Abstract
Several lifestyle and sociodemographic factors are associated with blood pressure (BP). The authors conducted a retrospective study of 4870 subjects from the National Health Survey 2009 in Chile to identify exposure factors associated with increasing BP levels. Subjects with isolated urinary excretion of sodium (n = 2873), potassium, and creatinine were included to estimate daily salt intake and urinary sodium/potassium (Na/K) ratio. Hypertension was defined according to European guidelines 2018 and American guidelines ACC/AHA 2017. Proportional odds models were developed to analyze education level, sedentarism, smoking, alcohol intake, estimated urinary Na/K ratio, estimated daily salt intake, and body mass index (BMI) as factors associated with increasing BP levels (from high‐normal BP to hypertension). Logistic regression models were checked for overdispersion. Mean age and BMI of the population were 42 years old and 27 kg/m2, respectively; 19% had low education level and 27% had hypertension according to European guidelines, whereas 47% according to ACC/AHA criteria. Mean estimated urinary Na/K ratio was 4 ± 2, and mean salt consumption was 10 ± 2 g/day. Estimated urinary Na/K ratio (OR, 1.11; 95% CI, 1.01‐1.21), BMI (OR, 1.10; 95% CI, 1.07‐1.13), estimated daily salt intake (OR, 1.10; 95% CI, 1.03‐1.17), and alcohol intake (OR, 1.03; 95% CI, 1.01‐1.05) were significantly associated with hypertension. This study highlights that a healthy diet and weight control should be important components of BP management plans, and it suggests that public policies should include close monitoring of these factors to reduce hypertension prevalence and improve its management in a Latino population.
Keywords: blood pressure, body mass index, diet, hypertension, potassium, sodium
1. INTRODUCTION
According to the World Health Organization, hypertension (HTN) is one of the chronic diseases that most contributes to the global burden of coronary heart disease, stroke, chronic kidney disease, and premature morbidity and mortality. The American College of Cardiology (ACC) and American Heart Association (AHA) guidelines (2017) and the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH) guidelines (2018) are the two leading criteria used to diagnose HTN. 1 , 2 In Chile, the ESC/ESH guidelines are used in clinical practice. Early detection, appropriate treatment, and strict monitoring of HTN may yield important health benefits as well as long‐term cost savings in developing countries. Unfortunately, detection, treatment, and effective control of HTN are deficient in Chile, as most developing countries. This situation requires urgent attention from health authorities.
Based on the preliminary results from the latest Chilean National Health Survey (NHS) 2017, 27.6% of the Chilean population has HTN, with prevalence rates of 73% in older adults and 57% in the lowest education level. 3 Chile has a high prevalence of overweight and obesity (74%), sedentary lifestyle (87%), smoking (33.3%), excessive consumption of salt (98%), and poor intake of fruits and vegetables (85%)—all of which results in high urinary sodium/potassium (Na/K) ratio for the population. 3 , 4 These factors all increase the risks of cardiovascular disease (CVD) and HTN. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 Given these data, education about and resources for a healthy lifestyle must be fundamental components of the efforts to reduce the rate of HTN in Chile.
The impact of sodium intake on BP is well established in the literature. 10 , 14 , 15 Traditionally, the principal recommended lifestyle modification for HTN focuses on sodium restriction. Less is known, however, about how increased potassium intake may counterbalance the harmful effects of salt on BP at a population level. Some studies report that high potassium intake favors renal sodium excretion and improves endothelial function, lowering BP, especially when it is higher than sodium intake. Therefore, a urinary Na/K ratio < 1 is crucial to achieving optimal BP levels. 13 , 14 , 15 Of note, the most recent ACC/AHA guidelines included a specific recommendation about enhanced intake of dietary potassium as one of the best proven non‐pharmacologic interventions for the prevention and treatment of HTN. 1
The first randomized trial to evaluate the effects of dietary patterns on blood pressure was the Dietary Approaches to Stop Hypertension (DASH) study. 16 Importantly, the DASH diet favors a urinary Na/K ratio < 1 through increased intake of dietary potassium and reduced sodium intake. It also results in increased intake of dietary calcium and magnesium, which can further boost BP. 16 , 17 Given these findings, the urinary Na/K ratio could be considered a biomarker of DASH diet adherence, since it reflects both potassium and sodium intake, which are both highly underestimated by dietary surveys. 18 Potassium‐rich foods are associated with reductions in BP and CV events, whereas the opposite is true for processed foods. 6 , 7 , 8 , 19 This information is important for clinicians in the everyday management of patients with or at‐risk for HTN to assess the contribution of dietary modification on BP.
The mean dietary sodium intake of the Chilean population is 3900 mg/day, which is well above the recommended range of 1500‐2300 mg/day. 1 , 2 , 3 , 4 , 20 Almost 96% of Chilean adults have a urinary Na/K ratio > 1, suggesting a deficient potassium intake, increased sodium intake and low consumption of fruits and vegetables. 4 Moreover, the obesity rate increased from 25% to 34% from 2009 to 2017. Together with the high rates of metabolic syndrome and diabetes in this population, obesity activates the sympathetic nervous and renin‐angiotensin systems predisposing to higher BP levels. 21 Finally, social determinants of cardiovascular (CV) risk, such as low education level, geographic place of residence, and social support, have also been associated with a higher risk of developing HTN. 22
The objective of this study was to identify exposure factors associated with the probability of being diagnosed with a high BP category (from normal‐high BP to HTN) in a Chilean Latino population. We considered the following factors in the study: education level, nutritional status, salt intake, sodium/potassium ratio, and alcohol consumption.
2. MATERIAL AND METHODS
This study was cross‐sectional, and it analyzed the database of the NHS 2009, which included an epidemiologically selected and representative sample of 5416 subjects. Four thousand seven hundred and eighty (4780) subjects had all the demographic, clinical, and biochemical samples, whereas only 2873 out of 3200 (urinary data sub‐sample) had all the urinary determinations. 4
The database was downloaded in SPSS software from the Chilean Ministry of Health website. We used data from 2009 because only the preliminary results were available for the NHS 2017, but not the database. Overall, the NHS collects demographic data, medical history, dietary data, physical activity, CV risk factors, drug and alcohol consumption, psychosocial factors, and determines lipid profile, glycemia, plasma creatinine level, and urinary spot samples of sodium, potassium, and creatinine from the Chilean population. The survey carries out every 6 years, and the data are used to assess and develop public health policies according to the epidemiologic data of the country. A trained surveyor collected health and demographical data from the subjects. All of them signed written informed consent. Later, a nurse interviewed each subject about medication intake and alcohol consumption and collected fasting venous blood and spot urine samples (this last one only assessed in a sub‐sample of 3200 subjects). Weight, height, waist circumference, and BP were measured during this visit. 4 Alcohol consumption was quantified as grams of alcohol during a typical day of drinking using the STEPs instrument proposed by the World Health Organization (WHO); we considered high alcohol consumption as > 20 g/day in women and > 30 g/day in men. 23 From dietary data, we only considered fruits and vegetable intake to compare with urinary potassium excretion. Grams of fruits and vegetable intake was estimated using the STEP instrument (WHO): The card pictures with the standard portion size of fruits and vegetables (1 portion = 80 g) were adapted to those consumed in Chile. Mean daily intake was calculated multiplying the number of portions consumed by the days of the week each subject ate fruits and vegetables. The result was then divided by 7. 4 , 23 Self‐reported physical activity was measured using the Global Physical Activity Questionnaire. 24 Sedentary subjects were those with an activity level of < 600 METs/minutes/week. Dyslipidemia was defined as triglycerides > 150 mg/dL, low‐density lipoprotein (LDL) >100 mg/dL, and/or high‐density lipoprotein (HDL) <50 mg/dL in women or < 40 mg/dL in men. 25 , 26 Diabetes was defined as subjects with a previous medical diagnosis and/or fasting glycemia ≥ 126 mg/dL. 25 WHO criteria: ≥25 kg/m2 and ≥ 30 kg/m2 defined overweight and obesity, respectively. Education level was defined as follows: low (<8 years of education), middle (8‐12 years of education), and high (>12 years of education).
2.1. Arterial hypertension criteria (NHS 2009)
During NHS 2009, seated BP was measured three times after 5 minutes resting (at 2‐minute intervals), using a device with a brachial cuff with automatic inflation (Omron HEM 742). 4 Optimal blood pressure was considered as < 120/80 mmHg. We used either an entry of previous diagnosis of hypertension or the average of the three BP measurements according to the criteria defined by the ESC/ESH 2018 and the AHA/ACC 2018 guidelines. 1 , 2
2.2. ESC 2018
High‐normal BP: 130‐139 mmHg and/or 85‐89 mmHg
HTN‐1:140‐159 mmHg and/or 90‐99 mmHg
HTN‐2: ≥160 mmHg and/or ≥ 100 mmHg.
2.3. ACC/AHA 2017
High BP: >120‐129 mmHg and < 80 mmHg
HTN‐1:130‐139 mmHg and/or 80‐89 mmHg
HTN‐2: ≥140 mmHg and/or ≥ 90 mmHg
Subjects with HTN and BP levels < 140 mmHg and/or < 90mmHg were considered as HTN‐1 according to ESC 2018 or HTN‐2 by ACC/AHA.
2.4. Estimation of sodium/potassium ratio and daily salt intake (NHS 2009)
During NHS 2009, samples of isolated urine (first‐void urine) were collected after measuring BP and before starting the interview with the nurse. Na, K, and creatinine levels were measured from this sample. 4 Urinary Na and K were determined with ion‐selective electrodes using an automatic analyzer, and urinary creatinine by the Jaffe method. Creatinine excretion was measured to standardize the excretion of electrolytes (eg, sodium/mg creatinine) and later to estimate 24‐hour excretion of them. The 24‐hour urinary excretion of sodium was estimated using the validated equations detailed below. 4 , 18 , 27
Sodium mEq/d multiplied by sodium atomic weight (23) was then converted to grams to calculate the estimated salt intake. Therefore 4 :
2.5. Statistical analysis
Proportional odds models were developed to calculate the probability of having high‐normal BP and HTN: a) optimal BP (<120/80) versus high‐normal BP (≥130/80); and b) normal BP (<120/80) versus HTN (≥140/90) according to the ESC/ESH guidelines. We calculated proportional odds cumulative logistic regression models (likelihood ratio P < .05) adjusted by age, sex, and antihypertensive treatment to determine the association with BP levels. The following factors were included in the models: BMI, education level, estimated urinary Na/K ratio, estimated daily salt intake, alcohol intake, sedentary lifestyle, and smoking. Estimated urinary Na/K and daily salt intake were included separately in the models to avoid collinearity. We performed a complimentary analysis of the patients who were not taking any antihypertensive medication. For this analysis, we only included subjects who had urinary samples with measurement of sodium, potassium, and creatinine excretion in addition to the demographic and clinical information listed previously.
Logistic regression models were checked for overdispersion. Specifically, in models including hypertension treatment as confounding variable quasibinomial models were considered with a dispersion parameter of 1.71.
3. RESULTS
Four thousand seven hundred and eighty (4780) subjects had all the demographic, clinical, and biochemical samples, whereas only 2873 out of 3200 (urinary data sub‐sample) had all the urinary determinations.
Table 1 provides the demographic and clinical characteristics of all the subjects (n = 4780). Overall, the mean age was 42 years, 60% were women, and 19% and 24.5%, respectively, belonged to low and high education levels. A total of 64% were overweight or obese, and 12.5% had diabetes. Of the 4780 subjects, 26.9% and 47% had HTN as defined by the ESC/ESH guidelines and the AHA/ACC guidelines, respectively, whereas 16% were taking antihypertensive medication. Three percent had high‐normal BP, 19% had HTN‐1, and 8% had HTN‐2 (all according to ESC/ESH guidelines). According to the ACC/AHA criteria, 13% of subjects had high BP, 20% had HTN‐1, and 27% had HTN‐2. The prevalence of HTN, at all stages, was higher in men than women.
Table 1.
Demographic and clinical characteristics of study subjects (N = 4780)
| Characteristic | Men (n = 1915) | Women (n = 2865) | P‐value a |
|---|---|---|---|
| Age, years | 41 ± 17 | 42 ± 18 | NS |
| Anthropometric variables | |||
| Waist circumference, cm | 93 ± 12 | 88 ± 14 | NS |
| BMI, kg/m2 | 27 ± 5 | 28 ± 6 | NS |
| Blood pressure | |||
| SBP, mmHg | 130 ± 19 | 122 ± 21 | .05 |
| DBP, mmHg | 78 ± 11 | 74 ± 10 | .04 |
| CV risk factors | |||
| Hypertension (ESC/ESH definition), % b | 28 | 25 | <.001 |
| Hypertension (ACC/AHA definition), % c | 52 | 42 | <.001 |
| Dyslipidemia, % | 86 | 79 | <.0001 |
| Diabetes, % | 11 | 14 | <.0001 |
| Smoking, % | 43 | 32 | <.01 |
| Overweight and obesity, % | 65 | 64 | NS |
| Sedentary, % d | 31 | 22 | NS |
| Educational level | |||
| Low (<8 years), % | 17 | 20 | |
| Middle (8‐12 years), % | 59 | 55 | |
| Higher (>12 years), % | 24 | 25 | <.01 |
| Dietary variables | |||
| Fruit and vegetables intake, g/day | 211 ± 131 | 241 ± 136 | .09 |
| Alcohol consumption, g/day | 11 ± 48 | 31 ± 70 | .07 |
| High alcohol consumption, % | 29 | 23 | <.0001 |
| Estimated daily salt intake, g/day e | 10 ± 3 | 9 ± 3.2 | .08 |
| Estimated urinary Na/K ratio e | 4 ± 2 | 3 ± 2 | .09 |
Data are expressed as mean ± standard deviation except where indicated.
Abbreviations: ACC, American College of Cardiology; AHA, American Heart Association; BMI, body mass index; DBP, diastolic blood pressure; ESC, European Society of Cardiology; ESH, European Society of Hypertension; Na/K, sodium/potassium; SBP, systolic blood pressure.
t Test was used for continuous variables and Fisher exact test for prevalence.
ESC/ESH definition of hypertension: SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg.
ACC/AHA definition of hypertension: SBP ≥ 130 mmHg and/or DBP ≥ 80mmHg.
Physical activity was measured using the Global Physical Activity Questionnaire, and subjects with an activity level < 600 METs/minutes/week were classified as sedentary.
Estimation of daily salt intake and urinary Na/K ratio was estimated from the sub‐sample who had spot urinary samples (n = 3200)
The mean estimated salt consumption in the population was 10 ± 2 g/day, with no significant differences between men and women, and the mean estimated urinary Na/K ratio was 4 ± 2 (Table 1). Daily intake of fruits and vegetables had a significant correlation with the urinary K excretion (r = .04; P = .03).
Table 2 shows the proportional odds model for the association of education level, BMI, physical activity, smoking, alcohol intake, estimated urinary Na/K ratio and estimated daily salt intake with BP levels adjusted by pharmacological treatment.
Table 2.
Proportional odds model for the association of education level, BMI, physical activity, smoking, alcohol intake, estimated urinary Na/K ratio, and estimated daily salt intake with blood pressure levels in all subjects adjusted by pharmacological treatment (n = 2873)
| Variable | Optimal BP vs high‐normal BP | Normal BP vs HTN | ||
|---|---|---|---|---|
| OR (95% CI) a | P‐value b | OR (95% CI) a | P‐value b | |
| Age | 1.06 (1.05‐1.07) | <.0001 | 1.09 (1.07‐1.10) | <.0001 |
| Sex (Women) | 0.39 (0.30‐0.51) | <.0001 | 0.43 (0.30‐0.61) | <.0001 |
| Low education level (<8 years) | 1.00 | ‐‐‐‐ | 1.00 | ‐‐‐‐ |
| Middle education level (8‐12 years) | 0.83 (0.58‐1.19) | NS | 1.16 (0.77‐1.74) | NS |
| High education level (>12 years) | 1.07 (0.72‐1.61) | NS | 0.90 (0.55‐1.47) | NS |
| BMI, kg/m2 | 1.10 (1.07‐1.13) | <.0001 | 1.10 (1.07‐1.14) | <.0001 |
| Estimated urinary Na/K ratio | 1.09 (1.03‐1.16) | <0.01 | 1.13 (1.05‐1.22) | <.01 |
| Estimated daily salt intake, g/day c | 1.06 (1.00‐1.11) | .04 | 1.10 (1.03‐1.17) | <.01 |
| Alcohol intake, 10 g | 1.02 (1.00‐1.04) | .07 | 1.03 (1.00‐1.05) | .02 |
| Sedentary lifestyle | 1.08 (0.80‐1.45) | NS | 0.90 (0.62‐1.32) | NS |
| Smoking | 0.91 (0.70‐1.18) | NS | 0.93 (0.65‐1.34) | NS |
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; HTN‐1, hypertension stage 1; HTN‐2, hypertension stage 2; Na/K, sodium/potassium; OR, odds ratio.
OR represents the probability of being at different BP levels where optimal BP is < 120 and < 80 mmHg; normal BP is 120 ‐ 129 mmHg and/or 80‐84 mmHg; high‐normal BP is 130‐139 mmHg and/or 85‐89 mmHg and HTN ≥ 140 mmHg and/or ≥ 90 mmHg (HTN‐1 is 140‐159 mmHg and/or 90‐99 mmHg, and HTN‐2 is 160‐179 mmHg and/or 100‐109 mmHg). OR for continuous variables is calculated for the increase of each unit: Age for each year, BMI for each 1 kg/m2, estimated urinary Na/K ratio for each unit, estimated daily salt intake for each 1 gram, and alcohol intake for each 10 grams.
Proportional odds cumulative logistic regression models (likelihood ratio P < .05) adjusted by antihypertensive pharmacological treatment.
Estimated daily salt intake was included in a separate model with the same variables (age, sex, level of education, BMI, alcohol intake, sedentary lifestyle, and smoking), excluding estimated Na/K ratio.
The following variables were factors significantly associated with HTN adjusted by pharmacological antihypertensive treatment: BMI (odds ratio [OR], 1.10; 95% confidence interval [CI], 1.07‐1.14; for each 1 kg/m2 increase); estimated urinary Na/K ratio (OR, 1.13; 95% CI, 1.05‐1.22; for each unit increase); estimated daily salt intake (OR, 1.10; 95% CI, 1.03‐1.17); and alcohol intake (odds ratio [OR], 1.03; 95% confidence interval [CI], 1.00‐1.05; for each 10 g). Education level, sedentary level, and smoking were not significantly associated with HTN in this model. We observed the same associations with the probability of being in the normal‐high BP category (Table 2).
Table 3 shows the same model for subjects who were not taking any antihypertensive medication (n = 2424). In this model, the same variables associated with different BP levels.
Table 3.
Proportional odds model for the association of education level, BMI, physical activity, smoking, alcohol intake, estimated urinary Na/K ratio, and estimated daily salt intake with blood pressure levels in subjects without pharmacological treatment (n = 2424)
| Variable | Optimal BP vs high‐normal BP | Normal BP vs HTN | ||
|---|---|---|---|---|
| OR (95% CI) a | P‐value b | OR (95% CI) a | P‐value b | |
| Age | 1.06 (1.05‐1.07) | <.0001 | 1.09 (1.08‐1.10) | <.0001 |
| Sex (Women) | 0.40 (0.32‐0.48) | <.0001 | 0.42 (0.32‐0.54) | <.0001 |
| Low education level (<8 years) | 1.00 | ‐‐‐‐ | 1.00 | ‐‐‐‐ |
| Middle education level (8‐12 years) | 0.79 (0.60‐1.05) | NS | 1.12 (0.83‐1.52) | NS |
| High education level (>12 years) | 1.17 (0.86‐1.61) | NS | 1.05 (0.73‐1.53) | NS |
| BMI, kg/m2 | 1.11 (1.08‐1.13) | <.0001 | 1.11 (1.09‐1.14) | <.0001 |
| Estimated urinary Na/K ratio | 1.11 (1.06‐1.16) | <.0001 | 1.15 (1.08‐1.21) | <.0001 |
| Estimated daily salt intake, g/day c | 1.06 (1.02‐1.11) | <.01 | 1.12 (1.06‐1.18) | <.0001 |
| Alcohol intake, each 10 g | 1.02 (1.00‐1.03) | .03 | 1.03 (1.01‐1.04) | <.01 |
| Sedentary lifestyle | 1.06 (0.85‐1.34) | NS | 0.90 (0.68‐1.18) | NS |
| Smoking | 0.93 (0.76‐1.13) | NS | 0.98 (0.75‐1.27) | NS |
Abbreviations: BMI, body mass index; BP, blood pressure; CI, confidence interval; HTN‐1, hypertension stage 1; HTN‐2, hypertension stage 2; Na/K, sodium/potassium; OR, odds ratio.
OR represents the probability of being at different BP levels where optimal BP is < 120 and < 80 mmHg; normal BP is 120 ‐ 129 mmHg and/or 80‐84 mmHg; high‐normal BP is 130‐139 mmHg and/or 85‐89 mmHg and HTN ≥ 140 mmHg and/or ≥ 90 mmHg (HTN‐1 is 140‐159 mmHg and/or 90‐99 mmHg, and HTN‐2 is 160‐179 mmHg and/or 100‐109 mmHg). OR for continuous variables is calculated for the increase of each unit: Age for each year, BMI for each 1 kg/m2, estimated urinary Na/K ratio for each unit, estimated daily salt intake for each 1 gram, and alcohol intake for each 10 grams.
Proportional odds cumulative logistic regression models (likelihood ratio P < .05) adjusted by antihypertensive pharmacological treatment.
Estimated daily salt intake was included in a separate model with the same variables (age, sex, level of education, BMI, alcohol intake, sedentary lifestyle, and smoking), excluding estimated Na/K ratio.
Figures 1 and 2 show the odds of having normal‐high BP, HTN 1, and HTN 2 in a 35‐year‐old man and woman.
Figure 1.
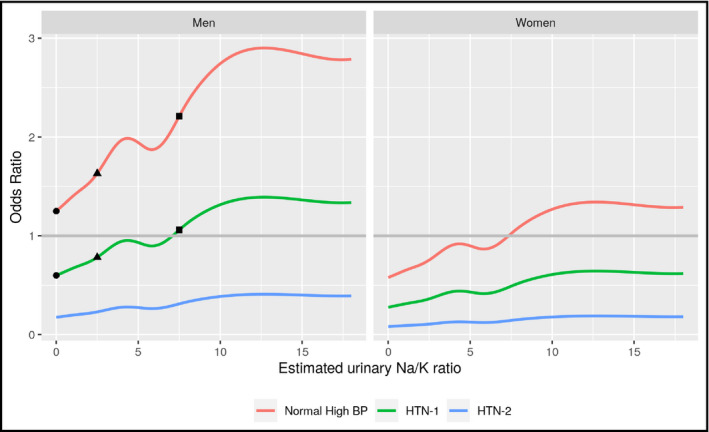
Charts represent average risk (%) of each hypertension stage (defined by ESC/ESH guidelines)4 in a person of 35 years old according to the urinary Na/K ratio. The reference is the probability of having normal‐high BP with an estimated urinary Na/K ratio < 1 (dot in red line). At this Na/K ratio, the probability of having HTN 1 reduces in about 50% (dot in green line), and HTN 2 in about 80% (blue line). When the estimated urinary Na/K ratio increases, the probability of being in each stage also increases. If the Na/K ratio increases to 2.5, the probability of having normal‐high BP increases about 50% (triangle in red line); whereas the probability of having HTN 1 increases 25% (triangle in green line) when compared with a ratio < 1 (dot in red line and green line). Finally, if the estimated urinary Na/K ratio increases to 7.5 (square), the probability of presenting normal‐high BP duplicates (square in red line), whereas the probability of HTN 1 increases by 50% (square in green line) when compared with a ratio < 1. As defined by ESC/ESH, high‐normal BP is 130‐139 mmHg and/or 85‐89 mmHg, HTN‐1 is 140‐159 mmHg and/or 90‐99 mmHg, and HTN‐2 is 160‐179 mmHg and/or 100‐109 mmHg. Abbreviations: BP, blood pressure; ESC, European Society of Cardiology; ESH, European Society of Hypertension; HTN‐1 = hypertension stage 1; HTN‐2 = hypertension stage 2; Na/K, sodium/potassium
Figure 2.
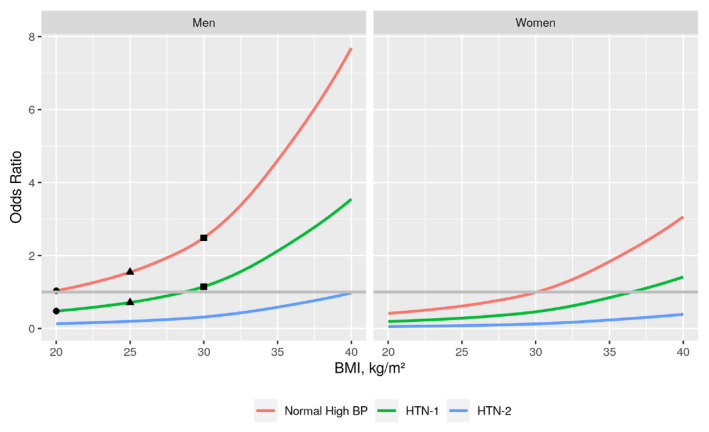
Charts represent average risk (%) of each hypertension stage (defined by ESC/ESH guidelines)4 in a person of 35 years old according to BMI. The reference is the probability of having normal‐high BP with BMI = 20 kg/m2 (circle in red line). At this BMI, the probability of having HTN 1 reduces in about 50% (dot in green line), and HTN 2 reduces over 90% (blue line). When the BMI increases, the probability of being in each stage also increases. If the BMI increases to 25 kg/m2 (overweight), the probability of having normal‐high BP increases about 70% (triangle in red line); whereas the probability of having HTN 1 increases about 30% (triangle in green line) compared with a BMI of 20 kg/m2 (circle in red line and green line). Finally, if the BMI increases to 30 kg/m2 (obesity), the probability of normal‐high BP increases by about 2.5‐fold (square in red line) and the probability of HTN 1 by about 60% (square in green line) when compared with a BMI of 20 kg/m2. As defined by ESC/ESH, high‐normal BP is 130‐139 mmHg and/or 85‐89 mmHg, HTN‐1 is 140‐159 mmHg and/or 90‐99 mmHg, and HTN‐2 is 160‐179 mmHg and/or 100‐109 mmHg. Abbreviations: BMI, body mass index; BP, blood pressure; ESC, European Society of Cardiology; ESH, European Society of Hypertension; HTN‐1 = hypertension stage 1; HTN‐2 = hypertension stage 2
4. DISCUSSION
Our study demonstrates that the main determinants associated with normal‐high BP levels and HTN 1 or 2 were BMI, estimated urinary Na/K ratio, estimated daily salt intake, and alcohol intake. The education level was not associated with increasing BP levels in this study when all the above variables were included in the model.
The Chilean NHS 2009, from which the data for this analysis are, reported a higher HTN prevalence in the lowest education level compared with the highest (57% vs. 15%, respectively). Besides, NHS 2009 data showed that individuals with low education level had a threefold higher risk of urinary Na/K ratio > 1 and they were 80% more likely to be obese than those with the highest education level. 4 In our analysis, we found that a high education level gave a 33% lower risk of high BP levels only when BMI, alcohol intake, the estimated daily sodium intake, and urinary Na/K ratio were excluded from the model (data not shown). These findings suggest that dietary variables could mediate in the association of the education level differences observed in the development of HTN . The published evidence indicates that low education level is associated with a worse lifestyle and higher stress, which are associated with obesity and higher CV risk; therefore, the effect of social determinants over BP might be mediated by diet and lifestyle. 22 , 28 Our results are similar to US data reported by Howard et al which suggested that dietary Na/K ratio, dietary pattern and BMI (for women) were crucial factors that mediated the higher incidence of HTN in black adults. 29
BMI as a continuous variable was significantly associated with high‐normal BP and HTN 1 and 2 in our study. A positive caloric balance increases visceral adipose tissue, which releases cytokines and decreases adiponectin. 21 Along with the increase in angiotensinogen synthesis, these cytokines increase vascular volume and BP due to higher sodium and water retention. 21 A meta‐analysis by Neter et al found that an average weight loss of 5.1 kg after a diet and/or exercise program reduced BP by 4.4/3.5 mmHg.30 In our study, individuals with a BMI ≥ 30 kg/m2 had systolic BP between 8 and 17 mmHg higher than those with normal BMI (ie, <25 kg/m2). This phenomenon occurred at all education levels (supplementary data). In general, weight loss greater than 5 kg significantly affects BP reduction. 30 Long‐term weight control is critically essential overall since evidence indicates that individuals who regain lost weight tend to experience increases in BP again. 31
Sodium and potassium kinetics are fundamental in the mechanisms related to HTN and should be the primary non‐pharmacological strategy for its management. 12 , 13 , 14 Studies in animals and humans have shown that potassium deficit induces renal vasoconstriction and increases the activity of the sodium‐chloride cotransporters leading to higher sodium retention and salt sensitivity. 13 , 32 , 33 , 34 Randomized studies have shown that potassium and sodium levels are both associated with effects on endothelial function: The flow‐mediated dilation of the brachial artery decreases with a high sodium intake (Na/K ratio > 1) and increases with high potassium intake (Na/K ratio < 1). 14 , 15 Hedayati et al reported in subjects from the Dallas Heart Study that for every 3 units of urinary Na/K ratio increase, BP rose 1.6/1 mmHg. 35 The current ACC/AHA and ESC/ESH guidelines emphasize the role of non‐pharmacological dietary interventions since they comprise the first step in the treatment of high BP. If the dietary reference intake (DRI) recommended by the Institute of Medicine is strictly applied, the optimal urinary Na/K ratio should be close to 0.3 (DRI for potassium = 4700 mg and sodium = 1500 mg). 20 Only 5% of the Chilean population meets a urinary Na/K ratio < 1, suggesting that a level of 0.3 is almost nonexistent in our population. 4 Indeed, the lowest tertile of urinary Na/K ratio in our study ranged from 0.14 to 2.35.
The weak correlation between urinary potassium and fruit and vegetable intake reaffirms that subjects’ perceptions and desirability might bias dietary data obtained from surveys. People tend to over‐report good behaviors and under‐report the bad. 36 For this reason, fruit and vegetable intake might not be a useful marker of BP unless it is obtained from a validated food frequency survey adjusted by caloric intake and corrected for bias, which is not the case for NHS 2009. Cornejo et al reported that sodium and potassium are underestimated by about 40% when estimated through 24‐hour recall dietary survey. Given that both are underestimated, the dietary sodium/potassium ratio was similar to the urinary ratio. 18 Therefore, urinary sodium and potassium adjusted by creatininuria are useful biomarkers of dietary intake for the clinical setting. In contrast, dietary Na/K estimation might be useful only if urinary samples are not available.
Several studies have reported that urinary Na/K ratio, sodium, and potassium excretion are all predictors of HTN and fatal and nonfatal CV events. 10 , 11 , 12 , 37 , 38 , 39 In our study, we found the same associations with HTN, although the effect of estimated salt intake disappeared when included in the same model with the estimated urinary Na/K ratio. The previous results might be explained by the collinearity of both variables when included in the same model. However, it also suggests that the estimated urinary Na/K ratio had a stronger association with BP levels than daily salt intake alone. The previous findings were somewhat anticipated because of renal pathophysiology, in which sodium closely associates with potassium intake. We also observed that the estimated urinary Na/K ratio remained associated with HTN only in the highest estimated salt intake tertile (≥10.4 grams/day) (data not shown). While the published data support that potassium intake has a more significant impact on HTN progression when sodium intake is high, we cannot explain why estimated urinary Na/K ratio was not associated with HTN in the moderate sodium intake tertile, which also had a high salt consumption (8.5‐10.3 grams of salt a day). 16 , 17 , 40 , 41 , 42 Further studies are warranted, considering other confounding variables.
A sedentary lifestyle was not associated with BP levels in our study. Similarly, Howard et al did not find an association between a sedentary lifestyle and HTN. Of note, however, another study from NHS 2009 data reported a 66% higher risk of HTN in physically inactive subjects. 43 Differences in designs of the two studies potentially explain this difference: We studied the association of several determinants with increasing BP levels (normal‐high BP and HTN 1 or 2) whereas Diaz‐Martinez and colleagues only evaluated the risk of prevalent HTN. 43 Finally, while it is well established that smoking is a CV risk factor and known to increase BP acutely, ours is not the first analysis to not demonstrate a relationship between smoking and HTN. 44 Although we did not measure the effect of smoke cessation, D’Elia et al reported that smoke cessation was associated with a lower risk of HTN compared to smokers. 45
Our study has limitations: It is a cross‐sectional study and, therefore, it can only determine associations and not causality. It is essential to compare these results with future prospective studies. Besides, we used an isolated urine sample for estimating sodium and potassium intake instead of 24‐hour urine excretion, which is the gold standard. However, according to validation studies, the equation of Tanaka et al used in this study is the least biased among the existent equations. As we stated previously, an excellent association exists between estimated Na/K urinary excretion calculated by an isolated spot urine sample with the 24‐hour urinary excretion in Chilean subjects (r = .87 for sodium and r = .86 for potassium, P < .01 for both). This study gives support to our results as a valid method when studying large population samples, as it is our NHS 2009.18 Besides, the MESA study demonstrated a significant relationship between urinary Na/K ratio and the incidence of stroke using a spot urine sample. 27 , 39 However, new equations with better associations with 24‐hour urine excretion are warranted. 46 , 47 Finally, we report a population in which we did not take out the individuals who were taking antihypertensive medication. However, we did adjust for this variable, and also, we did build a model excluding those subjects (Table 3). The results were very similar.
In conclusion, BMI, estimated urinary Na/K ratio, and alcohol intake are associated with increasing BP categories, but not education level nor estimated daily salt intake. This study highlights the importance of encouraging a healthy diet high in dietary potassium, low in daily salt intake, and weight loss in all the hypertensives and also the general population. If the governments do not curtail obesity, sedentary lifestyle, and diets that are high in caloric and processed food, the prevalence of HTN—one of the leading causes of death, heart failure, stroke, and kidney failure—is going to persist or increase in the years to come.
CONFLICT OF INTEREST
Professor Mónica Acevedo is currently participating in the new Hypertension Guidelines for the Chilean Ministry of Health. The other authors report no conflict of interest for this manuscript.
AUTHOR CONTRIBUTIONS
Giovanna Valentino, MSc and Mónica Acevedo, MD involved in conception and design of the work, collected and obtained the result, analyzed and interpreted the data, wrote and critically revised the manuscript, and approved the final version. Camila Hernández, MD involved in conception and design of the work, collected and obtained the result, analyzed and interpreted the data, critically revised the manuscript, and approved the final version. Rodrigo Tagle, MD analyzed and interpreted the data, critically revised the manuscript, provided technical advice and approved the final version. Lorena Orellana, RN, Fernando Baraona, MD, and Marcela Adasme, RN critically revised the manuscript, provided technical advice and approved the final version. Carlos Navarrete, PhD served as a Statistical advisor, collected and obtained the result, critically revised the manuscript, and approved the final version.
Supporting information
Fig S1
ACKNOWLEDGMENTS
This investigation used information from the Health Surveys for Epidemiological Surveillance of the Sub‐Secretary of Public Health. The authors thank the Chilean Ministry of Health for sharing the 2009 database. We thank Emily Donovan for editorial assistance.
Valentino G, Hernández C, Tagle R, et al. Urinary sodium‐to‐potassium ratio and body mass index in relation to high blood pressure in a national health survey in Chile. J Clin Hypertens. 2020;22:1041–1049. 10.1111/jch.13904
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e484‐e594. [DOI] [PubMed] [Google Scholar]
- 2. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Europ Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 3. Departamento de Epidemiología, Ministerio de Salud de Chile . Encuesta Nacional de Salud 2016‐2017: Segunda entrega de resultados. https://www.minsal.cl/wp-content/uploads/2018/01/2-Resultados-ENS_MINSAL_31_01_2018.pdf. Accessed December 29, 2018.
- 4. Departamento de Epidemiología, Ministerio de Salud de Chile . Encuesta Nacional de Salud ENS Chile 2009–2010. http://web.minsal.cl/portal/url/item/bcb03d7bc28b64dfe040010165012d23.pdf. Accessed December 29, 2018
- 5. Martínez‐González MA, Sánchez‐Tainta A, Corella D, et al. A provegetarian food pattern and reduction in total mortality in the Prevención con Dieta Mediterránea (PREDIMED) study. Am J Clin Nutr. 2014;100(Suppl 1):320S‐328S. [DOI] [PubMed] [Google Scholar]
- 6. Buil‐Cosiales P, Toledo E, Salas‐Salvadó J,. et al. Association between dietary fiber intake and fruit, vegetable or whole‐grain consumption and the risk of CVD: results from the PREvención con DIeta MEDiterránea (PREDIMED) trial. Br J Nutr. 2016;116:534‐546. [DOI] [PubMed] [Google Scholar]
- 7. Doménech M, Roman P, Lapetra J, et al. Mediterranean diet reduces 24‐hour ambulatory blood pressure, blood glucose, and lipids: one‐year randomized, clinical trial. Hypertension. 2014;64:69‐76. [DOI] [PubMed] [Google Scholar]
- 8. Miller V, Mente A, Dehghan M, et al. Prospective Urban Rural Epidemiology (PURE) study investigators. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390:2037‐2049. [DOI] [PubMed] [Google Scholar]
- 9. Diaz KM, Booth JN 3rd, Seals SR, et al. Physical activity and incident hypertension in African Americans: The Jackson Heart Study. Hypertension. 2017;69:421‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Intersalt Cooperative Research Group . Intersalt: an international study of electrolyte excretion and blood pressure: results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;297:319‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang Q, Liu T, Kuklina EV, et al. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1183‐1191. [DOI] [PubMed] [Google Scholar]
- 12. Castro H, Raij L. Potassium in hypertension and cardiovascular disease. Semin Nephrol. 2013;33:277‐289. [DOI] [PubMed] [Google Scholar]
- 13. Ellison DH, Terker AS. Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assoc. 2015;126:46‐55. [PMC free article] [PubMed] [Google Scholar]
- 14. Gijsbers L, Dower JI, Schalkwijk CG, et al. Effects of sodium and potassium supplementation on endothelial function: a fully controlled dietary intervention study. Br J Nutr. 2015;114:1419‐1426. [DOI] [PubMed] [Google Scholar]
- 15. Blanch N, Clifton PM, Petersen KS, Keogh JB. Effect of sodium and potassium supplementation on vascular and endothelial function: a randomized controlled trial. Am J Clin Nutr. 2015;101:939‐946. [DOI] [PubMed] [Google Scholar]
- 16. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117‐1124. [DOI] [PubMed] [Google Scholar]
- 17. Sacks FM, Svetkey LP, Vollmer WM,. et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001;344(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 18. Cornejo K, Pizarro F, Atalah E el al. Assessment of dietary intake and urinary excretion of sodium and potassium in adults. Rev Med Chile. 2014;142(6):687‐695. [DOI] [PubMed] [Google Scholar]
- 19. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Otten JJ, Pitzi Hellwig J, Meyers LD, eds. Dietary Reference Intakes (DRI). The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006: 370‐386. [Google Scholar]
- 21. Bray GA, Heisel WE, Afshin A, et al. The science of obesity management: an Endocrine Society scientific statement. Endocr Rev. 2018;39:79‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Havranek EP, Mujahid MS, Barr DA. Social determinants of risk and outcomes for cardiovascular disease. A scientific statement from the american heart association. Circulation. 2015;132:873‐898. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization . The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS). https://www.who.int/ncds/surveillance/steps/Part5.pdf. Accessed April 17, 2020
- 24. World Health Organization . Global Physical Activity Questionnaire (GPAQ) Analysis Guide. https://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf. Accessed December 15,2019.
- 25. Gandehari H, Kamal‐Bahl S, Wong ND. Prevalence and extent of dyslipidemia and recommended lipid levels in US adults with and without cardiovascular comorbidities: the National Health and Nutrition Examination Survey 2003–2004. Am Heart J. 2008;156:112‐119. [DOI] [PubMed] [Google Scholar]
- 26. Matsui S, Kajikawa M, Hida E, et al. Optimal target level of low‐density lipoprotein cholesterol for vascular function in statin naïve individuals. Sci Rep. 2017;7:8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16:97‐103. [DOI] [PubMed] [Google Scholar]
- 28. Eguchi E, Iso H, Honjo K, Yatsuya H, Tamakoshi A. No modifying effect of education level on the association between lifestyle behaviors and cardiovascular mortality: the Japan Collaborative Cohort Study. Sci Rep. 2017;7:39820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Howard G, Cushman M, Moy CS, et al. Association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA. 2018;320:1338‐1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta‐analysis of randomized controlled trials. Hypertension. 2003;42:878‐884. [DOI] [PubMed] [Google Scholar]
- 31. Stevens VJ, Obarzanek E, Cook NR, et al. Long‐term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Inter Med. 2001;134:1‐11. [DOI] [PubMed] [Google Scholar]
- 32. Terker AS, Zhang C, McCormick JA, et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21(1):39‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valdés G, Vio CP, Montero J, Avendaño R. Potassium supplementation lowers blood pressure and increases urinary kallikrein in essential hypertensives. J Hum Hypertens. 1991;5(2):91‐96. [PubMed] [Google Scholar]
- 34. Suga SI, Phillips MI, Ray PE, et al. Hypokalemia induces renal injury and alterations in vasoactive mediators that favor salt sensitivity. Am J Physiol Renal Physiol. 2001;281(4):F620‐F629. [DOI] [PubMed] [Google Scholar]
- 35. Hedayati SS, Minhajuddin AT, Ijaz A, et al. Association of urinary sodium/potassium ratio with blood pressure: sex and racial differences. Clin J Am Soc Nephrol. 2012;7:315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hebert JR, Hurley TG, Peterson KE, et al. Social desirability trait influences on self‐reported dietary measures among diverse participants in a multicenter multiple risk factor trial. The Journal of Nutrition. 2008;138(1):226S‐234S. [DOI] [PubMed] [Google Scholar]
- 37. Cook NR, Obarzanek E, Cutler JA,. et al. Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow‐up study. Arch Intern Med 2009;169(1):32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mirmiran P, Bahadoran Z, Nazeri P, Azizi F. Dietary sodium to potassium ratio and the incidence of hypertension and cardiovascular disease: a population‐based longitudinal study. Clin Exp Hypertens. 2018;40:772‐779. [DOI] [PubMed] [Google Scholar]
- 39. Averill MM, Young RL, Wood AC, et al. Spot urine sodium‐to‐potassium ratio is a predictor of stroke. Stroke. 2019;50(2):321‐327. 10.1161/STROKEAHA.118.023099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Appel LJ, Giles TD, Black HR, et al. ASH Position Paper: Dietary approaches to lower blood pressure. J Clin Hypertens (Greenwich). 2009;11:358‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Whelton PK, He J, Cutler JA, et al. Effects of oral potassium on blood pressure. Meta‐analysis of randomized controlled clinical trials. JAMA. 1997;277:1624‐1632. [DOI] [PubMed] [Google Scholar]
- 42. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296‐308. [DOI] [PubMed] [Google Scholar]
- 43. Díaz‐Martínez X, Petermann F, Leiva AM, et al. Association of physical inactivity with obesity, diabetes, hypertension and metabolic syndrome in the Chilean population. Rev Med Chil. 2018;146:585‐595. [DOI] [PubMed] [Google Scholar]
- 44. Primatesta P, Falaschetti E, Gupta S, Marmot MG, Poulter NR. Association between smoking and blood pressure: evidence from the health survey for England. Hypertension. 2001;37:187‐193. [DOI] [PubMed] [Google Scholar]
- 45. D’Elia L, De Palma D, Rossi G, et al. Not smoking is associated with lower risk of hypertension: results of the Olivetti Heart Study. Eur J Public Health. 2014;24(2):226‐230. [DOI] [PubMed] [Google Scholar]
- 46. Allen NB, Zhao L, Loria CM, et al. The validity of predictive equations to estimate 24‐hour sodium excretion: The MESA and CARDIA urinary sodium study. Am J Epidemiol. 2017;186(2):149‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wan ER, Cross J, Sofat R, Walsh SB. 24‐Hour vs. spot urinary sodium and potassium measurements in adult hypertensive patients: a cohort validation study. Am J Hypertens. 2019;32(10):983‐991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


