Abstract
Blood pressure (BP) measurements of pregnant women have been collected in offices and at home for previous research. However, it remains uncertain whether there is difference between research BP, defined as BP measured for the purpose of epidemiological research and BP measured at home or in an office. Therefore, the present study aimed to compare research BP with home and unstandardized office BP. Research, home, and office BP were measured among pregnant women who participated in the Tohoku Medical Megabank Project Birth and Three‐Generation Cohort Study (TMM BirThree Cohort Study). Research BP was measured twice at our research center while the participant was seated and after resting for 1‐2 minutes. Research, home, and office BP were compared and agreement among the values was assessed. Differences among research, home, and office BP values and possible factors affecting differences were analyzed. Among 656 pregnant women, the mean (± standard deviations) research systolic (S), diastolic (D) BP, home SBP, home DBP office SBP, and office DBP were 103.8 ± 8.5, 61.8 ± 7.3, 104.4 ± 9.2, 61.2 ± 6.8, 110.5 ± 10.8, and 63.8 ± 8.7mmHg, respectively. Research SBP value was lower than home value (P = .0072; difference between mean research and home BP: −0.61 ± 7.8 mmHg). Research SBP and DBP values were lower than office values (P < .0001 for both SBP and DBP; means ± standard deviations of differences between research and office BP: 6.7 ± 10.1 and 2.0 ± 8.5 mmHg for SBP and DBP, respectively). In conclusion, when research BP is measured under conditions controlled, research BP can give close values to home BP for pregnant women.
Keywords: blood pressure measurements, epidemiology, home blood pressure, office blood pressure, pregnancy
1. INTRODUCTION
Blood pressure (BP) measurement during pregnancy plays an important role because hypertensive disorders affect 5% to 10% of women during pregnancy. 1 Women who have hypertensive disorders of pregnancy (HDP) are at risk for intrauterine growth restriction, placental abruption, preterm, and cesarean birth. 1 , 2 Factors such as age, 3 body mass index (BMI), 4 parity, 5 family history of hypertension, 6 and smoking 7 affect BP during pregnancy. As BP is measured in offices (office BP) during routine prenatal checkups, office BP has often been used in research on pregnant women. However, previous studies have found that office BP is higher than home BP during pregnancy. 8 , 9 Home BP is more reproducible than office BP and it is applicable to monitoring BP during pregnancy, 10 , 11 , 12 whereas it has cumbersome aspect in daily measurements. BP should be monitored during pregnancy; therefore, it is worth investigating a measurement method which gives close BP value to home BP. BP value is sometimes included in measurement items of epidemiological research, and the BP is originally measured by the research. However, it remains uncertain whether BP defined as that measured for the purpose of epidemiological research (research BP) differs from office and home BP among pregnant women. The primary aim of the present study is to compare research BP values with home BP values and the secondary aim is to compare research BP with office BP among pregnant women.
2. METHOD
2.1. Study design
This cross‐sectional study is a part of the Tohoku Medical Megabank Project Birth and Three‐Generation Cohort Study (TMM BirThree Cohort Study), which recruited 22,493 pregnant women between July 19, 2013, and March 31, 2017, in Miyagi and Iwate prefectures, Japan. The Institutional Review Board at Tohoku University Graduate School of Medicine approved the study (May 27, 2013; Approval No: 2013‐1‐103‐1). Details of the TMM BirThree Cohort Study are described elsewhere. 13 , 14 As follow‐up is now ongoing, the present study implemented cross‐sectional analyses of baseline data.
2.2. Study population
Pregnant women who presented at obstetric clinics or hospitals provided written, informed consent to participate in the TMM BirThree Cohort Study. In our research centers, we also recruited pregnant women to the TMM BirThree Cohort Study, 13 and health assessment of the participants were performed once or twice during pregnancy. Exclusion criteria comprised >40 weeks of gestation, research, home, and office BP values not measured, office BP not measured within four weeks before to four weeks after from the day when research BP was measured, or a diagnosis of HDP. We did not impose any exclusion criteria regarding the number of BP measurements for each method. Data from 656 participants were analyzed in the present study.
2.3. Data collection
2.3.1. Research BP measurements
Research BP was measured in pregnant women before undergoing any other examinations at our research center. A trained nurse measured research BP twice using an HEM‐9000AI electronic upper arm‐cuff device (OMRON Corporation, Kyoto, Japan) 15 in seated participants after they had rested for 1 to 2 minutes. The means of BP values obtained from the pregnant women were analyzed.
2.3.2. Home BP measurement
Pregnant women measured home BP every morning for two weeks after presenting at our research center. Home BP was measured while the participant was seated, within one hour of waking and after 1 to 2 minutes of rest, before taking medicines and eating breakfast according to Japanese guidelines. 16 Our staff provided the participants with HEM‐7080IC electronic upper arm‐cuff devices (OMRON Corporation) 17 that measure BP based on cuff‐oscillometry and instructed them how to measure BP at home. The means of BP values measured for four weeks were analyzed.
2.3.3. Office BP measurements
Office BP was measured in pregnant women who presented at any of 39 participating hospitals or clinics in Miyagi prefecture. Some participants measured their own BP using automated devices in hospitals or clinics, whereas medical staff measured BP in others using an electrical device. We analyzed BP measured at hospitals or clinics within four weeks before to four weeks after from the day when research BP was measured. If BP was measured at hospitals or clinics more than twice in this period, the mean of all BP values was used.
2.3.4. Basic characteristics and outcomes
We collected information about smoking habits and pre‐pregnancy BMI from a self‐administered questionnaire. Information about age, expected date of delivery, and history of pregnancy and delivery was determined from medical records obtained at the time of registration in the TMM BirThree Cohort Study. A diagnosis of HDP was obtained from medical records at the time of delivery. Office BP data and gestational weeks at the time office BP was measured were collected from medical records.
2.4. Statistical analysis
Characteristics of the participants are shown as means ± standard deviation (SD), or as numbers (n) and ratios (%). We evaluated differences and similarities among research, home, and office BP as follows. We assessed the means of research, home, and office BP using Pearson correlation coefficients and Student's t tests and then analyzed agreement among combinations of the three types of BP measurements using Bland‐Altman plots. We also reconfirmed consistency using Bland‐Altman plots of geometric means and ratios (Supplemental, Figure S1). We then analyzed “differences” among research, home, and office BP values as follows. We defined the research‐home BP difference as research BP minus home BP, office‐research BP difference as office BP minus research BP, and office‐home BP difference as office BP minus home BP and determined Pearson correlation coefficients for office‐research vs. research‐home BP difference, office‐home vs. research‐home BP difference and office‐research vs. office‐home BP difference. Possible factors such as age, 18 , 19 , 20 parity, 5 , 9 pre‐pregnancy BMI, 4 pregnancy trimester, 21 and smoking habit 20 , 22 were assessed using multivariate analyses of covariance (ANCOVA). These factors were also used for adjustment in these analyses. Performing ANCOVA, we set cutoff as previous study to age and pre‐pregnancy BMI according to previous study. 5
All data were statistically analyzed using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina, USA) and Python Language Reference, version 3.6.2 (Python Software Foundation at http://www.python.org). Statistical significance was defined as P < .05.
3. RESULTS
3.1. Characteristics of participants
Table 1 shows that the mean age of the 656 participants was 32.2 ± 4.9 years and the mean gestational weeks at the time of research BP measurement was 14.5 ± 4.4 weeks. The mean research systolic (S) and diastolic (D) BP values were 103.8 ± 8.5 and 61.8 ± 7.3 mmHg, respectively. The average number of home BP measurements was 11.6 ± 3.0 and the mean home SBP and DBP values were 104.4 ± 9.2 and 61.2 ± 6.8 mmHg, respectively. The average number of office BP measurements was 1.9 ± 0.8, mean time interval between office and research BP measurements was 0.50 ± 2.2 weeks and mean office SBP and DBP values were 110.5 ± 10.8 and 63.8 ± 8.7 mmHg, respectively. The mean research‐home, office‐research, and office‐home SBP and DBP differences were −0.61 ± 7.8 and 0.58 ± 6.4, 6.7 ± 10.1 and 2.0 ± 8.5, and 6.2 ± 11.0 and 2.6 ± 8.7 mmHg, respectively. The rates of participants with positive research‐home, office‐research, and office‐home SBP and DBP differences were 49.4% and 52.7%, 74.2% and 61.9%, and 69.7% and 64.3%, respectively. The number of participants whose mean of research and home SBP was above 140 mmHg and that of DBP was above 90 mmHg was 0 and 2, respectively. The number of participants whose mean of research and office SBP was above 140 mmHg and that of DBP was above 90 mmHg was 1 and 1, respectively. The number of participants whose mean of office and home BP was above 140 mmHg and that of DBP was above 90 mmHg was 1 and 0, respectively.
Table 1.
Characteristics of the participants
| Variable | (N = 656) |
|---|---|
| Age (years) | 32.2 ± 4.9 |
| Gestational week at measurement (weeks) | 14.5 ± 4.4 |
| First trimester (5‐15 weeks) | 357 (57.2) |
| Second trimester (16‐27 weeks) | 281 (42.8) |
| Nulliparous | 267 (40.1) |
| Height (before pregnancy) (cm) | 158.5 ± 5.3 |
| Weight (before pregnancy) (kg) | 54.0 ± 9.1 |
| Body mass index (before pregnancy) (kg/m2) | 21.5 ± 3.3 |
| Smoking during pregnancy | 72 (11.0) |
| Research BP | |
| Number of measurements | 2.0 ± 0.0 |
| Systolic BP (mmHg) | 103.8 ± 8.5 |
| Diastolic BP (mmHg) | 61.8 ± 7.3 |
| Home BP | |
| Number of measurements | 11.6 ± 3.0 |
| Systolic BP (mmHg) | 104.4 ± 9.2 |
| Diastolic BP (mmHg) | 61.2 ± 6.8 |
| Office BP | |
| Number of measurements | 1.9 ± 0.8 |
| Interval between research BP measurement (weeks) | 0.50 ± 2.2 |
| Systolic BP (mmHg) | 110.5 ± 10.8 |
| Diastolic BP (mmHg) | 63.8 ± 8.7 |
| Research‐home BP difference | |
| Systolic BP (mmHg) | ‐0.61 ± 7.8 |
| Diastolic BP (mmHg) | 0.58 ± 6.4 |
| Office‐research BP difference | |
| Systolic BP (mmHg) | 6.7 ± 10.1 |
| Diastolic BP (mmHg) | 2.0 ± 8.5 |
| Office‐home BP difference | |
| Systolic BP (mmHg) | 6.2 ± 11.0 |
| Diastolic BP (mmHg) | 2.6 ± 8.7 |
Results are shown as means ± standard deviation or as n (%).BP, blood pressure.
3.2. Associations among research, home, and office BP
3.2.1. Research vs. home BP
Research BP was correlated with home SBP and DBP (r = 0.61 and 0.58 for SBP and DBP, respectively) (Figure 1A,B). We found that research SBP value was lower than home SBP (P = .046) (Figure 1G) and research DBP value was higher than home DBP (P = .023) (Figure 1H).
Figure 1.
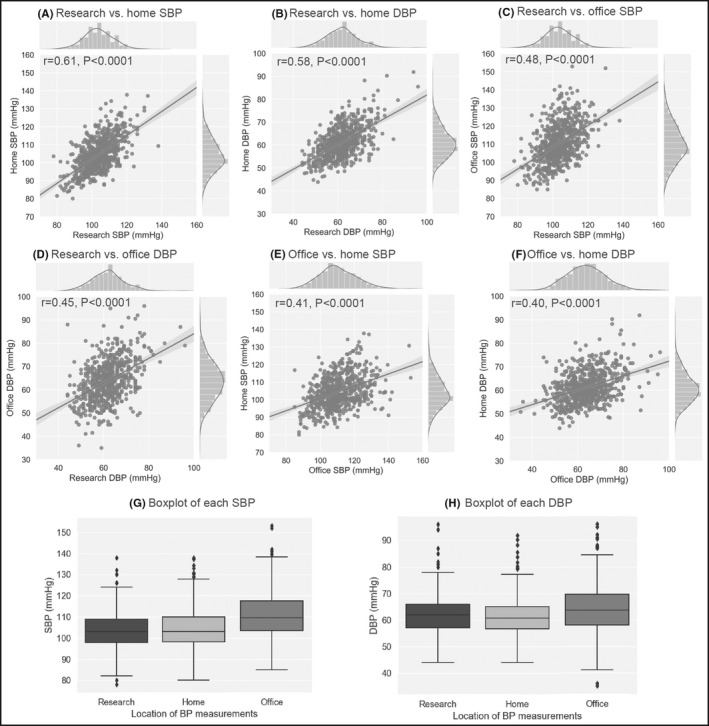
Scatter plots, histograms and regression lines of all BP combinations. (A) research vs. home SBP, (B) research vs. home DBP, (C) research vs. office SBP, (D) research vs. office DBP, (E) office vs. home SBP, and (F) office vs. home DBP. Lines in histograms represent kernel density estimation for BP distribution and gray area represents standard error (r, Pearson correlation coefficient; P, p‐value). Boxplots show SBP (G) and DBP (H). DBP, diastolic blood pressure; SBP, systolic blood pressure
3.2.2. Research vs. office BP
Research BP was correlated with office BP (r = 0.48 and 0.45 for SBP and DBP, respectively) (Figure 1C,D). Both SBP and DBP values were lower for research than office values (P < .0001 for both SBP and DBP) (Figure 1G,H).
3.2.3. Office vs. home BP
Office BP was correlated with home BP (r = 0.41 and 0.40 for SBP and DBP, respectively) (Figure 1E,F). Both SBP and DBP values were higher for office than home values (P < .0001 for both SBP and DBP) (Figure 1G,H).
3.3. Agreement determined using Bland‐Altman plots
3.3.1. Research vs. home BP
The average mean of research and home SBP and that of DBP were 104.1 ± 8.0 and 61.5 ± 6.3 mmHg, respectively, and the coefficients between the means and the differences for SBP and DBP were −0.10 (P = .0079) and 0.095, (P = .015), respectively (Figure 2A,B).
Figure 2.
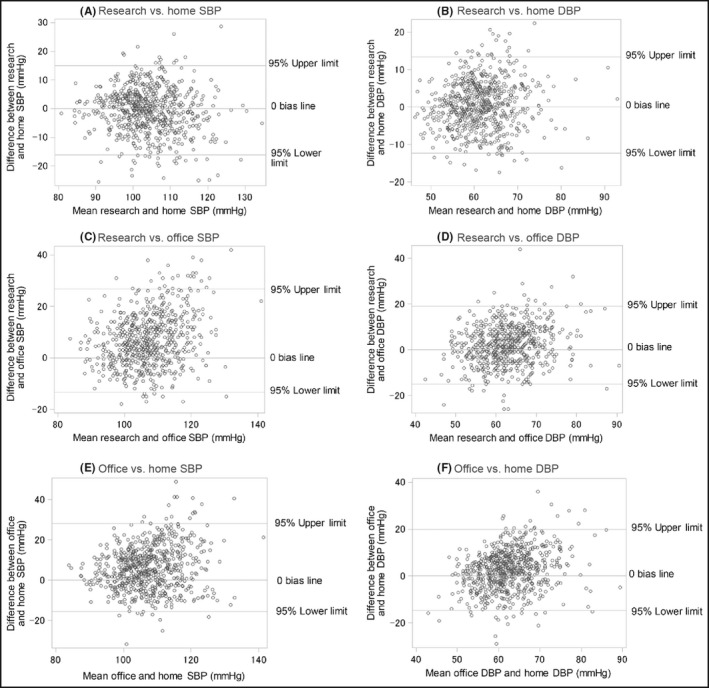
Bland‐Altman plots. (A) research vs. home SBP, (B) research vs. home DBP, (C) research vs. office SBP, (D) research vs. office DBP, (E) office vs. home SBP, and (F) office vs. home DBP. Horizontal axes, means of paired BP; vertical axes, difference in BP (r, Pearson correlation coefficient; P, p‐value). DBP, diastolic blood pressure; SBP, systolic blood pressure
3.3.2. Research vs. office BP
The average mean of research and office SBP and that of DBP were 107.1 ± 8.3 and 62.8 ± 6.8 mmHg, respectively, and the coefficients between the means and the differences for SBP and DBP were 0.26 and 0.19, respectively (P < .0001 for both SBP and DBP) (Figure 2C,D).
3.3.3. Office vs. home BP
The averaged mean of office and home BP and that of DBP were 107.0 ± 8.5 and 62.0 ± 6.4 mmHg, respectively, and the coefficient between the means and differences for SBP and DBP were 0.17 and 0.27, respectively (P < .0001 for both SBP and DBP) (Figure 2E,F).
3.4. Associations between BP difference combinations
Office‐research and research‐home BP differences were negatively correlated (r=−0.27 and −0.36 for SBP and DBP, respectively) (Figure 3A,B). Office‐research and office‐home BP differences were correlated (r = 0.73 and 0.72 for SBP and DBP, respectively) (Figure 3C,D). Office‐research and research‐home BP differences were correlated (r = 0.47 and 0.39 for SBP and DBP, respectively) (Figure 3E,F).
Figure 3.
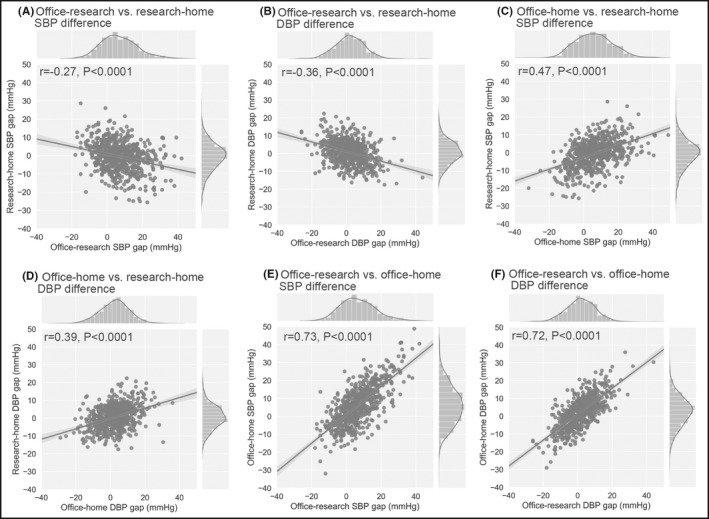
Scatter plots, histograms and regression lines of BP differences. (A) office‐research vs. research‐home SBP difference, (B) office‐research vs. research‐home DBP difference, (C) office‐research vs. office‐home SBP difference, (D) office‐research vs. office‐home DBP difference, (E) office‐home vs. research‐home SBP difference, and (F) office‐home vs. research‐home DBP difference. Lines in histograms represent estimated kernel density for BP difference distribution. Gray area represents standard error (r, Pearson correlation coefficient; P, P‐value). DBP, diastolic blood pressure; SBP, systolic blood pressure
3.5. Possible factors affecting BP differences
Table 2 shows the possible factors affecting BP differences. Age was positively associated with office‐research SBP difference and pre‐pregnancy BMI was positively associated with office‐research DBP difference. Smoking habit was negatively associated with office‐research and office‐home SBP and DBP differences.
Table 2.
Multivariate analysis of covariance among difference combinations
| BP difference (mmHg) | Systolic BP | Diastolic BP | ||||
|---|---|---|---|---|---|---|
| Age < 30 y | Age ≥ 30 y | P | Age < 30 y | Age ≥ 30 y | P | |
| Research‐home | ‐0.15 (0.55) | ‐0.82 (0.37) | .32 | 1.2 (0.45) | 0.31 (0.30) | .11 |
| Office‐research | 5.4 (0.71) | 7.4 (0.47) | .0021 | 1.2 (0.60) | 2.4 (0.40) | .11 |
| Office‐home | 5.2 (0.77) | 6.6 (0.51) | .16 | 2.4 (0.61) | 2.7 (0.41) | .69 |
| Nulliparous | Multiparous | Nulliparous | Multiparous | |||
| Research‐home | ‐0.79 (0.48) | ‐0.49 (0.40) | .64 | ‐0.79 (0.48) | ‐0.49 (0.40) | .64 |
| Office‐research | 7.5 (0.61) | 6.2 (0.51) | .11 | 2.0 (0.54) | 2.0 (0.43) | .94 |
| Office‐home | 6.7 (0.67) | 5.8 (0.55) | .26 | 2.5 (0.53) | 2.7 (0.44) | .77 |
| BMI < 22 | BMI ≥ 22 | BMI < 22 | BMI ≥ 22 | |||
| Research‐home | ‐0.30 (0.38) | ‐1.2 (0.52) | .16 | 0.23 (0.31) | 1.2 (0.43) | .064 |
| Office‐research | 6.7 (0.48) | 6.9 (0.67) | .83 | 2.5 (0.41) | 1.0 (0.56) | .034 |
| Office‐home | 6.4 (0.52) | 5.7 (0.72) | .42 | 2.8 (0.41) | 2.3 (0.58) | .48 |
| Non‐smoker | Smoker | Non‐smoker | Smoker | |||
| Research‐home | ‐0.61 (0.32) | ‐0.62 (0.93) | .99 | 0.57 (0.27) | 0.59 (0.77) | .98 |
| Office‐research | 7.1 (0.41) | 4.1 (1.2) | .020 | 2.3 (0.35) | ‐0.31 (1.0) | .016 |
| Office‐home | 6.5 (0.45) | 3.5 (1.3) | .032 | 2.9 (0.36) | 0.28 (1.0) | .019 |
| First trimester | Second trimester | First trimester | Second trimester | |||
| Research‐home | ‐0.51 (0.56) | ‐0.75 (0.70) | .83 | 0.52 (0.47) | 0.65 (0.58) | .89 |
| Office‐research | 6.5 (0.72) | 7.2 (0.90) | .63 | 2.3 (1.1) | 1.5 (0.038) | .51 |
| Office‐home | 6.0 (0.79) | 6.4 (0.98) | .77 | 2.9 (0.63) | 2.2 (0.78) | .58 |
Data are shown as means (standard error). First and second trimesters, 5 −15 and 16 ‐ 27 weeks, respectively.
Abbreviations: BMI, body mass index (kg/m2); BP, blood pressure; P, probability.
4. DISCUSSION
We found that research BP (if measured under conditions controlled as described herein) could give close values to home BP for pregnant women. The results of the present study revealed that office SBP and DBP were higher than home SBP and DBP (by 6 and 2 mmHg, respectively), which reconfirmed the white‐coat effect (WCE) among pregnant women. 8 , 12 , 23 Ishikuro et al 9 reported that home BP was 4 to 5 mmHg lower than office BP among Japanese pregnant women. The WCE manifests in 15% to 20% of the general population, 24 and in 30% of pregnant women. 25 , 26 Office BP can be measured easily and inexpensively, but it is affected by WCE. 27 Unattended office BP measurements have been studied in an effort to improve the accuracy of office BP. 28 However, Asayama et al 29 reported that unattended office BP was not a suitable alternative to home BP in a Japanese population. Evidence supporting the prognostic value of unattended office BP is limited 11 and its characteristics such as clinic effects 29 should be studied. Compared with office BP, home BP provides more reproducible data, 12 avoids the WCE and is more closely associated with hypertension‐mediated organ damage in the general population. 11 Iwama et al 23 found that a high maternal home BP before 20 weeks of gestation is associated with a higher risk of lower infant birth weight than office BP. However, home BP measurement has cumbersome aspect because participants must learn how to measure it for specific periods and measure BP repeatedly by themselves. 27 Tucker et al performed meta‐analysis and showed that no evidence of a systematic difference between home and office BP values but this may be attributed to the low BP values. 30 The effect of BP value in home BP may influence on our results. Home BP has merits, but an alternative measurement method with less burden and limited WCE should be identified. Myers et al 31 noted that BP tended to be lower when measured by research staff than family physician. We measured research BP in pregnant women for epidemiological investigations based on Japanese guidelines. 16 Under these conditions, research BP might be measured with limited WCE because the mean research and home BP values were similar in the present study. Previous findings have shown elevated sympathetic tone in patients with white‐coat hypertension. 32 An overactive sympathetic nervous system might be suppressed during research BP measurements to a level similar to that of home BP. Of course, there is a little feasibility for pregnant women to measure research BP out of office routinely; however, we can discuss relations between clinical manifestations and research BP in the sense of approximation to home BP. Women who have poorly controlled chronic hypertension are advised to use home blood pressure monitoring for pregnancy. 1 Research BP can be used to discuss factors related with HDP in the research context as approximation to home BP and this discussion may lead to reveal properties of hypertension‐related disorder during pregnancy. The practical use of home BP and improvements in office BP accuracy remain issues.
Bland‐Altman plots indicated no agreement among BP measurements in the sense that there was no proportional bias. Correlation coefficients in Bland‐Altman plots between research and office BP and office and home BP were relatively high among our results. This might reflect inadequate estimates generated by office BP 10 , 33 because participants with high mean BP tended to have wide differences. Correlation coefficients in Bland‐Altman plots between research and home SBP were negative. This result indicates that the difference between research and home SBP become smaller when mean of these become higher. This may indicate that among participants with high BP have close research BP to home BP. Since the geometric means and ratios confirmed the same results, they might provide a clue to understanding the features of research and home BP.
We analyzed associations among office‐home, office‐research, and research‐home BP differences. Associations were positive except for the association between office‐research and research‐home differences. This might mean that home and research BP values are similar. Office‐research and research‐home differences were negatively associated, whereas office‐home and research‐home gaps were positively associated. These results might reflect that about 50% of our participants had higher research BP than home BP, but the difference between the values was small. Further study is required to clarify the similarities and differences between research and home BP.
Multivariate ANCOVA revealed that BP differences are positively associated with age, pre‐pregnancy BMI, and negatively associated with smoking habit. Aging reportedly increases the WCE in general population. 19 White‐coat hypertension has been identified in 1.1% and 2.2% of women aged 18‐30 and 30‐40 years, respectively. 19 Rates of arteriosclerotic blood vessels increase with age 34 and BP is apt to be influenced by arteriosclerosis in blood vessels. However, whether such changes occur in younger population (age ± SD: 32.2 ± 4.9 years) discussed in the present study remains unknown. Some studies discussed that changes in metabolic and vascular systems induced by pregnancy 35 might unmask extant subclinical risks, which could manifest in later life as effects of aging. 36 This might reflect extant factors that accumulate not only with advancing age, but also during pregnancy. Obesity might be associated with BP 4 , 37 because abnormal renal sodium and water reabsorption and impaired pressure natriuresis play major roles in obesity‐related hypertension. 38 In the present study, pre‐pregnancy BMI was negatively associated with the office‐research DBP difference. However, it remains unclear whether BMI is associated with WCE. 39 Cigarette smoking reportedly decreases the WCE among adults. 20 , 22 Nicotine reduces anxiety through central nervous system effects; thus, the psychophysiological effects of nicotine might explain the limited WCE on smokers and the reduced BP measured in an office environment. 20 , 22 Another explanation might be that office BP tends to be measured when nicotine levels are low, whereas home BP can be measured under a patient's normal level of nicotine, which elevates BP. 40 In this study, smoking habit was associated with office‐research and office‐home SBP and DBP differences. Some participants in the present study might have smoked before research BP was measured. The present results indicate that the similarity between research and home BP values is due to the outcomes of both mechanisms.
This cross‐sectional study has some limitations. First, we cannot discuss time‐course of BP in this research. The BP of pregnant women changes according to gestational week 9 partly because the endocrine system influences BP and total peripheral vascular resistance also changes according to gestational week. 41 The mean gestational weeks of research BP measurement in the present study was 14.5 ± 4.4 weeks and physiological changes between the first and second trimester of pregnancy might affect results. Previous study also reported change of BP from first trimester to second trimester had various patterns, 42 and this could affect differences of BPs. However, mean interval between office and research BP measurements in the present study was 0.50 ± 2.2 weeks. The effect of interval might be limited. As another possibility, order effect might influence on our results. There were some patterns of orders among research, home, and office BP measurements because we analyzed all office and home BP measurements performed in defined period. Office BPs were measured before or after measurement of research BPs depending on participant's schedule of medical checkup. Home BPs were measured after research BPs as well as home BP depending on participant’s schedule of medical checkup. Home BPs were measured after research BPs. Further study is required to determine the time‐course of research BP and interrelations with office and home BP including timing of measurements during pregnancy. Second, measurement methods for research, home, and office BP had variation. BPs were measured according to Japanese guideline 16 and this guideline states that the difference between the BPs of the left and right arms should be confirmed at the first measurement. We measured BPs in the three contexts, research, home, and office. In each context, the same arm was always used to measure BPs. The arm used to measure research, home, and office BPs was not standardized. For research BP measurement, we recommended that participants come to our center in a fasting state but we did not insist on it to maintain the usual condition of pregnancy. Research BP might be affected by stimulants such as smoking, coffee, or behavioral factors, though pregnant women are usually recommended not to take coffee or smoke by medical staff in Japan. For office BP measurement, some participants measured their own BP at clinic or hospitals, others were measured by medical staff. We could not distinguish BPs measured by medical staff from BPs measured by participants themselves. Each clinic or hospital used the BP monitor adopted by each facility, and the BP monitors used to measure office BP were not standardized. We could not collect information on these devices. In office BP measurement, the time of measurement was not standardized and office BP might be affected by stimulants as research BP. These might result in variation of office BP. It is still unclear whether standardized office BP gives the same results reported in this study. However, all these situations can occur in research and clinical practice. The present results showed that the research BP value was close to the home BP value even if BPs were measured under non‐standardized conditions, with only research BP measured in a controlled situation according to measurement method of guideline. 16 Third, there might be selection bias because participants in the TMM BirThree Cohort Study participated voluntarily in the study and provided us with their data. One possibility is that participants were healthier because people who volunteer to participate in a study may be more health conscious than those who do not volunteer. On the other hand, another possibility is that people who volunteer to participate in a study may be at risk of some disease. The study population did not include women with high BP and this might result in similar BP level. However, selection bias might be limited because, in The TMM BirThree Cohort Study, about half of the pregnant women in Miyagi Prefecture were recruited, 13 and the characteristics of pregnant women were similar to vital statistics in Japan, 43 for example, the mean age of participants was 32.2 ± 4.9 years in this study and in the Japanese vital statistics, the mean maternal age at the time of birth of the first, second, and third child was born is 30.7, 32.6, and 33.6 years, respectively. Nevertheless, research BP was measured for participants who volunteer to visit our centers and research BP might be affected by selection bias above mentioned. The effect of selection bias should be evaluated in another study. In conclusion, we found that research BP was lower than office and home SBP and that the difference between research BP and home BP among pregnant women was small. Differences among research, home, and office BPs partly can be attributed to measurement situations, however, these differences may reflect variations in each measurement method.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Takuma Usuzaki, Mami Ishikuro, Taku Obara and Shinichi Kuriyama designed the study; Takuma Usuzaki conducted the systematic literature review, analyzed and interpreted the data, and wrote the manuscript; Mami Ishikuro, Hirohito Metoki, Keiko Murakami, Aoi Noda, Fumihiko Ueno, Masahiro Kikuya, Taku Obara, and Shinichi Kuriyama interpreted the data and critically reviewed the manuscript.
Supporting information
Figure S1.
ACKNOWLEDGEMENTS
We appreciate for participants to the TMM BirThree Cohort Study.
Usuzaki T, Ishikuro M, Metoki H, et al. Comparison among research, home, and office blood pressure measurements for pregnant women: The TMM BirThree Cohort Study. J Clin Hypertens. 2020;22:2004–2013. 10.1111/jch.14050
Funding information
This work was supported by the Japan Agency for Medical Research and Development (AMED), Japan [grant number, JP20km0105001].
REFERENCES
- 1. Folk D.M.. Hypertensive disorders of pregnancy: overview and current recommendations. J Midwifery Womens Health. 2018;63(3):289‐300. [DOI] [PubMed] [Google Scholar]
- 2. Seely E.W., Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254‐1261. [DOI] [PubMed] [Google Scholar]
- 3. Khalil A., Syngelaki A., Maiz N., Zinevich Y., Nicolaides K.H.. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol. 2013;42(6):634‐643. [DOI] [PubMed] [Google Scholar]
- 4. Thadhani R., Stampfer M.J., Hunter D.J., Manson J.E., Solomon C.G., Curhan G.C.. High body mass index and hypercholesterolemia: risk of hypertensive disorders of pregnancy. Obstet Gynecol. 1999;94(4):543‐550. [DOI] [PubMed] [Google Scholar]
- 5. Ishikuro M., Obara T., Metoki H., et al. Parity as a factor affecting the white‐coat effect in pregnant women: the BOSHI study. Hypertens Res. 2015;38(11):770‐775. [DOI] [PubMed] [Google Scholar]
- 6. Ness R.B., Markovic N., Bass D., Harger G., Roberts J.M.. Family history of hypertension, heart disease, and stroke among women who develop hypertension in pregnancy. Obstet Gynecol. 2003;102(6):1366‐1371. [DOI] [PubMed] [Google Scholar]
- 7. England L.J., Levine R.J., Qian C., et al. Smoking before pregnancy and risk of gestational hypertension and preeclampsia. Am J Obstet Gynecol. 2002;186(5):1035‐1040. [DOI] [PubMed] [Google Scholar]
- 8. Ishikuro M., Obara T., Metoki H., et al. Differences between clinic and home blood pressure measurements during pregnancy. J Hypertens. 2015;33(7):1492‐1493. [DOI] [PubMed] [Google Scholar]
- 9. Ishikuro M., Obara T., Metoki H., et al. Blood pressure measured in the clinic and at home during pregnancy among nulliparous and multiparous women: the BOSHI study. Am J Hypertens. 2013;26(1):141‐148. [DOI] [PubMed] [Google Scholar]
- 10. Pickering T.G., Miller N.H., Ogedegbe G., et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 11. Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 12. Imai Y., Obara T., Asamaya K., Ohkubo T. The reason why home blood pressure measurements are preferred over clinic or ambulatory blood pressure in Japan. Hypertens Res. 2013;36(8):661‐672. [DOI] [PubMed] [Google Scholar]
- 13. Kuriyama S., Metoki H., Kikuya M., et al. Cohort Profile: Tohoku Medical Megabank Project Birth and Three‐Generation Cohort Study (TMM BirThree Cohort Study): Rationale, Progress and Perspective. Int J Epidemiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kuriyama S., Yaegashi N., Nagami F., et al. The Tohoku Medical Megabank Project: Design and Mission. J Epidemiol. 2016;26(9):493‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schillaci G., Mannarino E., Cavallini C., Notaristefano S., Battista F., Pucci G.. P2.08 central‐to‐peripheral blood pressure amplification: invasive validation of two devices (Sphygmocor and Omron Hem9000ai). Artery Research. 2012;6(4):165. [Google Scholar]
- 16. Shimamoto K., Ando K., Fujita T., et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37(4):253‐390. [DOI] [PubMed] [Google Scholar]
- 17. Brown M.A., Roberts L., Davis G., Mangos G.. Can we use the Omron T9P automated blood pressure monitor in pregnancy? Hypertens Pregnancy. 2011;30(2):188‐193. [DOI] [PubMed] [Google Scholar]
- 18. Horikawa T., Obara T., Ohkubo T., et al. Difference between home and office blood pressures among treated hypertensive patients from the Japan home versus office blood pressure measurement evaluation (J‐HOME) study. Hypertens Res. 2008;31(6):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 19. Conen D., Aeschbacher S., Thijs L., et al. Age‐specific differences between conventional and ambulatory daytime blood pressure values. Hypertension. 2014;64(5):1073‐1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hozawa A., Ohkubo T., Nagai K., et al. Factors affecting the difference between screening and home blood pressure measurements: the Ohasama Study. J Hypertens. 2001;19(1):13‐19. [DOI] [PubMed] [Google Scholar]
- 21. Ishikuro M., Obara T., Metoki H., et al. Blood pressure changes during pregnancy. Hypertens Res. 2012;35(5):563‐564. author reply 534. [DOI] [PubMed] [Google Scholar]
- 22. Manios E.D., Koroboki E.A., Tsivgoulis G.K., et al. Factors influencing white‐coat effect. Am J Hypertens. 2008;21(2):153‐158. [DOI] [PubMed] [Google Scholar]
- 23. Iwama N., Metoki H., Ohkubo T., et al. Maternal clinic and home blood pressure measurements during pregnancy and infant birth weight: the BOSHI study. Hypertens Res. 2016;39(3):151‐157. [DOI] [PubMed] [Google Scholar]
- 24. Asayama K., Thijs L., Li Y., et al. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white‐coat and masked hypertension in the population. Hypertension. 2014;64(5):935‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellomo G., Narducci P.L., Rondoni F., et al. Prognostic value of 24‐hour blood pressure in pregnancy. JAMA. 1999;282(15):1447‐1452. [DOI] [PubMed] [Google Scholar]
- 26. Izzo J.L., Sica D.A., Black H.R.. Hypertension primer : the essentials of high blood pressure : basic science, population science, and clinical management. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 27. Pickering T.G., White W.B., Giles T.D., et al. When and how to use self (home) and ambulatory blood pressure monitoring. J Am Soc Hypertens. 2010;4(2):56‐61. [DOI] [PubMed] [Google Scholar]
- 28. Myers M.G.. Automated office blood pressure‐incorporating SPRINT into clinical practice. Am J Hypertens. 2017;30(1):8‐11. [DOI] [PubMed] [Google Scholar]
- 29. Asayama K., Ohkubo T., Rakugi H., et al. Comparison of blood pressure values‐self‐measured at home, measured at an unattended office, and measured at a conventional attended office. Hypertens Res. 2019;42(11):1726‐1737. [DOI] [PubMed] [Google Scholar]
- 30. Tucker K.L., Sheppard J.P., Stevens R., et al. Self‐monitoring of blood pressure in hypertension: a systematic review and individual patient data meta‐analysis. PLoS Medicine. 2017;14(9):e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Myers M.G.. A short history of automated office blood pressure ‐ 15 Years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palatini P., Julius S.. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep. 2009;11(3):199‐205. [DOI] [PubMed] [Google Scholar]
- 33. Stergiou G.S., Asayama K., Thijs L., et al. Prognosis of white‐coat and masked hypertension: International database of home blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63(4):675‐682. [DOI] [PubMed] [Google Scholar]
- 34. Casalnuovo G., Gerdts E., de Simone G., et al. Arterial stiffness is associated with carotid atherosclerosis in hypertensive patients (the Campania Salute Network). Am J Hypertens. 2012;25(7):739‐745. [DOI] [PubMed] [Google Scholar]
- 35. Williams D.. Pregnancy: a stress test for life. Curr Opin Obstet Gynecol. 2003;15(6):465‐471. [DOI] [PubMed] [Google Scholar]
- 36. Sattar N., Greer I.A.. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Brien T.E., Ray J.G., Chan W.S.. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368‐374. [DOI] [PubMed] [Google Scholar]
- 38. Hall J.E.. Mechanisms of abnormal renal sodium handling in obesity hypertension. Am J Hypertens. 1997;10(5 Pt 2):49S‐55S. [PubMed] [Google Scholar]
- 39. Ben‐Dov I.Z., Mekler J., Ben‐Arie L., Bursztyn M. Lack of association between body‐mass index and white‐coat hypertension among referred patients. Blood Press Monit. 2007;12(2):95‐99. [DOI] [PubMed] [Google Scholar]
- 40. Papathanasiou G., Zerva E., Zacharis I., et al. Association of high blood pressure with body mass index, smoking and physical activity in healthy young adults. Open Cardiovasc Med J. 2015;9:5‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calina D., Docea A.O., Golokhvast K.S., Sifakis S., Tsatsakis A., Makrigiannakis A. Management of endocrinopathies in pregnancy: a review of current evidence. Int J Environ Res Public Health. 2019;16(5):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salles G.F., Schlussel M.M., Farias D.R., et al. Blood pressure in healthy pregnancy and factors associated with no mid‐trimester blood pressure drop: a prospective cohort study. Am J Hypertens. 2015;28(5):680‐689. [DOI] [PubMed] [Google Scholar]
- 43. Ministry of Health, Labour and Welfare, Vital Statistics Japan . https://www.mhlw.go.jp/english/database/db‐tm/index.html. Accessed June, 6, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.


