Abstract
Thyroid dysfunction plays a role in blood pressure (BP) regulation. However, the associations between thyroid function and BP and arterial stiffness in the general Chinese population without thyroid disease are unknown. This population‐based cross‐sectional study aimed to investigate the association between thyroid function and peripheral and central BP and arterial stiffness in Chinese individuals. After excluding those who had thyroid diseases or incomplete clinical measurements, this study included 691 participants. Of the participants, 444 (64.2%) were women and 215 (31.1%) had hypertension. After adjustment for covariates, serum FT3 was significantly associated with a higher pulse rate in both sexes. In men, each 2.72‐fold increase in serum FT4 levels was associated with higher peripheral systolic BP (+10.82 mmHg, p = .005) and pulse pressure (+5.71 mmHg, p = .03). Each 2.72‐fold increase in serum FT4 levels was associated with higher central systolic BP (+8.03 mmHg, p = .03) and pulse pressure (+3.89 mmHg, p = .05). In women, serum FT4 was only associated with a higher central pulse pressure (+2.96 mmHg, p = .04). After adjustment for covariates, serum FT4 was significantly associated with a faster cfPWV exclusively in men. Our study showed that serum FT4 is associated with higher peripheral and central BP and faster cfPWV in men, whereas serum FT3 is positively associated with a higher pulse rate in both sexes, indicating that the effects of thyroid function on BP and arterial stiffness are more significant in men than in women.
Keywords: arterial stiffness, blood pressure, Chinese, population, thyroid hormone
1. INTRODUCTION
Thyroid dysfunction (hyperthyroidism and hypothyroidism) may increase the risk of hypertension. 1 Hyperthyroidism and hypothyroidism are also common among hypertensive patients, 1 and hypertension guidelines suggest the need for evaluating the thyroid functions of hypertensive patients. 2 From the perspective of preventing hypertension induced by thyroid dysfunction, early intervention should be considered. Thus, it is essential to investigate the association between thyroid function and the indicators of hypertension incidence, particularly from the standpoint of preventing hypertension and related cardiovascular events.
Central (aortic) blood pressure (BP), which is the pressure exerted on the heart and brain, is different from the pressure measured in the arm. Emerging evidence now suggests that central BP is a better cardiovascular prognostic marker than conventional brachial cuff BP. 3 In addition, currently, central hemodynamics may be reliably assessed noninvasively using a few devices. 4 , 5 As arterial stiffening and central hemodynamics are markers and manifestations of organ damage, 6 it is of interest to investigate the association between thyroid function and arterial stiffening and central hemodynamics.
Several studies have examined the association between thyroid function and brachial BP 7 , 8 , 9 , 10 and hypertension. 11 , 12 , 13 However, most of these study cohorts were restricted to patients with hyperthyroidism, hypothyroidism, or subclinical hypothyroidism. 8 , 9 , 12 , 13 Moreover, very few previous studies have examined the relationship between thyroid function (including thyroid antibody levels) and central hemodynamics and arterial stiffness measured by pulse wave velocity (PWV). 14 The present population‐based study aimed to investigate the association between thyroid function and peripheral and central BP and arterial stiffness, as assessed by carotid‐femoral PWV (cfPWV) and brachial‐ankle PWV (baPWV).
2. MATERIALS AND METHODS
2.1. Study participants
The present cross‐sectional analysis was based on the data from an ongoing population study on multiple cardiovascular risk factors in Dali, Yunnan Province, China. The study participants were recruited from two communities in Dali. From October to December 2018, we invited all inhabitants aged 18 years or older to participate in the study. Of those invited, 764 (70%) participated. The ethics committee of the Dali University approved the study protocol. All participants provided written informed consent. We excluded 26 participants with a history of hyperthyroidism (n = 8), hypothyroidism (n = 4), Hashimoto's thyroiditis (n = 3), or surgical resection of thyroid cancer (n = 2) or thyroid nodule (n = 9). We further excluded 47 participants from our analysis because they did not have blood samples collected (n = 4) or arterial (n = 38) or anthropometric measurements (n = 5). Thus, a total of 691 subjects were included in the present analysis.
2.2. Fieldwork
Two experienced physicians measured each participant's BP five times consecutively using a mercury sphygmomanometer after the participants had rested for at least 5 min in the sitting position. The average of these five BP readings was used in the analysis. The same physicians also administered a standardized questionnaire to collect information on medical history, smoking habits, alcohol intake, and the use of medications. Hypertension was defined as a peripheral systolic BP of at least 140 mmHg or a diastolic BP of 90 mmHg in the sitting position or the use of antihypertensive drugs. A trained physician performed anthropometric measurements. The body mass index was calculated by dividing the body weight in kilograms by the square of the height in meters.
Venous blood samples were drawn after overnight fasting to measure the levels of plasma glucose, serum thyroid hormone, total cholesterol, and other biochemical markers. Diabetes mellitus was defined as a fasting plasma glucose level of at least 7.0 mmol/L, a hemoglobin A1c level of at least 6.5%, or the use of antidiabetic agents.
2.3. Central BP and cfPWV measurement
To ensure a steady state, one trained physician performed all arterial measurements using applanation tonometry after the participants had rested for 15 min in the supine position. The participants were informed to refrain from smoking, vigorous exercise, and drinking alcohol or caffeine‐containing beverages for at least 2 h before the examination. We used a high‐fidelity SPC‐301 micromanometer (Millar Instruments) interfaced with a laptop computer running the SphygmoCor version 7.1 (AtCor Medical) to record arterial waveforms. Recordings were discarded when the variability of consecutive waveforms exceeded 5% or when the amplitude of the pulse wave signal was <80 mV. We calibrated the pulse wave with the brachial BP (the average of two consecutive readings) measured in the supine position immediately before the SphygmoCor recordings, using a validated Omron HEM‐7051 oscillometric BP monitor (Omron). From the radial signal, the SphygmoCor software calculates the aortic pulse wave using a validated generalized transfer function. The central systolic and diastolic BP values were derived from the aortic pulse wave.
To measure the cfPWV, the physician recorded the right carotid and femoral waveforms (for 12 s each). With the simultaneously recorded electrocardiogram (lead 2), the time delay between the feet of the two pressure waveforms was taken as the transit time between the carotid and femoral pressure waves. The distance traveled by the pressure wave was the difference between the distances from the sternal notch to the femoral location and from the sternal notch to the carotid location. The PWV was calculated by dividing the distance traveled by the wave by the transit time.
2.4. baPWV measurement
Pulse wave measurements were performed using the Vascular Profiler‐1000 device (Omron), which operates based on the oscillometric cuff technique. Trained physicians placed the pressure cuffs on both arms and both ankles and performed the measurements after the subjects had rested for 10 min in the supine position. The device simultaneously and automatically recorded pulse waves in both ankles and measured the BP in the four limbs.
2.5. Serum thyroid hormone measurement
Serum thyroid hormone levels were measured, and autoantibodies were detected using electrochemiluminescence immunoassays. At our hospital, the normal ranges of serum thyroid‐stimulating hormone (TSH), FT4, FT3, TT4, and TT3 levels were 0.35–4.94 μIU/ml, 9–26 pmol/L, 1.34–6.79 pmol/L, 45–133 nmol/L, and 0.66–2.3 nmol/L, respectively. Based on the serum TSH and FT4 levels, we classified the participants into the following three categories: (i) euthyroidism, with TSH levels of 0.35–4.94 μIU/ml; (ii) subclinical hypothyroidism, with TSH levels >4.94 μIU/ml and FT4 levels of 9–26 pmol/L; and (iii) subclinical hyperthyroidism with TSH levels <0.35 μIU/ml and FT4 levels of 9–26 pmol/L.
2.6. Statistical analysis
The distribution of the data was assessed for normality using the Shapiro‐Wilk test. Thyroid hormone concentrations were not normally distributed and therefore logarithmically transformed for statistical analysis. Means and proportions were compared using the Student t test and Fisher's exact test, respectively.
Compared with women, men have distinct differences in thyroid hormone levels and the prevalence of thyroid dysfunction. Thus, we performed sex‐specific analyses to investigate the relationship between thyroid hormone levels and BP and arterial stiffness. Spearman's correlation analysis was performed to investigate the sex‐specific relationship between thyroid hormone levels and BP phenotypes and arterial stiffness. We performed multivariate‐adjusted linear regression analysis to study the association of each 2.72‐fold increase in the serum thyroid hormone levels with central and peripheral BP and arterial stiffness. Database management and statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). p values <.05 were considered statistically significant.
3. RESULTS
3.1. Characteristics of the study population
The 691 participants included 444 (64.2%) women, 215 (31.1%) patients with hypertension, and 75 (10.8%) patients with diabetes. Table 1 shows the characteristics of the study participants according to sex. Current smoking, alcohol intake, hypertension, and diabetes mellitus were more common in men; further, the men included in this study were also older and had a higher body mass index, waist circumference, hip circumference, fasting blood glucose level, low‐density lipoprotein cholesterol level, triglyceride level, peripheral systolic and diastolic BP, central systolic and diastolic BP, baPWV, and cfPWV (all p ≤ .02) than the women in this study. Other characteristics were similar between both sexes. Men had a lower glomerular filtration rate and lower levels of high‐density lipoprotein cholesterol, FT4, TT3, TSH, and TgAb than women (p ≤ .04). Men and women had similar levels of serum total cholesterol, FT3, TT4, TMAb, TPOAb, peripheral pulse pressure, pulse rate, and central pulse pressure (p ≥ .13).
TABLE 1.
Characteristics of the study population
| Variable | Men (n = 247) | Women (n = 444) | p |
|---|---|---|---|
| Current smoking, n (%) | 137 (55.5) | 6 (1.4) | <.0001 |
| Alcohol intake, n (%) | 73 (29.6) | 8 (1.8) | <.0001 |
| Hypertension, n (%) | 100 (40.5) | 115 (25.9) | <.0001 |
| Diabetes mellitus, n (%) | 40 (16.2) | 35 (7.9) | .0008 |
| Age, years | 53.5 ± 13.5 | 51.0 ± 11.9 | .02 |
| Body mass index, kg/m2 | 24.5 ± 3.4 | 23.6 ± 3.3 | .0007 |
| Waist circumference, cm | 89.0 ± 9.0 | 82.9 ± 9.0 | <.0001 |
| Hip circumference, cm | 96.7 ± 7.3 | 94.6 ± 7.4 | .0003 |
| Fasting blood glucose, mmol/l | 5.9 ± 1.8 | 5.5 ± 1.4 | .0007 |
| Total cholesterol, mmol/l | 4.8 ± 1.0 | 4.8 ± 0.9 | .97 |
| HDL‐C, mmol/l | 1.3 ± 0.2 | 1.4 ± 0.2 | <.0001 |
| LDL‐C, mmol/l | 2.7 ± 0.7 | 2.5 ± 0.7 | .004 |
| Triglyceride, mmol/l | 1.7 (1.2–2.4) | 1.4 (1.0–1.9) | .003 |
| GFR, ml/min/1.73 m2 | 87.1 ± 18.0 | 95.2 ± 18.4 | <.0001 |
| FT3, pmol/l | 4.3 (3.6–5.0) | 4.3 (3.6–5.0) | .29 |
| FT4, pmol/l | 16.7 (13.5–19.4) | 16.9 (14.5–20.0) | .04 |
| TT3, nmol/l | 1.0 (0.8–1.3) | 1.1 (0.9–1.3) | .03 |
| TT4, nmol/l | 80.6 (69.8–92.7) | 83.3 (73.1–94.2) | .21 |
| TSH, μIU/ml | 1.0 (0.6–1.5) | 1.3 (0.8–2.3) | .008 |
| TgAb, IU/ml | 16.8 (11.9–22.9) | 19.5 (14.1–51.9) | .007 |
| TMAb, IU/ml | 0.8 (0.5–1.0) | 0.8 (0.5–1.2) | .37 |
| TPOAb, S/CO | 6.2 (5.2–8.4) | 6.8 (5.4–11.7) | .18 |
| Peripheral SBP, mmHg | 122.4 ± 17.5 | 117.0 ± 17.7 | .0001 |
| Peripheral DBP, mmHg | 79.8 ± 11.8 | 75.8 ± 10.8 | <.0001 |
| Peripheral PP, mmHg | 42.7 ± 11.9 | 41.2 ± 10.9 | .10 |
| Central SBP, mmHg | 118.9 ± 15.8 | 114.0 ± 16.7 | .0002 |
| Central DBP, mmHg | 81.8 ± 12.2 | 76.5 ± 11.4 | <.0001 |
| Central PP, mmHg | 36.6 ± 8.3 | 37.0 ± 8.9 | .56 |
| Pulse rate, beats/min | 71.3 ± 9.2 | 72.3 ± 8.5 | .13 |
| baPWV, cm/s | 1613 ± 436 | 1501 ± 341 | .006 |
| cfPWV, m/s | 11.2 ± 2.2 | 9.9 ± 1.7 | <.0001 |
The values are expressed as means ± standard deviations, medians (interquartile ranges), or numbers s(%).
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; FT3, free T3; FT4, free T4; GFR, glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PP, pulse pressure; SBP, systolic blood pressure; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid‐stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
3.2. Spearman's correlation analysis of the relationship between serum thyroid hormone levels and peripheral and central BP
Among men, the serum FT3 level was positively correlated with the pulse rate (r = .150, p = .018), whereas the FT4 level was positively correlated with peripheral and central systolic BP, diastolic BP, and peripheral pulse pressure (r ≥ .129, p ≤ .043). Serum TT3 levels were significantly associated with peripheral diastolic BP (r = .132, p = .038), whereas serum TT4 levels were associated with higher peripheral and central systolic and diastolic BP values (r ≥ .137, p ≤ .032). The serum TSH level was marginally associated with higher peripheral and central systolic and diastolic BP (r ≥ .117, p ≤ .066) (Table 2). Serum TMAb concentration was negatively associated with central pulse pressure among men (r = −.135, p = .035) (Table 2).
TABLE 2.
Association between thyroid function and peripheral and central blood pressure in men
| Peripheral SBP, mmHg | Peripheral DBP, mmHg | Peripheral PP, mmHg | Pulse rate, beats/min | Central SBP, mmHg | Central DBP, mmHg | Central PP, mmHg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| FT3, pmol/l | .048 | .448 | .100 | .119 | −.013 | .839 | .150 | .018 | .053 | .403 | .080 | .210 | .019 | .766 |
| FT4, pmol/l | .205 | .001 | .171 | .007 | .129 | .043 | .109 | .089 | .166 | .009 | .163 | .010 | .110 | .084 |
| TT3, nmol/l | .109 | .088 | .132 | .038 | .045 | .478 | .099 | .120 | .029 | .650 | .069 | .281 | .002 | .974 |
| TT4, nmol/l | .160 | .012 | .143 | .025 | .103 | .106 | .031 | .633 | .137 | .032 | .138 | .030 | .060 | .346 |
| TSH, μIU/ml | .159 | .012 | .117 | .066 | .124 | .051 | −.102 | .109 | .119 | .062 | .125 | .049 | .057 | .369 |
| TgAb, IU/ml | −.004 | .949 | .040 | .531 | −.055 | .393 | −.048 | .456 | .001 | .993 | .038 | .553 | −.024 | .708 |
| TMAb, IU/ml | −.090 | .160 | −.077 | .227 | −.047 | .464 | −.086 | .176 | −.110 | .083 | −.076 | .231 | −.135 | .035 |
| TPOAb, S/CO | −.009 | .887 | .046 | .476 | −.069 | .283 | .042 | .514 | .025 | .694 | .035 | .581 | .015 | .814 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; FT3, free T3; FT4, free T4; PP, pulse pressure; SBP, systolic blood pressure; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid‐stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
Among women, serum FT3 levels were positively correlated with pulse rate (r = .109, p = .022), whereas FT4 levels were positively correlated with central systolic BP and pulse pressure (r ≥ .109, p ≤ .021). Serum TT3 levels were significantly associated with peripheral pulse pressure (r = .102, p = .032), whereas serum TT4 levels were associated with higher peripheral and central systolic and diastolic BP, and central pulse pressure (r ≥ .095, p ≤ .045). Serum TSH levels were positively associated with higher peripheral diastolic BP (r = .114, p = .017) (Table 3). None of the autoantibody markers showed an association with BP among women (Table 3).
TABLE 3.
Association between thyroid function and peripheral and central blood pressure in women
| Peripheral SBP, mmHg | Peripheral DBP, mmHg | Peripheral PP, mmHg | Pulse rate, beats/min | Central SBP, mmHg | Central DBP, mmHg | Central PP, mmHg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| FT3, pmol/l | .064 | .179 | .030 | .531 | .073 | .125 | .109 | .022 | .046 | .338 | .055 | .246 | .008 | .864 |
| FT4, pmol/l | .012 | .802 | −.021 | .667 | .047 | .319 | −.013 | .791 | .109 | .021 | .063 | .183 | .123 | .010 |
| TT3, nmol/l | .088 | .065 | .042 | .380 | .102 | .032 | .032 | .498 | .070 | .143 | .073 | .124 | .035 | .467 |
| TT4, nmol/l | .120 | .012 | .095 | .045 | .076 | .112 | .077 | .104 | .159 | .001 | .114 | .016 | .145 | .002 |
| TSH, μIU/ml | .042 | .379 | .114 | .017 | −.045 | .350 | −.066 | .165 | .040 | .397 | .066 | .167 | −.003 | .956 |
| TgAb, IU/ml | .047 | .325 | −.003 | .951 | .069 | .148 | −.003 | .944 | −.025 | .605 | −.034 | .476 | −.012 | .806 |
| TMAb, IU/ml | .014 | .770 | −.012 | .806 | .033 | .486 | .023 | .634 | −.014 | .770 | −.006 | .907 | −.017 | .726 |
| TPOAb, S/CO | .019 | .690 | .024 | .613 | .014 | .768 | −.021 | .655 | .018 | .703 | .013 | .783 | .012 | .794 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; FT3, free T3; FT4, free T4; PP, pulse pressure; SBP, systolic blood pressure; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid‐stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
3.3. Spearman's correlation analysis of the relationship between serum thyroid hormone levels and arterial stiffness
In Spearman's correlation analyses, serum FT4 and TT4 levels were positively associated with cfPWV (r ≥ .128, p ≤ .044) among men. Serum TSH levels were positively associated with both baPWV and cfPWV (r ≥ .164, p ≤ .010) (Table 4). Among women, serum FT4 and TT4 levels were positively associated with cfPWV (r ≥ .097, p ≤ .041) (Table 5).
TABLE 4.
Associations between thyroid function and arterial stiffness in men
| baPWV, cm/s | cfPWV, cm/s | |||
|---|---|---|---|---|
| r | p | r | p | |
| FT3, pmol/l | −.041 | .523 | −.022 | .733 |
| FT4, pmol/l | .095 | .140 | .133 | .037 |
| TT3, nmol/l | .033 | .610 | .031 | .623 |
| TT4, nmol/l | .109 | .092 | .128 | .044 |
| TSH, μIU/ml | .246 | .0001 | .164 | .010 |
| TgAb, IU/ml | .098 | .130 | .119 | .061 |
| TMAb, IU/ml | −.109 | .091 | −.070 | .275 |
| TPOAb, S/CO | .036 | .583 | .037 | .564 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; cfPWV, carotid‐femoral pulse wave velocity; FT3, free T3; FT4, free T4; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid‐stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
TABLE 5.
Associations between thyroid function and arterial stiffness in women
| baPWV, cm/s | cfPWV, cm/s | |||
|---|---|---|---|---|
| r | p | r | p | |
| FT3, pmol/l | .039 | .412 | .009 | .858 |
| FT4, pmol/l | .032 | .503 | .097 | .041 |
| TT3, nmol/l | .081 | .090 | .056 | .236 |
| TT4, nmol/l | .107 | .024 | .140 | .003 |
| TSH, μIU/ml | .018 | .707 | .063 | .184 |
| TgAb, IU/ml | .046 | .333 | .000 | .993 |
| TMAb, IU/ml | .042 | .374 | .023 | .630 |
| TPOAb, S/CO | .009 | .849 | .008 | .871 |
baPWV, brachial‐ankle pulse wave velocity; cfPWV, carotid‐femoral pulse wave velocity; FT3, free T3; FT4, free T4; TgAb, thyroglobulin antibody; TMAb, thyroid microsomal antibody; TPOAb, thyroid peroxidase antibody; TSH, thyroid‐stimulating hormone; TT3, total triiodothyronine; TT4, total thyroxine.
3.4. Multivariable‐adjusted association between thyroid hormone levels and peripheral and central BP
The serum FT3 level was significantly associated with a higher pulse rate among both men and women after adjusting for age, body mass index, alcohol intake, current smoking, former smoking, antihypertensive treatment, and diabetes mellitus. Indeed, each 2.72‐fold increase in the serum FT3 level led to 5.77 beats/min (p = .02) and 4.65 beats/min (p = .006) increases in pulse rate among men and women, respectively (Tables 6 and 7).
TABLE 6.
Multivariable‐adjusted associations between thyroid function and blood pressure and pulse rate in men
| Peripheral SBP, mmHg | Peripheral DBP, mmHg | Peripheral PP, mmHg | Pulse rate, beats/min | Central SBP, mmHg | Central DBP, mmHg | Central PP, mmHg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | |
| lnFT3, pmol/l | 4.80 ± 4.13 | .25 | 2.93 ± 2.93 | .32 | 1.86 ± 2.74 | .46 | 5.77 ± 2.45 | .02 | 2.77 ± 3.89 | .48 | .53 ± 2.85 | .85 | 1.93 ± 2.10 | .36 |
| lnFT4, pmol/l | 10.82 ± 3.80 | .005 | 5.11 ± 2.72 | .06 | 5.71 ± 2.54 | .03 | 3.85 ± 2.32 | .10 | 8.03 ± 3.60 | .03 | 4.18 ± 2.65 | .12 | 3.89 ± 1.95 | .05 |
| lnTT3, nmol/l | 6.32 ± 3.44 | .07 | 4.11 ± 2.43 | .09 | 2.21 ± 2.29 | .34 | 3.33 ± 2.07 | .11 | .85 ± 3.25 | .79 | .54 ± 2.38 | .82 | 0.17 ± 1.76 | .92 |
| lnTT4, nmol/l | 8.06 ± 4.92 | .10 | 4.89 ± 3.49 | .16 | 3.17 ± 3.27 | .33 | 2.20 ± 2.99 | .46 | 4.64 ± 4.64 | .32 | 3.10 ± 3.40 | .36 | 1.32 ± 2.52 | .60 |
| lnTSH, μIU/ml | 0.89 ± 1.32 | .50 | 1.37 ± 0.93 | .14 | −0.48 ± 0.88 | .58 | −0.77 ± 0.80 | .33 | 1.52 ± 1.24 | .22 | 1.87 ± 0.90 | .04 | −0.34 ± 0.67 | .62 |
Values were adjusted for age, body mass index, alcohol intake, current smoking, former smoking, antihypertensive treatment, diabetes mellitus, and heart rate (where applicable).
TABLE 7.
Multivariable‐adjusted associations between thyroid function and blood pressure and pulse rate in women
| Peripheral SBP, mmHg | Peripheral DBP, mmHg | Peripheral PP, mmHg | Pulse rate, beats/min | Central SBP, mmHg | Central DBP, mmHg | Central PP, mmHg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | β ± SE | p | |
| lnFT3, pmol/l | 3.92 ± 2.88 | .17 | 1.05 ± 1.95 | .58 | 2.87 ± 1.85 | .12 | 4.65 ± 1.68 | .006 | 2.24 ± 2.73 | .41 | 1.19 ± 1.94 | .54 | 0.93 ± 1.49 | .53 |
| lnFT4, pmol/l | −0.40 ± 2.83 | .89 | −0.86 ± 1.87 | .64 | 0.46 ± 1.82 | .80 | −0.58 ± 1.68 | .73 | 4.54 ± 2.67 | .09 | 1.56 ± 1.91 | .42 | 2.96 ± 1.46 | .04 |
| lnTT3, nmol/l | 1.89 ± 2.45 | .44 | 0.71 ± 1.62 | .66 | 1.18 ± 1.57 | .45 | 2.06 ± 1.44 | .15 | 1.83 ± 2.31 | .43 | 1.60 ± 1.65 | .33 | 0.25 ± 1.27 | .84 |
| lnTT4, nmol/l | 0.16 ± 3.74 | .97 | 1.34 ± 2.47 | .59 | −1.80 ± 2.40 | .62 | 3.74 ± 2.20 | .09 | 3.17 ± 3.53 | .37 | 1.04 ± 2.51 | .68 | 2.20 ± 1.93 | .26 |
| lnTSH, μIU/ml | −0.05 ± 0.87 | .95 | 1.18 ± 0.57 | .04 | −1.23 ± 0.55 | .03 | −0.50 ± 0.51 | .33 | −0.52 ± 0.82 | .52 | 0.29 ± 0.58 | .61 | −0.85 ± 0.45 | .06 |
Values were adjusted for age, body mass index, alcohol intake, current smoking, former smoking, antihypertensive treatment, diabetes mellitus, and heart rate (where applicable).
Among men, each 2.72‐fold increase in the serum FT4 level was associated with higher peripheral systolic BP (+10.82 mmHg, p = .005) and a higher pulse pressure (+5.71 mmHg, p = .03) in multivariable‐adjusted linear regression analyses. In addition, each 2.72‐fold increase in the serum FT4 level was also associated with higher central systolic BP (+8.03 mmHg, p = .03) and pulse pressure (+3.89 mmHg, p = .05). Furthermore, each 2.72‐fold increase in the serum TSH level was associated with higher central diastolic BP (+1.87 mmHg, p = .04) (Table 6).
Among women, the serum FT4 level was significantly associated with a higher central pulse pressure (+2.96 mmHg, p = .04) in the multivariable‐adjusted regression analyses. Each 2.72‐fold increase in the serum TSH level was positively associated with higher peripheral diastolic BP (+1.18 mmHg, p = .05) and lower peripheral pulse pressure (–1.23 mmHg, p = .03) among women (Table 7).
3.5. Multivariable‐adjusted association between thyroid hormone levels and arterial stiffness
Only the serum FT4 level was significantly associated with a higher cfPWV among men but not among women, after adjustment for age, height, heart rate, alcohol intake, current smoking, former smoking, antihypertensive treatment, and diabetes mellitus. Indeed, each 2.72‐fold increase in the FT4 level was associated with a 0.98 m/s faster cfPWV among men (p = .02) (Tables 8 and 9). Other thyroid hormone indices were not significantly associated with baPWV or cfPWV.
TABLE 8.
Multivariable‐adjusted association between thyroid function and arterial stiffness in men
| baPWV | cfPWV | |||
|---|---|---|---|---|
| β ± SE | p | β ± SE | p | |
| lnFT3, pmol/l | −54.9 ± 90.9 | .55 | 0.24 ± 0.48 | .62 |
| lnFT4, pmol/l | 137.2 ± 84.5 | .11 | 0.98 ± 0.44 | .02 |
| lnTT3, nmol/l | −18.9 ± 76.5 | .80 | 0.04 ± 0.40 | .93 |
| lnTT4, nmol/l | 70.8 ± 107.5 | .51 | 0.80 ± 0.56 | .16 |
| lnTSH, μIU/ml | 53.7 ± 28.9 | .06 | 0.06 ± 0.15 | .68 |
Values were adjusted for age, height, heart rate, alcohol intake, current smoking, former smoking, antihypertensive treatment, and diabetes mellitus.
TABLE 9.
Multivariable‐adjusted association between thyroid function and arterial stiffness in women
| baPWV | cfPWV | |||
|---|---|---|---|---|
| β ± SE | p | β ± SE | p | |
| lnFT3, pmol/l | −30.0 ± 49.2 | .54 | −0.14 ± 0.28 | .63 |
| lnFT4, pmol/l | 26.9 ± 48.2 | .58 | 0.52 ± 0.28 | .06 |
| lnTT3, nmol/l | −23.5 ± 41.9 | .58 | −0.06 ± 0.24 | .79 |
| lnTT4, nmol/l | 19.4 ± 63.2 | .76 | 0.56 ± 0.36 | .13 |
| lnTSH, μIU/ml | −7.7 ± 14.7 | .60 | 0.11 ± 0.08 | .18 |
Values were adjusted for age, height, heart rate, alcohol intake, current smoking, former smoking, antihypertensive treatment, and diabetes mellitus.
3.6. Categorical analyses of the relationship between serum FT4 level and BP and arterial stiffness
To analyze the associations between serum FT4 level and BP and arterial stiffness, we also classified the participants into the following four groups according to the quartiles of serum FT4 level: among men, Q1 (n = 62, FT4 9.01–13.46 pmol/l), Q2 (n = 62, FT4 13.58–16.67 pmol/l), Q3 (n = 61, FT4 16.68–19.37 pmol/l), and Q4 (n = 62, FT4 19.41–25.98 pmol/l); among women, Q1 (n = 111, FT4 9.02–14.45 pmol/l), Q2 (n = 111, FT4 14.48–16.86 pmol/l), Q3 (n = 111, FT4 16.87–19.96 pmol/l), and Q4 (n = 111, FT4 19.97–25.98 pmol/l).
After adjusting for covariates, only peripheral systolic BP was significantly increased across the quartiles of serum FT4 level among men but not among women (p trend = 0.009) (Figure 1). Similarly, cfPWV generally increased across the quartiles of serum FT4 levels (p trend = 0.01) only among men (Figure 2).
FIGURE 1.
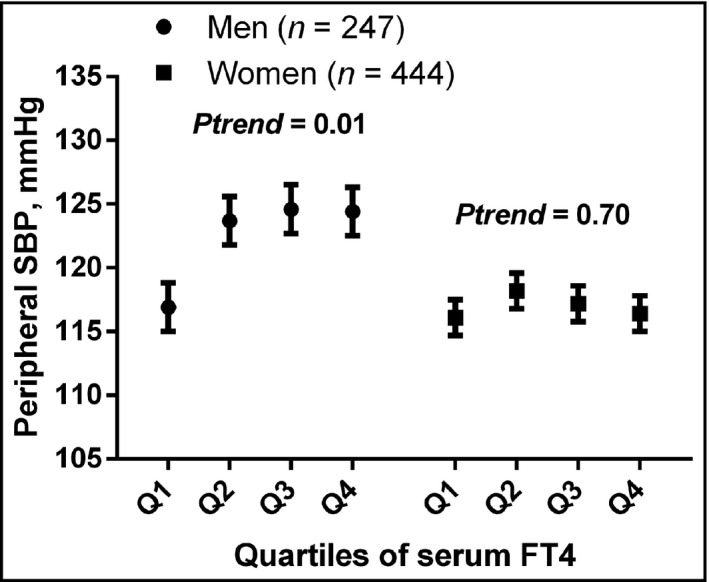
Peripheral systolic blood pressure across quartiles of serum FT4 levels among men and women. The values were adjusted for age, body mass index, alcohol intake, current smoking, former smoking, heart rate, antihypertensive treatment, and diabetes mellitus. Among men: Q1 (n = 62, FT4 9.01–13.46 pmol/l), Q2 (n = 62, FT4 13.58–16.67 pmol/l), Q3 (n = 61, FT4 16.68–19.37 pmol/l), and Q4 (n = 62, FT4 19.41–25.98 pmol/l). Among women: Q1 (n = 111, FT4 9.02–14.45 pmol/l), Q2 (n = 111, FT4 14.48–16.86 pmol/l), Q3 (n = 111, FT4 16.87–19.96 pmol/l), and Q4 (n = 111, FT4 19.97–25.98 pmol/l). SBP, systolic blood pressure
FIGURE 2.
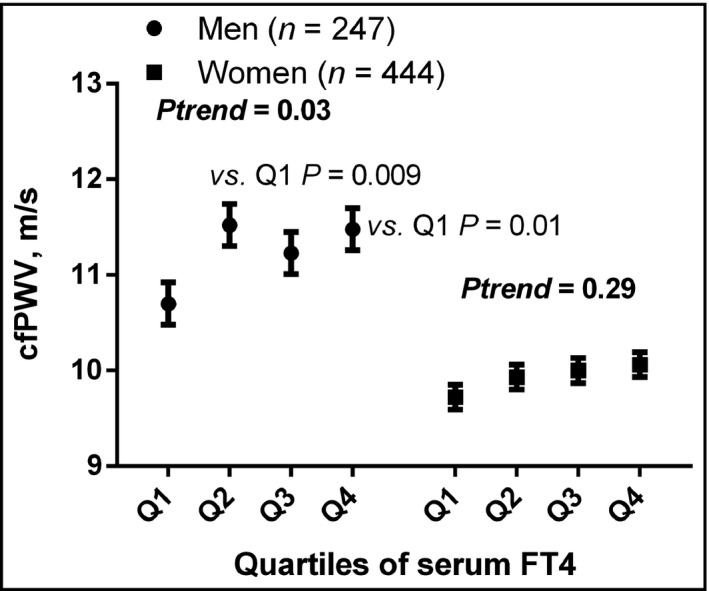
cfPWV across quartiles of serum FT4 among men and women. The values were adjusted for age, height, heart rate, alcohol intake, current smoking, former smoking, antihypertensive treatment, and diabetes mellitus. Among men: Q1 (n = 62, FT4 9.01–13.46 pmol/l), Q2 (n = 62, FT4 13.58–16.67 pmol/l), Q3 (n = 61, FT4 16.68–19.37 pmol/l), and Q4 (n = 62, FT4 19.41–25.98 pmol/l). Among women: Q1 (n = 111, FT4 9.02–14.45 pmol/l), Q2 (n = 111, FT4 14.48–16.86 pmol/l), Q3 (n = 111, FT4 16.87–19.96 pmol/l), and Q4 (n = 111, FT4 19.97–25.98 pmol/l). cfPWV, carotid‐femoral pulse wave velocity
The presence of hypertension defined by peripheral BP did not differ significantly across the quartiles of serum FT4 levels or other thyroid function indices. There were no significant differences in BP and arterial stiffness between participants with euthyroidism and those with subhyperthyroidism and subhypothyroidism.
4. DISCUSSION
The present population‐based study of Chinese persons without overt thyroid dysfunction showed that the serum FT3 level was significantly positively associated with a higher pulse rate among both men and women, albeit the association was slight. There was a significant but minor positive association between the serum FT4 level and peripheral and central systolic BP and pulse pressure among men. Among women, the serum FT4 level was associated with higher central pulse pressure. In addition, the serum FT4 level was positively associated with a faster cfPWV among men but not among women. In contrast to other studies, the serum TSH level was only marginally associated with a higher peripheral diastolic BP and lower pulse pressure among women but not among men. Our findings that thyroid function among participants without overt thyroid dysfunction are significantly associated with peripheral BP, central hemodynamics, and arterial stiffness are of clinical significance.
Thyroid function has direct and indirect effects on BP regulation and cardiac function. 1 , 15 Many mechanisms have been proposed to explain the role of thyroid function in the regulation of BP. 1 , 15 There is well‐documented evidence on the changes in BP among patients with thyroid dysfunction, including hyperthyroidism, subclinical hyperthyroidism, and hypothyroidism. 1 , 9 , 12 , 13 However, there is conflicting evidence in cross‐sectional and longitudinal studies regarding BP changes across the range of euthyroidism. 11 , 15 , 16 , 17 Moreover, none of the previous studies have considered central hemodynamics and arterial stiffness, which may play an important role in mediating the effects of thyroid function on BP regulation.
In particular, FT4 exerts more notable effects on both peripheral and central systolic BP in men than serum TSH. Our findings are consistent with those of most, but not all, previous studies. 8 , 16 , 18 Among 2282 euthyroid individuals, Abdi and colleagues 16 found that a serum FT4 level within the reference range was positively associated with all BP parameters in the entire population and men. However, serum TSH had a statistically significant mild increasing effect only on systolic and diastolic BP and mean BP in men. The multivariate transitional model found no association between serum TSH level within the reference range and BP status. Indeed, a 1 ng/dl increase in the FT4 level was associated with a 40%‐increased risk of prehypertension (odds ratio, 1.40; 95% confidence interval: 1.02–1.90) but not with an increased risk of hypertension (odds ratio, 0.93; 95% confidence interval: 0.80–1.09). 16 Among 12,487 euthyroid Chinese adults, Gu and colleagues 18 demonstrated that FT3 and FT4 levels are positively related to the prevalence of elevated BP; however, no significant relationship was found between TSH levels and elevated BP. 16 Among 1319 Chinese participants, there was no relationship between serum TSH levels and BP. However, in the same study, the prevalence of hypertension in the subclinical hypothyroidism group was significantly higher than that in the euthyroid group (41.3% vs. 25.6%) in females. The risk of hypertension in the subclinical hypothyroidism group was significantly higher than that in the euthyroid group after adjustment for confounding factors. 8
The FT4 level is currently the best measure of thyroid status along with serum TSH levels. FT4 level remains fairly stable in individuals over the years at an optimal “set point” for thyroid function. 19 In the evaluation of the association between thyroid function and BP, serum TSH level may be more sensitive in patients with overt thyroid dysfunction, such as hyperthyroidism and hypothyroidism. However, serum FT4 level is more sensitive than TSH level in euthyroid participants, especially in men. Thus, our study further supports the need to assess both serum TSH and FT4 levels simultaneously in the initial evaluation of thyroid dysfunction in the general population.
In the present study, we found that the serum FT4 level was not only associated with a higher peripheral systolic BP and pulse pressure but also a higher central systolic BP, central pulse pressure, and cfPWV in men. No previous study has investigated the relationship between thyroid function and central hemodynamics and arterial stiffness in the general population. Our findings further provide evidence that thyroid function, to at least a small degree, affects both peripheral and central BP, which may be mediated by a deterioration in central arterial stiffness. Besides, among 221 healthy men from the general population, both the TSH level and FT4·TSH product were positively associated with fasting and post‐load insulin concentrations and negatively associated with insulin sensitivity. 20 Subjects with an FT4·TSH product exceeding the median value showed reduced endothelium‐dependent vasodilation. 20 In a small study that assessed arterial stiffness in 102 volunteers (30 with overt hyperthyroidism, 28 with subclinical hyperthyroidism, 14 with euthyroidism induced by antithyroid therapy, and 30 healthy controls), there was a negative correlation between heart rate‐corrected augmentation index (Aix@75) and TSH levels and a positive correlation between Aix@75 and free thyroid hormone concentrations. 14 Together with our findings, the evidence suggests that elevated FT4, even within the normal range, may accelerate endothelial dysfunction and central arterial stiffness and thus increase BP.
Our study findings indicate that the effects of thyroid function on BP and arterial stiffness are more significant in men than in women. Thyroid dysfunction is generally more prevalent among women than among men. 21 Women also have higher rates of thyroid autoimmunity. 22 The mechanism underlying the sex difference in the association between thyroid function and BP and arterial stiffness is largely unknown. The higher prevalence of thyroid autoimmunity among women may complicate and weaken the association between FT4 and TSH levels and BP and cfPWV. Besides, sex differences in sympathetic neural‐hemodynamic balance could partly explain the observed sex difference in the association between serum FT4 and TSH levels and BP. 23
Our findings should be interpreted within the context of the strengths and limitations of our study. Our study comprehensively assessed thyroid function and BP phenotypes as well as central hemodynamics in a general Chinese population. However, our study has some limitations. First, the cross‐sectional design of our study did not allow for any causal inferences. Second, only a single measurement was performed for thyroid function, which is influenced by many factors and may fluctuate with time. Third, our study's sample size was relatively small, and the possibility of type I error cannot be excluded. Fourth, our study included some hypertensive patients who were receiving antihypertensive treatment. Though we adjusted for this in multivariate analyses, we cannot completely exclude the effect of antihypertensive treatment on thyroid hormone levels. Finally, according to the retrospective power analyses, our study had ≥51.2% (51.2%–80.9%) power to detect a significant association between serum FT3, FT4, pulse rate, and peripheral and central blood pressure. The possibility that the null findings in women and other thyroid hormones might be attributable to insufficient power cannot be excluded.
In the present study of middle‐aged Chinese individuals without overt thyroid dysfunction, the serum FT4 level is minorly associated with higher peripheral and central BP and faster cfPWV among men. The serum FT3 level is also minorly positively associated with a higher pulse rate in both men and women. Compared with FT4, TSH might be a less sensitive marker for evaluating the association between thyroid function and BP, particularly among women. Further longitudinal studies are required to determine whether a causal relationship exists between these parameters.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTION
LHL involved in the study design. MTJ, WYL, LHW, JHW, QLL, QYL, NQH, QL, and YZZ involved in data collection. LHL analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Funding information
The present study was supported financially by grants from the National Natural Science Foundation of China (81460084, 81660072, and 81860084), the Young and Middle‐aged Academic Leader Training Foundation of Yunnan Province (2015HB056), the Medical Academic Leader Foundation of Yunnan Provincial Bureau of Health (D‐201672), the Innovation Team of Hypertension Prevention and Treatment of Dali University (ZKPY2019304), and the Ten‐thousand Talents Program of Yunnan Province.
ACKNOWLEDGMENT
The authors gratefully acknowledge the voluntary participation of all study participants.
Jamal MT, Li Q‐L, Li Q‐Y, et al. Association of thyroid hormones with blood pressure and arterial stiffness in the general population: The Dali study. J Clin Hypertens. 2021;23:363–372. 10.1111/jch.14154
Md Tasneem Jamal, Qing‐Lu Li, Qi‐Yan Li contributed equally to this work.
REFERENCES
- 1. Berta E, Lengyel I, Halmi S, et al. Hypertension in thyroid disorders. Front Endocrinol (Lausanne). 2019;10:482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joint Committee for Guideline R . 2018 Chinese guidelines for prevention and treatment of hypertension‐a report of the Revision Committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol. 2019;16:182‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agabiti‐Rosei E, Mancia G, O'Rourke MF, et al. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154‐160. [DOI] [PubMed] [Google Scholar]
- 4. Ding FH, Fan WX, Zhang RY, Zhang Q, Li Y, Wang JG. Validation of the noninvasive assessment of central blood pressure by the SphygmoCor and Omron devices against the invasive catheter measurement. Am J Hypertens. 2011;24:1306‐1311. [DOI] [PubMed] [Google Scholar]
- 5. Ding FH, Li Y, Zhang RY, Zhang Q, Wang JG. Comparison of the SphygmoCor and Omron devices in the estimation of pressure amplification against the invasive catheter measurement. J Hypertens. 2013;31:86‐93. [DOI] [PubMed] [Google Scholar]
- 6. Spartano NL, Augustine JA, Lefferts WK, et al. Arterial stiffness as a noninvasive tissue biomarker of cardiac target organ damage. Curr Biomark Find. 2014;4:23‐34. [Google Scholar]
- 7. Xu R, Huang F, Zhang S, Lv Y, Liu Q. Thyroid function, body mass index, and metabolic risk markers in euthyroid adults: a cohort study. BMC Endocr Disord. 2019;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu D, Jiang F, Shan Z, et al. A cross‐sectional survey of relationship between serum TSH level and blood pressure. J Hum Hypertens. 2010;24:134‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaminski G, Makowski K, Michałkiewicz D, et al. The influence of subclinical hyperthyroidism on blood pressure, heart rate variability, and prevalence of arrhythmias. Thyroid. 2012;22:454‐460. [DOI] [PubMed] [Google Scholar]
- 10. Neves JS, Fontes‐Carvalho R, Borges‐Canha M, et al. Thyroid hormones within the normal range and cardiac function in the general population: the EPIPorto study. Eur Thyroid J. 2020. 10.1159/000508407. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang YC, Hua SC, Chang CH, et al. High TSH level within normal range is associated with obesity, dyslipidemia, hypertension, inflammation, hypercoagulability, and the metabolic syndrome: a novel cardiometabolic marker. J Clin Med. 2019;8:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jian WX, Jin J, Qin L, et al. Relationship between thyroid‐stimulating hormone and blood pressure in the middle‐aged and elderly population. Singapore Med J. 2013;54:401‐405. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Huang C, Meng Z, et al. Gender‐specific differences on the association of hypertension with subclinical thyroid dysfunction. Int J Endocrinol. 2019;2019:6053068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yildiz C, Altay M, Yildiz S, et al. Arterial stiffness in hyperthyroid patients is deteriorated due to thyroid hormones. Arch of Endocrinol and Metab. 2019;63:258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5:513‐520. [DOI] [PubMed] [Google Scholar]
- 16. Abdi H, Gharibzadeh S, Tasdighi E, et al. Associations between thyroid and blood pressure in euthyroid adults: a 9‐year longitudinal study. Horm Metab Res. 2018;50:236‐241. [DOI] [PubMed] [Google Scholar]
- 17. Langén VL, Niiranen TJ, Puukka P, Sundvall J, Jula AM. Association between thyroid‐stimulating hormone and blood pressure in adults: an 11‐year longitudinal study. Clin Endocrinol. 2016;84:741‐747. [DOI] [PubMed] [Google Scholar]
- 18. Gu Y, Zheng L, Zhang Q, et al. Relationship between thyroid function and elevated blood pressure in euthyroid adults. J Clin Hypertens (Greenwich). 2018;20:1541‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andersen S, Bruun NH, Pedersen KM, Laurberg P. Biologic variation is important for interpretation of thyroid function tests. Thyroid. 2003;13:1069‐1078. [DOI] [PubMed] [Google Scholar]
- 20. Fernández‐Real JM, López‐Bermejo A, Castro A, Casamitjana R, Ricart W. Thyroid function is intrinsically linked to insulin sensitivity and endothelium‐dependent vasodilation in healthy euthyroid subjects. J Clin Endocrinol Metab. 2006;91:3337‐3343. [DOI] [PubMed] [Google Scholar]
- 21. Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty‐year follow‐up of the Whickham Survey. Clin Endocrinol (Oxf). 1995;43:55‐68. [DOI] [PubMed] [Google Scholar]
- 22. Calcaterra V, Nappi RE, Regalbuto C, et al. Gender differences at the onset of autoimmune thyroid diseases in children and adolescents. Front Endocrinol (Lausanne). 2020;11:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Briant LJB, Charkoudian N, Hart EC. Sympathetic regulation of blood pressure in normotension and hypertension: when sex matters. Exp Physiol. 2016;101:219‐229. [DOI] [PubMed] [Google Scholar]


