertension diagnosed by a health professional – hypertension may be self‐reported and antihypertensive drugs may or may not be used for management. There is no entry criterion based upon blood pressure measurements made at the baseline survey.
Eat most of their meals in the home.
Consent to participate.
Individuals were excluded if they or other household members used a potassium‐sparing diuretic, but not if they used other hypertensive medications. Participants were also excluded if they or other household members used potassium supplements, had any known acute or chronic kidney disease, 21 had other reason for concern about use of salt substitute, were not expected to live longer than 6 months from the date of assessment, or another member of the household was already enrolled in the study.
After we approached the households, each household decided for themselves which of the adult member of the household (who also met eligibility criteria) will be the study participant. One qualified study physician was available to guide and monitor the enrolment of participants to enhance the validity of enrolling those with self‐reported hypertension, as well as maximizing the safety of the study participants and their families. In particular, the physician determined the presence or absence of self‐reported kidney disease in potential participants as well as members in their household, given the concern that salt substitute use may increase the risk of hyperkalemia in patients with kidney disease. 21
2.5. Randomization
Individuals were randomized in a 1:1 ratio through a central computerized process to assign treatment packs provided in masked identical packaging and distinguished only by a unique identification number. An independent biostatistician generated random allocation sequence and designed the allocation sequence list. The blinded study team enrolled participants and assigned participants to intervention group according to the list.
2.6. Intervention and control
Once randomized, participants received treatment packs containing 5000 grams of study salts. The salt substitute and regular salt were provided free of charge in masked, identical packaging without the manufacturer’s name, except distinguished only by a unique identification number on each pack. The regular salt and salt substitute used in the study also contained iodine with fortification levels according to Indian regulatory requirements (File No. 3/DFS/FFRC/Fortification/FSSAI‐2017).
2.7. Intervention
The sodium reduction intervention is based upon the provision of reduced‐sodium, added‐potassium salt substitute (70% sodium chloride/30% potassium chloride blend). 22 Participants were advised to replace all discretionary salt use with the salt substitute. As the salt substitute used in the study was not available in the Indian market, Siddharth Starch Pvt. Ltd. company based in Maharashtra, India was commissioned to blend and supply the product.
2.8. Control
Regular salt (100% sodium chloride) was provided to participants in the control group, who were advised to replace discretionary salt use at home with the salt provided. Salt used in the study was provided free of charge also by Siddharth Starch Pvt. Ltd. company.
Sufficient salt substitute or salt was provided to cover the household cooking, seasoning, and food preservation requirements, at an average of 20 g/person/day to a maximum of 5 kg per 3 months for a household. Directions were given to continue the use of the study salt substitute or normal salt until the day that the final 24‐hour urine sample is scheduled to be collected at the end of study follow‐up.
2.9. Data collection and follow‐up
Across the study period, there were five visits scheduled for each participant (Table 1). After confirming eligibility criteria on the day of recruitment, informed consent was obtained from each study participant and each of their household members prior to commencement of any data collection or other study procedure. For household members under 18 years of age, informed consent was provided by a parent or guardian. Hereafter, the remaining study procedures applied only to the main study participant but not their household members. Personal information, including participant demographics, behaviors related to salt consumption and history of any past illnesses were ascertained via a questionnaire. A clinical assessment was also undertaken by trained study personnel and included measures of height (FREEMANS MEASURES PVT. LTD), weight, blood pressure, and pulse rate. In addition, directions were provided regarding the collection of a 24‐hour urine sample, and another in‐person appointment was made for the following day (second visit). Additional details about these study procedures are provided below.
Table 1.
Schedule of the Salt Substitute in India Study procedures
| Registration | Randomization | Intervention period include randomization column as part of intervention period | End of study | ||
|---|---|---|---|---|---|
| One‐month follow‐up | Three‐month follow‐up | ||||
| Visit Number | 1 | 2 | 3 | 4 | 5 |
| Visit time | Day 0 | Day 1‐3 | Day 25‐35 | Day 80‐100 | Day 85‐105 |
| Assessed for eligibility | X | ||||
| Informed consent | X | ||||
| Demographics data collection | X | ||||
| Behaviors related to salt consumption survey | X | X | |||
| Disease history | X | ||||
| Medication use | X | X | X | ||
| Blood pressure | X | X | X | ||
| Pulse rate | X | X | X | ||
| Anthropometric measurements a | X | ||||
| Distribute study salt | X | ||||
| Collect 24‐hour urine sample | X | X | |||
| 24‐hour dietary recall | X | X | |||
| Acceptability and adherence survey | X | X | |||
| Adverse events report | X | X | |||
Weight, height measurements.
At the second study visit, participants who provided complete 24‐hour urine specimens were randomized and provided with study salt substitute or regular salt for the next three months, and an appointment was made for the 1‐month follow‐up visit (third visit). Additionally, dietary sources of sodium were assessed through an interviewer‐administered 24‐hour dietary recall survey. 14
On the third study visit at 1 month, participants were asked by trained study personnel about the acceptability of the study salt, their usage and the occurrence of any adverse events. Blood pressure and heart rate were measured again following the same procedure as implemented on the second visit. An appointment was then made for the 3‐month follow‐up visit (fourth visit). For the fourth visit, participants were asked similar questions on acceptability of the study salt, their usage and the occurrence of any adverse or serious adverse events. Blood pressure and heart rate were again measured, as well as dietary intake assessed using the 24‐hour dietary recall questionnaire. Directions were provided regarding collection of a 24‐hour urine sample, and an appointment to collect the sample was made (fifth and final study visit). The 24‐hour urine sample was collected on the final study visit.
Study personnel who conducted interviews were blinded to the participants’ group allocation. All study contact was made via face‐to‐face home visits. If an individual was not present in the village on the day of the scheduled study visits, then a follow‐up visit was re‐scheduled for another time. For participants unable or unwilling to continue with the standard follow‐up schedule, alternative options were made available, including flexibility regarding the collection date of the 24‐hour urine samples.
2.10. Blood pressure measurement
At the first, third, and fifth study visits, blood pressure was measured three times for each participant using an automated BP monitor (A&D Medical, validated by British Hypertension Society and European Society of Hypertension standards.) according to established standardized methods, and the mean of the last two measurements was recorded. 23 BP monitors were validated and calibrated regularly. The examination took place in a quiet room, with the participant sitting comfortably in a chair for at least 5 minutes before measurement with back and arm supported and feet flat on the floor. The participant was instructed to avoid, for at least 30 minutes before the BP measurement, strenuous exercise, caffeine, smoking or a full bladder. Neither the patient nor study personnel spoke during the rest period or during the BP measurement. The participant rested for one minute between each of the readings.
2.11. 24‐hour urine collection and urine electrolytes measurement
Participants were given written and verbal instructions from trained study staff on how to collect 24‐hour urine samples. Participants were asked to not change their usual dietary and lifestyle habits. At study visits 1 and 4, they were provided with new containers to collect 24‐hour urine samples and instructed to collect all urine voided during a 24‐hour period. Before starting collection, participants were asked to go to the toilet and void urine, which was not included in the final sample. The time and date that the participant passed this urine was recorded on the 24‐hour urine bottle. Participants were then instructed to collect all urine in the subsequent 24‐hour period into the provided container without omission and were asked to return the 24‐hour urine on the second visit and the final visit. Study staff recorded the start and finishing time of the collection and total urine volume. The total urine volume was used to assess the completeness of 24‐hour urine collections, and samples were excluded from analysis if the total 24‐hour urine volume was less than 500 mL or greater than 6000 mL. 24
Analysis of urine electrolytes was performed at the study visit site using automated electrolyte meters (Compact Water Quality Meter, LAQUAtwin‐Na‐11 and LAQUAtwin‐K‐11m, HORIBA Scientific), which use a direct ion‐selective electrode technique to measure sodium and potassium concentrations.
Estimates of salt intake per 24‐hours (in grams) were derived from urine sodium concentration (in grams/L) multiplied by 2.54, 10 then multiplied by urine volume (L). Estimates of potassium (in grams) were similarly derived from urine potassium (in grams/L) by multiplying by 1.91, 25 then multiplied by urine volume (L). In accordance with previous reports, 10 , 26 we made best estimates of 24‐hour intake by inflating measured values by 10% to account for likely non‐urinary losses of sodium through sweat and feces. 26
2.12. Salt substitute acceptability and usage and 24‐hour dietary recall
Acceptability and use of the salt substitute or regular salt were assessed through an interviewer‐administered questionnaire with 12 questions 27 that inquired about the health effects of salt, recommended salt intake levels, approaches used to control the amount of salt consumed, the taste of the salt substitute/salt and the proportion of household salt use that was replaced by study salt during the trial intervention period (Supplementary Material).
A multiple pass 24‐hour dietary recall method 14 was used, through a questionnaire that was administered via face‐to‐face interviews by field staff. Data from 24‐hour dietary recall surveys were transcribed into a purpose‐built nutrient database, with nutrient composition data based on the Indian Food Composition Tables, 28 which was developed by the Indian National Institute of Nutrition. Staff were trained in the administration of dietary recall surveys following the methodology developed by the Agricultural Research Service of the US Department of Agriculture. 29 Members of the research team have conducted such dietary surveys previously in India. 14 Discretionary salt intake was estimated by asking participants about the quantity of salt added during food preparation and prior to consumption. Participants were asked to estimate the amount of salt added with the use of a food model booklet. 14 Interviewer prompts were provided to account for variable recipe ingredients and food preparation techniques. 14 , 30
2.13. Primary and secondary efficacy outcomes
The primary efficacy outcome will be the effect of the salt substitute on SBP over time. The secondary efficacy outcomes will be the effect of the salt substitute on DBP, levels of 24‐hour urinary sodium and 24‐hour urinary potassium. Acceptability of salt substitute and identification of major dietary sources of sodium will be exploratory outcomes.
2.14. Safety
The salt substitute used in this study is considered generally safe, but has a potential risk of causing hyperkalemia (defined as serum potassium level more than 6.5 mmol/L) if consumed in very large quantities (more than 50 g/day). 31 However, this risk is considered to be very low, 32 , 33 and concordantly, reduced‐sodium and added‐potassium products that are currently available to the general public in India does not carry specific warnings on product packaging. 34 We additionally mitigated potential adverse effects of the salt substitute by excluding participants with renal disease and concurrent use of potassium‐sparing or potassium‐supplementing medicines. Aside from hyperkalemia, there were no other specific adverse effects anticipated based on prior human trials. 19 , 32
Any suspected cases of hyperkalemia among study participants or household members identified during the scheduled study visits triggered an additional home visit to collect additional information. All other adverse events or serious adverse events among study participants or household members that occurred during the study, whether likely to be related to the study intervention or not, were recorded according to standard processes for serious adverse event reporting. The occurrence of serious adverse events were reported to the local ethics committee in line with their requirements. Furthermore, the study staff collected and removed the study salt from participants who experienced a serious adverse event.
2.15. Sample size
The planned sample size was 498 participants, which would provide 80% power, with a significance level of 5% (one‐sided test) to detect a 5 mmHg or greater difference in mean SBP between groups. The hypothesized effect size was based on result of a previous systematic review and meta‐analysis of salt substitute and blood pressure, 35 which is also a clinically meaningful SBP effect. The power estimate assumes mean baseline blood pressure of 140 mmHg, a standard deviation of 20 mmHg (19) and up to 20% loss to follow‐up for the primary outcome.
2.16. Statistical analysis
Mean levels (SDs) for continuous variables and frequency (n and %) were calculated for baseline and characteristics. Differences between groups at baseline were evaluated using t test for continuous variables, and Pearson chi‐square test (or Fisher exact test for cells < 10) for categorical variables. Statistical analyses were carried out using SAS version 7.1 (SAS Institute Inc, Cary, North Carolina, USA).
We are currently cleaning and collating all outcome data. Once outcome data are finalized, the effect of the salt substitute intervention on SBP will be assessed using the principle of intention‐to‐treat, and differences in average SBP between treatment groups will be analyzed using mixed linear models. This analytic approach allows the incorporation of baseline, 1‐month, and 3‐month measures of SBP to maximize the power of the analysis. Analysis of treatment effects on DBP will also use mixed linear models, whereas treatment effects on 24‐hour urine sodium and potassium will be assessed using analysis of covariance.
3. PARTICIPANT CHARACTERISTICS
The study design and number of participants recruited for the SSiIS study are shown in Figure 1.
Figure 1.
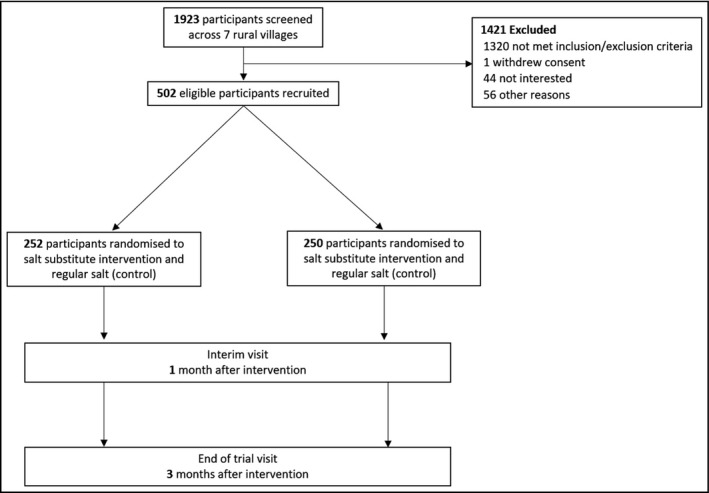
Study design and number of participants recruited for the Salt Substitute in India Study
Participant recruitment started on 25th November 2019 in the 7 rural Indian villages, and the last participant completed the enrolment process on 8th January 2020. In total, 1,923 individuals were screened and 502 met eligibility criteria. Baseline characteristics of the participants are shown in Table 2. Mean (SD) age of participants was 61.6 (12.0) years, 58.8% were female, and 6.0% were current smokers. Most participants achieved primary school education (87%). All participants had hypertension, while few (1.4%) had a history of CVD which was defined as a participant’s self‐reported history of stroke, coronary artery disease, myocardial infarction or peripheral arterial disease, and 22.0% also had a history of diabetes mellitus. Mean (SD) body mass index was 23.4 (4.5) kg/m2, and mean (SD) SBP/DBP was 133.5 (21.1)/83.6 (12.2) mmHg. Use of antihypertensive medications at baseline was 40.6% for alpha‐blockers, 29.9% for angiotensin‐converting enzyme inhibitor or angiotensin II receptor antagonist, 22.5% for beta‐blockers, 3.0% for calcium channel antagonist and 0.2% for diuretics. There were 4.2% participants who did not use any medication to control blood pressure. Participants had similar baseline demographic and medical characteristics across the randomized groups.
Table 2.
Baseline characteristics of randomized participants in the Salt Substitute in India Study d
|
Group 1 (n = 252) |
Group 2 (n = 250) |
Total (n = 502) |
|
|---|---|---|---|
| Age, years, mean (SD) | 61.5 (11.1) | 61.7 (12.9) | 61.6 (12.0) |
| Female, no. (%) | 147 (58.3) | 148 (59.2) | 295 (58.8) |
| Education, no. (%) | |||
| Primary school or lower | 220 (87.3) | 217 (86.8) | 437 (87.1) |
| High school a | 29 (11.5) | 28 (11.2) | 57 (11.4) |
| College | 2 (0.8) | 3 (1.2) | 5 (1.0) |
| University or higher | 1 (0.4) | 2 (0.8) | 3 (0.6) |
| Current Smoker, no. (%) | 16 (6.3) | 14 (5.6) | 30 (6.0) |
| BMI, kg/m2, mean (SD) | 23.1 (4.7) | 23.6 (4.2) | 23.4 (4.5) |
| SBP, mm Hg, mean (SD) | 133.9 (20.0) | 133.2 (22.5) | 133.5 (21.2) |
| DBP, mm Hg, mean (SD) | 84.1 (11.5) | 83.1 (12.9) | 83.6 (12.2) |
| History of CVD, no. (%) | 3 (1.2) | 4 (1.6) | 7 (1.4) |
| History of diabetes, no. (%) | 59 (23.4) | 51 (20.5) | 110 (22.0) |
| 24‐hour urine electrolytes concentration, mean (SD) | |||
| Sodium concentration, mg/L | 2818.0 (1120.1) | 2831.80 (1213.4) | 2824.82 (1165.8) |
| Potassium concentration, mg/L | 612.7 (311.0) | 657.5 (355.3) | 634.8 (334.0) |
| Salt intake, g per day, mean (SD) b , c | 10.62 (5.19) | 10.18 (4.82) | 10.40 (5.00) |
| Potassium intake, g per day, mean (SD) b | 1.57 (0.85) | 1.63 (1.02) | 1.60 (0.94) |
Descriptive data for participants were generated by an independent biostatistician. Both the statistician and the investigators remain blinded to participant treatment assignment.
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation.
High school includes junior high school, senior high school and technical secondary school.
Total numbers of eligible urine samples are 460, including 233 in group 1 and 227 in group 2.
Inflation by 10% applies to salt intake in grams per day.
None of the between‐group differences were significant.
Among the 502 participants who collected 24‐hour urine samples at study baseline, 42 samples were excluded from analyses due to total urine volume less than 500 mL. Mean (SD) 24‐hour urinary sodium excretion at baseline was 2,825 (1,166) mg/L, which corresponded to a mean (SD) salt intake of 9.45 (4.55) g/day. 36 With 10% inflation to adjust for minimum likely non‐urinary losses of sodium in sweat and feces, the corresponding best mean (SD) estimate of daily salt intake in the study population was 10.40 (5.00) g/day.
4. DISCUSSION
The SSiIS trial is the first randomized‐controlled trial of a salt substitution intervention among individuals with hypertension in rural India. This study has successfully recruited 502 eligible participants among whom the hypotheses can be examined, and there has been no attrition to date.
Excessive sodium intake causes hypertension, which is one of the most prevalent conditions in India (30.7% overall prevalence, defined as systolic BP ≥ 140 mm Hg or a diastolic BP ≥ 90 mm Hg or being treated for hypertension). 37 Approximately 1.65 million annual cardiovascular disease deaths have been attributed to excess sodium consumption (>2g/day) worldwide 5 and nearly 200 000 per year in India. 38 Antihypertensive drugs are highly effective for blood pressure lowering and are recommended by guidelines 23 to reduce the risks of serious cardiovascular complications. Meanwhile, behavioral approaches to blood pressure control based upon sodium reduction are also recommended. 23 Previous studies have demonstrated that reduced‐sodium, added‐potassium salt substitutes lowers BP, 15 , 17 , 18 especially in settings with high discretionary salt use. 16 If this study provides favorable results in terms of efficacy, safety, and acceptability of a salt substitute in India, then results will have important implications for using salt substitutes to control hypertension in India, and also potentially stimulate further large‐scale trials testing the effectiveness of salt substitutes to reduce BP in other Indian populations and settings.
Strengths of this study include the randomized‐controlled design that ensured similar baseline characteristics across groups and hence allow a causal examination of the effect of salt substitute on the primary and secondary outcomes. Double blinding of the intervention for participants and study personnel, including outcome assessors, will also minimize the risk of bias. The recruited sample size allows for 20% drop‐out to safeguard statistical power. Further, we recruited a similar proportion of men and women, which allows for generalizing our finding across genders. The blood pressure measurements are conducted following standard hypertension guidelines using validated devices. While collection of 24‐hour urine samples is onerous, it is the gold‐standard approach to assess usual dietary sodium consumption 39 which is another key strength of our study.
A limitation of our study is that we did not administer para‐aminobenzoic acid to the participants to objectively assess the completeness of the 24‐hour urine collection. 40 It is likely some of the collections were incomplete and hence overall there may be an underestimate of total salt consumption, although we expect this to be balanced between the study groups and hence not influence our outcome analyses comparing the salt substitute and control groups. Furthermore, although the risk is low, there is the potential that salt substitute use could cause hyperkalemic in specific populations (eg, those with impaired renal function), and a limitation of our study was that we did not regularly assess serum potassium levels of the participants.
The current study also had operational challenges that are typical of randomized‐controlled trials: for example, ensuring recruited participants met eligibility criteria, maintaining blinding of participants and study personnel and maximizing adherence to the standardized protocols. The study team endeavored to manage these challenges by developing a detailed work schedule and in‐depth protocols before recruitment and also targeted employment of local study staff to minimize language and cultural barriers. We also provided comprehensive training to study staff, in particular in relation to blood pressure measurement, 24‐hour urine sample collection and the use of automated meters to measure urine electrolytes. These efforts likely contributed to the successful and rapid recruitment of participants and adherence to the study procedures.
5. CONCLUSION
In conclusion, the SSiIS trial has already provided data on the feasibility of recruitment of Indian hypertensive patients into a trial of reduced‐sodium, added‐potassium salt substitution. Going forward, the trial will provide robust data on the effects of this form of salt substitution on blood pressure. Findings from this trial will provide much‐needed evidence to evaluate the efficacy, safety and acceptability of reduced‐sodium added‐potassium salt substitutes as a possible strategy to reduce hypertension in Indian hypertensive patients.
CONFLICT OF INTEREST
The study protocol received ethics approval from the George Institute Ethics Committee (Project Number 09‐2019). Written informed consent was obtained from all study participants and their household members. An external Independent Safety Monitor who is an expert in clinical trials has been appointed. The monitor will be provided with data about all Serious Adverse Events as they accrue during the study and will be asked to advise the investigators if at any point during the course of the study there is any reason for concern. The Independent Safety Monitor will be supported by a trial statistician who will provide the data required from the relevant participant and perform additional analyses as might be requested by the Independent Safety Monitor.
AUTHOR CONTRIBUTIONS
BN and JY conceived of the study. BN, JY, SRT, MT and JHYW participated in the study design. QL provided biostatistical support. JY and SRT drafted the manuscript. BN, JHYW, MDH, CA and SP participated in the critical review of the manuscript. All authors read and approved the final manuscript. MDH has received support from the American Heart Association, Verily, and AstraZeneca for work unrelated to this research. The other authors declare no conflict of interest. The funders and NuTek Natural Ingredients had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Supporting information
Supplementary Material
Acknowledgements
The authors gratefully thank all field investigators, study team, all participants and their household members for participating in the study. We thank NuTek Natural Ingredients for providing the salt and the salt substitute free of charge.
Thout SR, Yu J, Tian M, et al. Rationale, design, and baseline characteristics of the Salt Substitute in India Study (SSiIS): The protocol for a double‐blinded, randomized‐controlled trial. J Clin Hypertens. 2020;22:1504–1512. 10.1111/jch.13947
Sudhir Raj Thout MA and Jie Yu are contributed equally to this work.
Funding information
The study was supported by the George Institute for Global Health Seed Grant, grant number 0141030.
REFERENCES
- 1. Patel V, Chatterji S, Chisholm D, et al. Chronic diseases and injuries in India. Lancet (London, England). 2011;377(9763):413–28. [DOI] [PubMed] [Google Scholar]
- 2. Midha T, Idris MZ, Saran RK, Srivastav AK, Singh SK. Prevalence and determinants of hypertension in the urban and rural population of a north Indian district. East African journal of public health. 2009;6(3):268–73. [PubMed] [Google Scholar]
- 3. Moser KA, Agrawal S, Davey Smith G, Ebrahim S. Socio‐demographic inequalities in the prevalence, diagnosis and management of hypertension in India: analysis of nationally‐representative survey data. PLoS ONE. 2014;9(1):e86043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. R. k. Suma, T. r. Mayamol, Divakaran Binoo, Karunakaran Usha, A. K. Jayasree. Hypertension: prevalence, awareness, treatment and control in a rural area of North Kerala, India. Int J Commun Med Public Health. 2017;4(10):3561–3567. [Google Scholar]
- 5. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–34. [DOI] [PubMed] [Google Scholar]
- 6. Ministry of Health & Family Welfare Government of India . Screening, Diagnosis, Assessment, and Management of Primary Hypertension in Adults in India. February 2016.
- 7. Arcand J, Wong MMY, Santos JA, et al. More evidence that salt increases blood pressure and risk of kidney disease from the Science of Salt: A regularly updated systematic review of salt and health outcomes (April‐July 2016). J Clin Hypertens (Greenwich). 2017;19(8):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strazzullo P, D'Elia L, Kandala NB. Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson C, Mohan S, Rogers K, et al. Mean dietary salt intake in urban and rural areas in India: a population survey of 1395 persons. J Am Heart Assoc. 2017;6(1):e004547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson C, Santos JA, McKenzie B, et al. The Science of Salt: A regularly updated systematic review of the implementation of salt reduction interventions (September 2016‐February 2017). J Clin Hypertens (Greenwich). 2017;19(10):928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhat S, Marklund M, Henry ME, et al. A systematic review of the sources of dietary salt around the world. Adv Nutr. 2020;11(3):677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol. 2009;38(3):791–813. [DOI] [PubMed] [Google Scholar]
- 14. Johnson C, Santos JA, Sparks E, et al. Sources of dietary salt in north and south India estimated from 24 hour dietary recall. Nutrients. 2019;11(2):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hernandez AV, Emonds EE, Chen BA, et al. Effect of low‐sodium salt substitutes on blood pressure, detected hypertension, stroke and mortality: A systematic review and meta‐analysis of randomised controlled trials. Heart (British Cardiac Society). 2019;105(12):953–960. [DOI] [PubMed] [Google Scholar]
- 16. Aburto N j, Ziolkovska A, Hooper L, Elliott P, Cappuccio F p, Meerpohl J j. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Group TCSSSC . Salt substitution: a low‐cost strategy for blood pressure control among rural Chinese. A randomized, controlled trial. J Hypertens. 2007;25(10):2011–8. [DOI] [PubMed] [Google Scholar]
- 18. Li N, Yan LL, Niu W, et al. A large‐scale cluster randomized trial to determine the effects of community‐based dietary sodium reduction–the China Rural Health Initiative Sodium Reduction Study. Am Heart J. 2013;166(5):815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li N, Yan LL, Niu W, et al. The effects of a community‐based sodium reduction program in rural china ‐ a cluster‐randomized trial. PLoS ONE. 2016;11(12):e0166620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nan Y, Tian HG, Shao RC, et al. Assessment of sodium and potassium in processed foods in an urban area in China. Eur J Clin Nutr. 1995;49(4):299–306. [PubMed] [Google Scholar]
- 21. Levey AS, Levin A, Kellum JA. Definition and classification of kidney diseases. Am J Kidney Dis. 2013;61(5):686–688. [DOI] [PubMed] [Google Scholar]
- 22. Cepanec K, Vugrinec S, Cvetković T, Ranilović J. Potassium chloride‐based salt substitutes: a critical review with a focus on the patent literature. Comp Rev. 2017;16(5):881–894. [DOI] [PubMed] [Google Scholar]
- 23. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 24. Ma W, Yin X, Zhang R, et al. Validation and assessment of three methods to estimate 24‐h urinary sodium excretion from spot urine samples in high‐risk elder patients of stroke from the rural areas of Shaanxi Province. Int J Environ Res Public Health. 2017;14(10):1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yin X, Neal B, Tian M, et al. Sodium and potassium content of 24 h urinary collections: a comparison between field‐ and laboratory‐based analysers. Public Health Nutr. 2018;21(6):1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014;6(11):4651–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li N, Prescott J, Wu Y, et al. The effects of a reduced‐sodium, high‐potassium salt substitute on food taste and acceptability in rural northern China. Br J Nutr. 2009;101(7):1088–93. [DOI] [PubMed] [Google Scholar]
- 28. Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian Food Composition Tables. NationalInstitute of Nutrition. Hyderabad, India: 2017:1–578. [Google Scholar]
- 29. Moshfegh AJ, Rhodes DG, Baer DJ, et al. The US Department of Agriculture Automated Multiple‐Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 30. Johnson C, Mohan S, Praveen D, et al. Protocol for developing the evidence base for a national salt reduction programme for India. BMJ Open. 2014;4(10):e006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soar J, Perkins GD, Abbas G, et al. European Resuscitation Council Guidelines for Resuscitation 2010 Section 8. Cardiac arrest in special circumstances: Electrolyte abnormalities, poisoning, drowning, accidental hypothermia, hyperthermia, asthma, anaphylaxis, cardiac surgery, trauma, pregnancy, electrocution. Resuscitation. 2010;81(10):1400–1433. [DOI] [PubMed] [Google Scholar]
- 32. Neal B, Tian M, Li N, et al. Rationale, design, and baseline characteristics of the Salt Substitute and Stroke Study (SSaSS)‐A large‐scale cluster randomized controlled trial. Am Heart J. 2017;188:109–117. [DOI] [PubMed] [Google Scholar]
- 33. Greer RC, Marklund M, Anderson CAM, et al. Potassium‐enriched salt substitutes as a means to lower blood pressure. Hypertension. 2020;75(2):266–274. [DOI] [PubMed] [Google Scholar]
- 34. Food Safety and Standards (Packaging) Regulation. 2018. Available from: https://www.fssai.gov.in/upload/uploadfiles/files/Gazette_Notification_Packaging_03_01_2019.pdf
- 35. Peng Y‐G, Li W, Wen X‐X, Li Y, Hu J‐H, Zhao L‐C. Effects of salt substitutes on blood pressure: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2014;100(6):1448–1454. [DOI] [PubMed] [Google Scholar]
- 36. Peng Y, Li W, Wang Y, et al. Validation and Assessment of Three Methods to Estimate 24‐h Urinary Sodium Excretion from Spot Urine Samples in Chinese Adults. PLoS ONE. 2016;11(2):e0149655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramakrishnan S, Zachariah G, Gupta K, et al. Prevalence of hypertension among Indian adults: Results from the great India blood pressure survey. Indian Heart J. 2019;71(4):309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Institute for Health Metrics and Evaluation (IHME) . GBD Compare Data Visualization. Seattle WI, University of Washington, 2017. Available at: http://vizhub.healthdata.org/gbd-compare. Accessed April 26, 2018. doi:10.4103/0971‐4065.111871. [Google Scholar]
- 39. Land MA, Webster J, Christoforou A, et al. Salt intake assessed by 24 h urinary sodium excretion in a random and opportunistic sample in Australia. BMJ Open. 2014;4(1):e003720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wielgosz A, Robinson C, Mao Y, et al. The impact of using different methods to assess completeness of 24‐hour urine collection on estimating dietary sodium. J Clin Hypertens (Greenwich). 2016;18(6):581–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


