Abstract
Hypertension is an important cause of cerebral small vessel disease, especially of white matter hyperintensity (WMH). The ability of intensive blood pressure (BP) control in preventing this pathological progression remains unclear. The authors systematically searched PubMed, EMBASE, SCOPUS, and Cochrane library for publications until July 20, 2020. Studies included were clinical trials with random allocation to an antihypertensive medication against placebo, or different treatment targets. The primary outcome was intergroup differences in the change of WMH volume. A random‐effect model was applied for pooling effect measures. Subgroup analysis and meta‐regression were conducted to explore heterogeneity. Seven studies with 2693 patients were identified. Compared with the control group, patients in the intensive BP control group had a slower progression of WMH, with a pooled intergroup standard mean difference (SMD) for WMH change of −0.22 (95% CI: −0.35 ~ −0.09, I2 = 63%). For studies comparing intensive and standard BP target, the pooled SMD is −0.37 (95% CI:‐0.50~‐0.24, I2 = 0%), while the pooled SMD of studies comparing active antihypertensive medication and placebo was only −0.08 (95% CI: −0.17 ~ 0.01, I2 = 0%). Meta‐regression analysis showed that the reduction in WMH progression is proportional to the magnitude of intensive BP control (β = −0.028, P < .001). In conclusion, intensive BP control prevents WMH progression, and its effect is associated with the magnitude of intensive BP control.
Keywords: hypertension, intensive blood pressure control, white matter hyperintensity
1. INTRODUCTION
Cerebral small vessel disease (CSVD) is very common among human populations, especially among the elderly and those with vascular risk factors. CSVD is an important cause of dementia and stroke 1 ; it is often ignored in clinical practice due to its insidious process of progression. White matter hyperintensity (WMH) is one of the most important MRI marker of CSVD. It is associated with higher risk of stroke, cognitive decline, dementia, and death and therefore is commonly used as a surrogate end point for brain health in research settings. 2 Adjustment of modifiable risk factors is the priority in the treatment of CSVD. 3
Hypertension is the leading risk factor of WMH. 4 , 5 Even stage 1 hypertension (SBP 130‐139 mm Hg or DBP 80‐89 mm Hg) is associated with accelerated WMH progression. 6 Meanwhile, the relative risk of WMH progression depends on the duration of hypertension and quality of blood pressure (BP) management. 7 Therefore, BP control is crucial for the prevention of WMH. Despite the presence of a few conflicting voice, in recent years, emerging evidence has shown the benefit of more intensive BP control in terms of cerebrovascular events and WMH. 8 , 9 , 10 , 11 , 12 , 13 , 14
In the present study, we systematically reviewed randomized trials investigating the effect of intensive BP control on the prevention of WMH progression.
2. METHODS
2.1. Searching strategy
This systematic review and meta‐analysis were conducted under the guidance of Preferred Reporting Items for Systematic Reviews and Meta‐Analyses statement (Appendix Table S1). And the protocol has been registered on the PROSPERO (CRD42020184962). We systematically searched PubMed, EMBASE, SCOPUS, and Cochrane Central Register of Controlled Trials for publications until July 20, 2020. The search was performed by two authors independently (YW‐L and C‐J). The detailed electronic searching strategy was shown in Appendix Table S2 in the supplement. The references of relevant articles were also reviewed.
2.2. Inclusion criteria and study selection
Eligible studies should be clinical trials enrolling adults with hypertension or other vascular risk factors including elderly, diabetes, stroke or TIA, and cardiovascular diseases. The study population should be randomly allocated to an antihypertensive medication against placebo on the basis of background antihypertensive therapy, or different treatment targets. Meanwhile, eligible studies should include baseline and follow‐up MRI assessment of white matter lesions. Inclusion was restricted to trials reported in English.
Two authors (YW‐L and C‐J) independently reviewed all the retrieved titles and abstracts for eligible studies. The full text of eligible studies was further acquired and reviewed.
2.3. Data extraction and quality assessment
Two authors independently reviewed the full text of eligible studies to extract necessary data and assess their quality. Baseline characteristics of studies including date of publication, sample size, duration of follow‐up, mean age of population, baseline SBP, antihypertensive medications, or BP goals were documented. SBP achieved after the intervention and the SBP change was also assessed; differences in achieved SBPs between intervention and control group were also calculated to describe the magnitude of intensive BP control. The outcome was defined as changes of total WMH volume from baseline to the last MRI scanning.
The quality of studies was assessed using Cochrane Collaboration's tool for assessing risk of bias. A third author (X‐D) was involved in the discussion if there was any disagreement on the quality of the study.
2.4. Data synthesis and statistical analysis
Continuous variables are presented as mean (SD) or median (range); categorical variables are presented as numbers (frequency). Effect size of outcome is presented as standard mean difference of WML changes between intensive and control group. Random‐effect model was used for pooling the effect measures, and the result for fixed‐effect model was also provided to illustrate the robustness of the pooled effect. Forest plots were used to summarize individual and pooled effect measures of the studies. Both funnel plot and Egger's linear regression were applied to assess publication bias.
Heterogeneity was investigated using I‐square test. Further, the subgroup analysis was conducted in studies comparing intensive and standard BP target as well as studies comparing active antihypertensive medication and placebo. The Meta‐regression was also conducted to further explore the relation between the effect measure and mean age of population, duration of follow‐up and magnitude of SBP control. One‐study‐omitted sensitivity analysis was conducted to investigate the robustness of the meta‐analysis.
Statistical analysis was performed in the R software. A 2‐sided P < .05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of the studies
The flow diagram of literature search and review is shown in Figure 1. Among 1408 initially retrieved items, 181 were excluded due to duplication. After review of titles and abstracts, 1227 was further excluded. In the end, 23 studies underwent full‐text evaluation, of which seven met the inclusion criteria.
Figure 1.
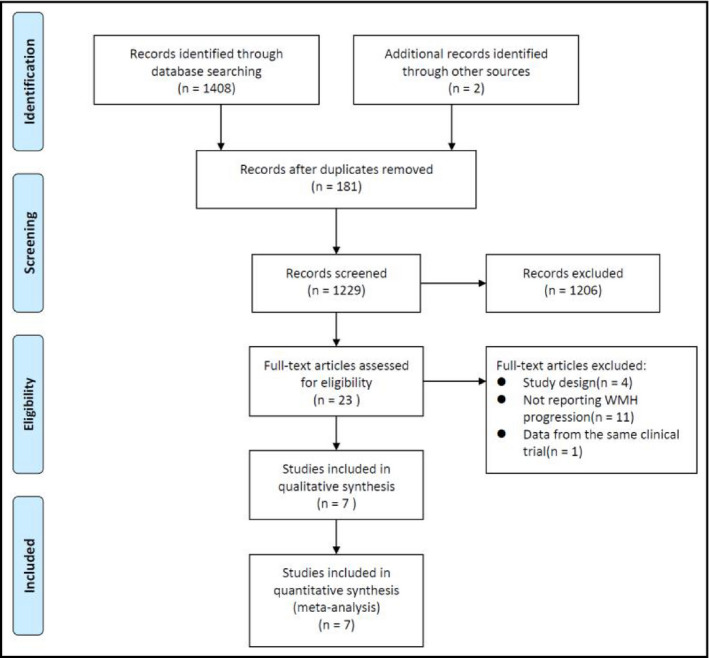
Flowchart of literature review and study selection
Characteristics of eligible studies are shown in Table 1. A total of 2693 participants were enrolled from the 7 eligible RCTs, with a median age of 67.1 years (range: 60.8‐80.6 years). Among them, 2314 (85.9%) had diagnosed hypertension at baseline. Three studies (INFINITY, SCOPE, and Zhang (2018)) focused on elderly hypertensive patients, two (PROGRESS and PRoFESS) enrolled patients with history of stroke, one (ACCORD MIND) enrolled patients with diabetes, and one (SPRINT MIND) enrolled patients at high risk of cardiovascular disease. Three studies compared patients with different SBP goals, and the other four compared an active hypertensive medication with placebo on the top of background treatment. The median follow‐up was 36 months (range: 23.7‐60). During the study period, SBP in control groups was all controlled at a reasonable level. And interventions further reduced SBP by a median of 9.1 mm Hg (range: 2.5‐14.9 mm Hg). One study (PROFESS) failed to achieve significant SBP difference between intervention and control group. Risk of bias of each study is shown in Appendix Table S3.
Table 1.
Characteristics of eligible studies
| Trial | Year | Sample size | Population |
Age |
Hypertension |
Follow‐up (months) |
Intervention | Control | Achieved SBP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Intensive | Control | |||||||||
| SPRINT MIND | 2019 | 449 |
Patients ≥ 50 y with SBP 130‐180 mm Hg and had increased cardiovascular risk |
67.1 | 449 (100.0%) | 48 | SBP < 120 mm Hg | SBP < 140 mm Hg | 120.7 | 134.9 |
| ACCORD MIND | 2019 | 314 | 45‐79 y Diabetes patients at a high cardiovascular risk | 62.3 | 305 (97.1%) | 40 | SBP < 120 mm Hg | SBP < 140mm Hg | 118.6 | 133.5 |
| INFINITY | 2019 | 199 | Patients > 75 y with hypertension and baseline WMH | 80.6 | 199 (100.0%) | 36 | SBP < 130 mm Hg | SBP < 145mm Hg | 132.6 | 145.6 |
| PROGRESS | 2005 | 192 | Patients with a history of cerebrovascular disease in previous 5 y | 60.8 | 103 (53.6%) | 36 | Perindopril with or without indapamide | Placebo | 131.8 | 140.9 |
| PRoFESS | 2015 | 771 | Patients > 50 y with recent ischemic stroke of non‐cardioembolic origin | 65.3 | 582 (75.5%) | 27.6 | Telmisartan | Placebo | 134.9 | |
| SCOPE | 2007 | 92 | Patients at age of 70‐89, with BP 160‐179/90‐99 mm Hg | 77.0 | 92 (100.0%) | 23.7 | Candesartan | Placebo | 141.0 | 147.0 |
| Zhang 2018 | 2018 | 676 | Community hypertensive patients aged ≥ 60 y | 70.7 | 676 (100.0%) | 60 | Telmisartan | Placebo | 138.8 | 144.0 |
3.2. Effect of intensive BP control on WML progression
The pooled standard mean difference for WML progression was −0.22 (95% CI: −0.35~‐0.09) for random‐effect model, with a moderate heterogeneity (I2 = 63%). In subgroup of studies comparing intensive and standard BP goals, the pooled SMD was −0.37 (95% CI: −0.50~‐0.24, I2 = 0.0%). However, in subgroup of studies comparing active antihypertensive medication and placebo, the pooled SMD for WML progression was only −0.08 (95% CI:‐0.17 ~ 0.01, I2 = 0.0%) which narrowly missed the threshold to be statistically significant (Figure 2). Funnel plot of the eligible studies is shown in Appendix Figure S1; Egger's linear regression suggested no publication bias (P = .278).
Figure 2.
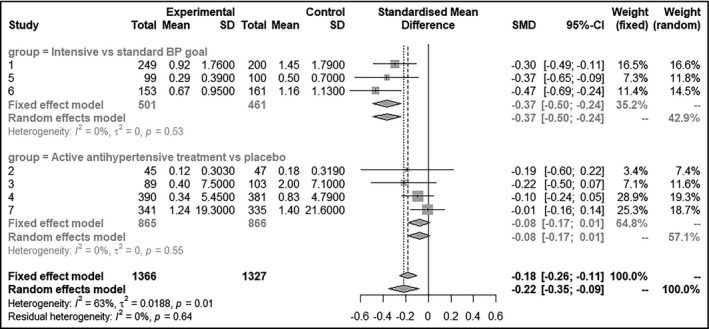
Summary of effect measures and pooled effect of intensive BP control on prevention of WMH progression
In meta‐regression, the magnitude of intensive BP control is associated with slower WML progression (β = −0.028, P < .001) (Figure 3). However, the age of the population (P = .91) and the period of follow‐up (P = .65) are not associated with the effect measures.
Figure 3.
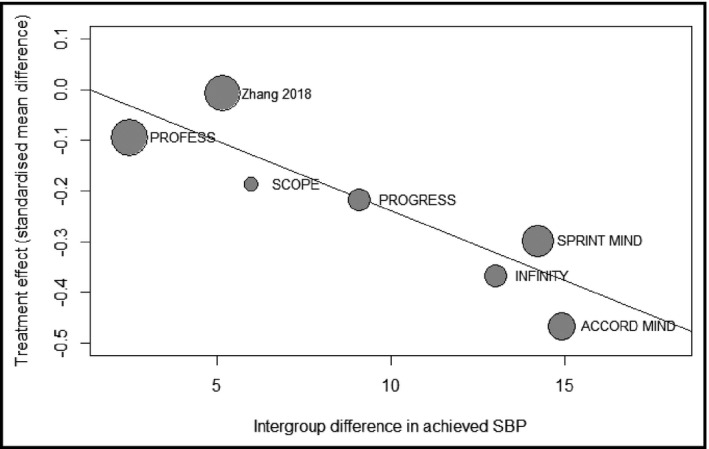
Bubble plots exhibiting the relation between the magnitude of intensive BP control and the effect measure
Sensitivity analysis (Appendix Figure S2) showed that the pooled SMD remained stable with the exclusion of any one of the studies.
4. DISCUSSION
The present systematic review provides further evidence for intensive BP control in preventing WMH progression, and a dose relation that more intensive BP control is associated with better prevention of WMH progression is also revealed.
4.1. Clinical considerations
Hypertension results in impairment of vascular integrity including arteriosclerosis, microatheroma, and microaneurysms, which decrease cerebral blood flow and as a result cause loss of myelin and gliosis. 15 , 16 These pathological changes manifest on MRI as WMH. 17 , 18 Therefore, WMH is an important marker of hypertensive brain injury. A previous meta‐analysis by Middelaar et. al. has preliminarily revealed a tendency of intensive BP control to prevent WMH progression. 19 However, only four studies were eligible at that time and the magnitude of intensive BP control was unsatisfactory in two studies (SCOPE and PRoFESS). As studies continue to emerge, the present meta‐analysis further clarified this beneficial effect and confirmed the robustness of the effect through one‐study‐omitted sensitivity analysis.
Meanwhile, a wider range of the magnitude of intensive BP control allowed us to more reliably describe the dose relationship. Adequate magnitude of intensive BP control may be the premise of WMH prevention. In studies with prespecified antihypertensive medication, the magnitude of intensive BP control varied and is usually less intensive. The intergroup SBP difference in one study (PRoFESS) did not even achieve statistical significance. This may explain why pooled effect measure in this subgroup was not significant. In comparison, studies targeting more intensive SBP goals had more reliable effect of BP control and therefore prevented WMH progression to a larger extent.
4.2. Knowledge gaps and future perspectives
Current studies also unveiled several knowledge gaps. Other MRI markers of CSVD are inadequately investigated in these studies. Of note, with 3 studies reporting the effect of intensive BP control on total brain volume, the results were conflicting. One study (SCOPE) reported less brain atrophy in active treatment group, while the other two (SPRINT MIND and ACCORD MIND) found paradoxically accelerated brain atrophy with intensive BP control. The potential cause and hazard of this phenomenon remain unclear. Previous RCTs have reported that patients receiving intensive BP control had maintained or even improved cerebral perfusion, 20 , 21 making it unreasonable to result in brain atrophy. However, one observational study also reported that low diastolic BP (≤70 mm Hg) is associated with more progression in subcortical brain atrophy. 22 Further studies should focus on the potential risk of hypotension, specifically low diastolic BP, during intensive BP control and its impact on CSVD and cognitive function.
Whether intensive BP control preserves cognitive function or not remains unclear. Previous RCTs failed to find significant reductions in cognitive impairment or incidence of dementia in patients receiving active antihypertensive medication. 23 The investigation of this relationship is hindered by several barriers. The incidence of dementia is quite low, meaning a large population is required for clinical trials. Moreover, cognitive screening strategies in previous studies were not sensitive enough for mild cognitive impairment. In recent studies, a battery of widely validated neuropsychological tests has been applied to assess general cognitive status and separated cognitive domains, which is able to discriminate mild cognitive impairment (MCI) and provide detailed cognitive information. MCI is a crucial stage of early intervention including risk factor management, lifestyle change, and cognitive training during cognitive decline. 24 It is closely conjoined with WMH progression in hypertensive patients. Recently, the SPRINT MIND trial has reported that intensive BP control reduced the incidence of MCI by 15%, though the effect on dementia was still not significant due to its low incidence 25 In the future, further studies on the effect of intensive BP control on mild cognitive impairment and its cause‐effect relation with WMH prevention will be of great clinical importance.
5. LIMITATION
The present meta‐analysis has several limitations. Evidence has been accumulating to suggest that patients with older age, diabetes, stroke, etc, also have a high burden of WMH, 3 but the targets of intensive BP control in these patients may be different. However, due to the limited number of studies, we failed to further discuss the effect of intensive BP control on the prevention of WMH progression in specific populations like the elderly, patients with diabetes or cerebrovascular disease. Meanwhile, we did not analyze the pooled effect on cognitive function for the heterogeneity of cognitive scales used in these studies.
6. CONCLUSION
In conclusion, intensive BP control significantly prevents WMH progression in patients with hypertension or other vascular risk factors. This protective effect, measured as intergroup SMD, is in proportion to the magnitude of intensive BP control. However, further studies are required to reveal the possible benefit in cognitive function, as well as the safety of intensive BP control.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
YW‐L, C‐J, CH‐S, and CS‐M are responsible for the conception and design of the systemic review and meta‐analysis. YW‐L and C‐J systematically performed the literature searching, reviewed the retrieved items for eligibility, and evaluated the quality of eligible studies. And X‐D was involved in the discussion if there was any disagreement on the eligibility and quality of the studies. YW‐L and XY‐G were responsible for the statistical analysis. X‐D, R‐B, RB‐T, and JZ‐D provided important advice on the methodology of meta‐analysis and the data interpretation. YW‐L and C‐J drafted the manuscript. CH‐S and CS‐M critically appraised the manuscript and approved the final version. All authors have the access to all of the data, read the manuscript, and agreed to be accountable for all aspects of the work.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
We sincerely thank Ms Yu Bai from the University of Sydney for her valuable advice on improving the grammar and readability of our manuscript.
Lai Y, Jiang C, Du X, et al. Effect of intensive blood pressure control on the prevention of white matter hyperintensity: Systematic review and meta‐analysis of randomized trials. J Clin Hypertens. 2020;22:1968–1973. 10.1111/jch.14030
Yiwei Lai and Chao Jiang contributed equally to this article.
Funding information
The work was supported by the National Key Research and Development Program of China (2017YFC0908803, 2016YFC0900901, 2018YFC1312501), the National Natural Science Foundation of China (81700299), and grant from Beijing Municipal Commission of Science and Technology (D171100006817001).
Contributor Information
Caihua Sang, Email: chshma@vip.sina.com, Email: sch9613070@sina.com.
Changsheng Ma, Email: chshma@vip.sina.com, Email: sch9613070@sina.com.
REFERENCES
- 1. Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all‐cause mortality: A systematic review and meta‐analysis. Neurosci Biobehav Rev. 2018;90:164‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta‐analysis. BMJ. 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease: A clinical review. Neurology. 2019;92:1146‐1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry. 2017;88:669‐674. [DOI] [PubMed] [Google Scholar]
- 5. Khan U, Porteous L, Hassan A, Markus HS. Risk factor profile of cerebral small vessel disease and its subtypes. J Neurol Neurosurg Psychiatry. 2007;78:702‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. Cerebral Small Vessel Disease and Stage 1 Hypertension Defined by the 2017 American College of Cardiology/American Heart Association Guidelines. Hypertension. 2019;73:1210‐1216. [DOI] [PubMed] [Google Scholar]
- 7. de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765‐772. [DOI] [PubMed] [Google Scholar]
- 8. Nasrallah IM, Pajewski NM, Auchus AP, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA. 2019;322(6):524‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Havenon A, Majersik JJ, Tirschwell DL, McNally JS, Stoddard G, Rost NS. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology. 2019;92:e1168‐e1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. White WB, Wakefield DB, Moscufo N, et al. Effects of Intensive Versus Standard Ambulatory Blood Pressure Control on Cerebrovascular Outcomes in Older People (INFINITY). Circulation. 2019;140:1626‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644‐1650. [DOI] [PubMed] [Google Scholar]
- 12. Weber R, Weimar C, Blatchford J, et al. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the prevention regimen for effectively avoiding second strokes (PRoFESS) MRI substudy. Stroke. 2012;43:2336‐2342. [DOI] [PubMed] [Google Scholar]
- 13. Firbank MJ, Wiseman RM, Burton EJ, Saxby BK, O'Brien JT, Ford GA. Brain atrophy and white matter hyperintensity change in older adults and relationship to blood pressure. Brain atrophy, WMH change and blood pressure. J Neurol. 2007;254:713‐721. [DOI] [PubMed] [Google Scholar]
- 14. Zhang H, Cui Y, Zhao Y, et al. Effects of sartans and low‐dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: a randomized, double‐blind and placebo‐controlled clinical trial. Hypertens Res. 2019;42:717‐729. [DOI] [PubMed] [Google Scholar]
- 15. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689‐701. [DOI] [PubMed] [Google Scholar]
- 16. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12:483‐497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804‐811. [DOI] [PubMed] [Google Scholar]
- 18. Arfanakis K, Evia AM, Leurgans SE, et al. Neuropathologic correlates of white matter hyperintensities in a community‐based cohort of older adults. J Alzheimer's Dis. 2020;73:333‐345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Middelaar T, Argillander TE, Schreuder F, Deinum J, Richard E, Klijn CJM. Effect of antihypertensive medication on cerebral small vessel disease: a systematic review and meta‐analysis. Stroke. 2018;49:1531‐1533. [DOI] [PubMed] [Google Scholar]
- 20. Croall ID, Tozer DJ, Moynihan B, et al. Effect of Standard vs Intensive Blood Pressure Control on Cerebral Blood Flow in Small Vessel Disease: The PRESERVE Randomized Clinical Trial. JAMA Neurol. 2018;75:720‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tryambake D, He J, Firbank MJ, O'Brien JT, Blamire AM, Ford GA. Intensive blood pressure lowering increases cerebral blood flow in older subjects with hypertension. Hypertension. 2013;61:1309‐1315. [DOI] [PubMed] [Google Scholar]
- 22. Jochemsen HM, Muller M, Visseren FL, et al. Blood pressure and progression of brain atrophy: the SMART‐MR Study. JAMA Neurol. 2013;70:1046‐1053. [DOI] [PubMed] [Google Scholar]
- 23. McGuinness B, Todd S, Passmore P, Bullock R. Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev. 2009:CD004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia. JAMA. 2019;321(6):553‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


