Abstract
We investigated association between blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy (LVH) in patients with hypertension and diabetes. Our study participants were treated patients with both diseases, enrolled in a China nationwide registry. The 773 patients were classified into four groups according to the control status of hypertension (systolic/diastolic blood pressure [BP] ≤140/90 mm Hg) and diabetes (HbA1c <7.0%): both uncontrolled (n = 208), only diabetes (n = 175) or hypertension controlled (n = 172), and both controlled (n = 218). Albuminuria was defined as a urinary albumin‐to‐creatinine ratio of ≥30 mg/g. LVH was assessed by the electrocardiogram Cornell product method. Antihypertensive therapy was not different between the four groups (P ≥ .48). The use of insulin alone or insulin plus oral antidiabetic agents was significantly higher than those with both diseases controlled (P ≤ .02). Patients with controlled hypertension and diabetes had a significantly (P < .0001) lower prevalence of albuminuria (odds ratio 0.22, 95% confidence interval 0.11‐0.43) than those with both diseases uncontrolled. Intensive BP control to <130/80 mm Hg was associated with lower risks of albuminuria in all patients (P = .001) and patients with HbA1c <7.0% (P = .048). Intensive glycemic control to HbA1c <6.5% was also associated with a significantly lower risk of albuminuria in all patients (P = .01), but not those with controlled BP (P = .43). Similar trends were observed for LVH, but statistical significance was not achieved on either intensive control condition (P ≥ .07). In patients with hypertension and diabetes, blood pressure and glucose control were associated with a lower prevalence of albuminuria and LVH, especially when achieving a more stringent target.
Keywords: albuminuria, controlled, diabetes mellitus, hypertension, left ventricular hypertrophy, uncontrolled
1. INTRODUCTION
With the changes in lifestyle, the prevalence of hypertension and diabetes mellitus increases rapidly in China.1, 2 Many people develop both diseases in their early or midlife. In a recent China nationwide registry of patients with either hypertension or diabetes mellitus, the prevalence of both hypertension and diabetes mellitus was 45.3%.3 Coexistence of hypertension and diabetes mellitus significantly increases the risk of microvascular and macrovascular complications in comparison with the presence of either disease alone.4 In addition, in the joint presence of hypertension and diabetes mellitus, the management of either disease is difficult and complicated, because drugs for one may compromise another.5, 6 Combination of thiazides with β‐blockers effectively lowers blood pressure, but increases the risk of diabetes mellitus.5 Some antidiabetic agents do induce fluid retention and potentially increase blood pressure.6
Several clinical trials investigated the combined antihypertensive and antidiabetic effect in diabetic patients.7, 8, 9 With less stringent targets, approximately 140 mm Hg of systolic blood pressure7 and 7.0% of glycosylated hemoglobin A1c,8, 9 such as in the United Kingdom Prospective Diabetes Study (UKPDS),7, 8, 9 both therapeutic approaches showed benefit of cardiovascular outcomes. However, with more stringent targets, 6.5% of HbA1c such as in the PreterAx and DiamicroN modified‐release controlled evaluation (ADVANCE) trial,10 or 120 mm Hg of systolic blood pressure,11 and 6.0% of HbA1c12 such as in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial11, 12 both treatments had no or limited benefit in cardiovascular prevention. It is therefore still a matter of debate whether intensive blood pressure and glucose lowering would be beneficial in patients with hypertension and diabetes mellitus even though current hypertension guidelines recommend similar stringent therapeutic target in this patient group.13, 14, 15
In the present cross‐sectional analysis in a group of patients treated with both antihypertensive and antidiabetic drug therapy, we investigated the association between blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy.
2. METHODS
2.1. Study population
Our study participants were recruited from a cross‐sectional, multicentre registry in China, which was carried out in the departments of cardiovascular and endocrine medicine of hospitals from June 2011 to March 2012. The study protocol of the registry had been described in detail previously.3 In brief, we registered consecutive patients with previously diagnosed hypertension from the departments of cardiovascular medicine and patients with previously diagnosed diabetes mellitus from the departments of endocrine medicine. The ethics committees of all participating hospitals approved the study protocol. All subjects gave written informed consent.
To be eligible for inclusion, a patient had to be at least 20 years old and was able to participate in two clinic visits two to 5 days apart. At the first clinic visit, physicians administered a standardized questionnaire to collect information on medical history, lifestyle, and use of medications. Blood pressure and anthropometry were measured. At the second clinic visit, blood pressure was measured for the second time. Venous blood samples were drawn after overnight fasting for measurements of plasma glucose, glycosylated hemoglobin A1 (HbA1c), and serum lipids. Morning void urine samples were collected for urinary measurements.
The registry included a total of 2510 patients. The present analysis included 773 patients with previously diagnosed and treated both hypertension and diabetes mellitus seen in cardiology or endocrinology.
2.2. Clinical and biochemical measurements
Blood pressure was measured using a validated Omron HEM‐7201 automatic oscillometric blood pressure monitor (Omron Healthcare) at the first and second clinic visits. On each of the two occasions, three blood pressure readings were obtained in the seated position after the subjects had rested for at least 5 minutes. These six readings on two clinic visits were averaged for statistical analysis. Control of hypertension was defined as a blood pressure below 140 mm Hg systolic and 90 mm Hg diastolic. Plasma glucose concentration was measured in all patients. Control of diabetes mellitus was defined as a plasma glycosylated hemoglobin A1c <7.0%.
Standard 12‐lead electrocardiogram (ECG) was recorded in all subjects. ECG‐left ventricular hypertrophy was defined according to the Cornell product index, as (RaVL + SV3) × QRS duration >244 mV·ms.13
Urinary routine test was performed on fresh spot urine samples at the laboratory of each participating hospitals. Urinary albumin and creatinine excretions were measured using the immunochemical method in a core laboratory certified by the College of American Pathologists (www.cap.org). In the absence of apparent urological infections on urine samples, albuminuria was defined as a urinary albumin‐to‐creatinine ratio ≥30 mg/g. Albuminuria included both microalbuminuria (30‐299 mg/g) and macroalbuminuria (≥300 mg/g).
2.3. Other measurements
Anthropometric measurements included body weight, body height, and waist and hip circumferences. Body mass index was calculated as the body weight in kilograms divided by the body height in meters squared. Overweight was defined as a body mass index of 24‐28 kg/m2 and obesity of 28 kg/m2 or greater. Central obesity was defined as a waist circumference ≥90 centimeters for men and ≥85 centimeters for women.
Dyslipidemia was defined as a serum triglycerides concentration of 1.70 mmol/L or higher, a serum total cholesterol concentration of 5.18 mmol/L or higher, a serum LDL cholesterol concentration of 3.37 mmol/L or higher, or a serum HDL cholesterol concentration of 1.04 mmol/L or lower, or as the use of statin or other lipid‐lowering agents.16
Ischemic heart disease included myocardial infarction and angina. Both ischemic heart disease and stroke were self‐reported.
2.4. Statistical analysis
For database management and statistical analysis, we used SAS software (version 9.4, SAS Institute). Means and proportions across the groups were compared by the analysis of variance (ANOVA) and Fisher's exact test, respectively. Continuous measurements with a skewed distribution were logarithmically transformed and represented by geometric mean and 95% confidence interval (CI). Logistic regression analyses were performed to study the associations of interest.
3. RESULTS
3.1. Clinical characteristics of patients
Table 1 shows the characteristics of patients according to the control status of hypertension and diabetes mellitus. Patients with uncontrolled hypertension and diabetes mellitus had a significantly (P ≤ .002) greater body mass index, higher serum triglycerides and total cholesterol concentrations, and faster heart rate than those with controlled hypertension and diabetes mellitus.
Table 1.
Characteristics of patients according to the control status of hypertension and diabetes mellitus
| Characteristic | Both hypertension and diabetes uncontrolled (n = 208) | Only diabetes controlled (n = 175) | Only hypertension controlled (n = 172) | Both hypertension and diabetes controlled (n = 218) | P value (ANOVA) |
|---|---|---|---|---|---|
| Men, n (%) | 90 (43.3) | 67 (38.3) | 76 (44.2) | 97 (44.5) | .60 |
| Age, years | 62.6 ± 9.8 | 63.6 ± 9.4 | 61.5 ± 10.3 | 61.7 ± 9.8 | .17 |
| Body mass index, kg/m2 | 26.6 ± 3.9 | 25.5 ± 3.2 | 25.7 ± 3.2 | 25.4 ± 3.4 | .002 |
| Current smoking, n (%) | 28 (13.5) | 18 (10.3) | 26 (15.1) | 29 (13.3) | .60 |
| Alcohol intake, n (%) | 23 (11.1) | 19 (10.9) | 21 (12.2) | 29 (13.3) | .86 |
| Systolic blood pressure, mm Hg | 152.5 ± 12.8 | 152.1 ± 13.1 | 127.2 ± 9.2 | 125.7 ± 9.1 | <.0001 |
| Diastolic blood pressure, mm Hg | 83.7 ± 11.3 | 81.9 ± 11.2 | 75.0 ± 8.7 | 73.8 ± 7.8 | <.0001 |
| Heart rate, beats/min | 76.0 ± 14.0 | 72.6 ± 13.2 | 74.3 ± 12.1 | 70.7 ± 11.9 | <.0001 |
| Plasma fasting glucose, mmol/L | 8.83 (7.47‐10.82) | 6.18 (5.51‐7.10) | 8.19 (6.90‐9.88) | 6.18 (5.59‐6.98) | <.0001 |
| Plasma glycosylated hemoglobin A1c, % | 8.58 ± 1.50 | 6.18 ± 0.49 | 8.20 ± 1.19 | 6.18 ± 0.43 | <.0001 |
| Serum triglycerides, mmol/L | 1.68 (1.10‐2.43) | 1.34 (0.95‐1.96) | 1.48 (1.03‐2.11) | 1.33 (0.90‐1.88) | <.0001 |
| Serum total cholesterol, mmol/L | 4.97 ± 1.27 | 4.79 ± 1.12 | 4.70 ± 1.11 | 4.42 ± 0.88 | <.0001 |
Values are arithmetic mean ± standard deviation, median (interquartile range), or number of subjects (%).
Control of hypertension was defined as a blood pressure below 140 mm Hg systolic and 90 mm Hg diastolic. Control of diabetes mellitus was defined as a plasma glycosylated hemoglobin A1c <7.0%.
Table 2 shows the antihypertensive and antidiabetic therapy according to the control status of hypertension and diabetes mellitus. Although the use of antihypertensive therapy was not significantly different, the use of insulin alone or insulin plus oral antidiabetic drugs was highest in patients with uncontrolled hypertension and diabetes mellitus (19.2% and 29.3%, respectively), and significantly higher than in patients with controlled hypertension and diabetes mellitus (9.6% and 6.4% respectively, P ≤ .02 vs both uncontrolled).
Table 2.
Treatment of hypertension and diabetes mellitus according to the control status of both diseases
| Treatment |
Both hypertension and diabetes uncontrolled (n = 208) |
Only diabetes controlled (n = 175) |
Only hypertension controlled (n = 172) |
Both hypertension and diabetes controlled (n = 218) |
P value (ANOVA) |
|---|---|---|---|---|---|
| Treatment of hypertension, n (%) | |||||
| Monotherapy | 126 (60.6) | 103 (58.9) | 98 (57.0) | 128 (58.7) | .92 |
| Combination therapy | 82 (39.4) | 72 (41.1) | 74 (43.0) | 90 (41.3) | .92 |
| Use of RAS inhibitors | 124 (59.6) | 103 (58.9) | 114 (66.3) | 135 (61.9) | .48 |
| Treatment of diabetes mellitus, n (%) | |||||
| Oral antidiabetic drugs alone | 105 (50.5) | 134 (76.6) | 95 (55.2) | 180 (82.6) | <.0001 |
| Insulin alone | 40 (19.2) | 23 (13.1) | 32 (18.6) | 21 (9.6) | .02 |
| Oral antidiabetic drugs plus insulin | 61 (29.3) | 17 (9.7) | 44 (25.6) | 14 (6.4) | <.0001 |
Abbreviation: RAS, renin‐angiotensin system.
Values are number of subjects (%).
For further explanations, see Table 1.
3.2. Association between blood pressure and glucose control and the prevalence of albuminuria and ECG‐left ventricular hypertrophy
Patients with controlled hypertension and diabetes mellitus had the lowest prevalence of albuminuria, being significantly (P < .0001) lower than those with uncontrolled hypertension and diabetes mellitus (14.0% vs 41.7%, Table 3). After adjustment for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, heart rate, and the use of inhibitors of the renin‐angiotensin system, the odds ratio for controlled versus uncontrolled hypertension and diabetes mellitus was 0.25 (95% CI 0.12‐0.49, P < .0001).
Table 3.
Risk of albuminuria and left ventricular hypertrophy according to the control status of hypertension and diabetes mellitus
| Both hypertension and diabetes uncontrolled | Only diabetes controlled | Only hypertension controlled | Both hypertension and diabetes controlled | |
|---|---|---|---|---|
| Albuminuria | ||||
| Number of study subjects (n = 428) | 108 | 99 | 92 | 129 |
| Prevalence of albuminuria (%) | 41.7 | 21.2 | 18.5 | 14.0 |
| Odds ratio (95% confidence interval)* | ‐ | 0.40 (0.21‐0.76) | 0.31 (0.16‐0.61) | 0.22 (0.11‐0.43) |
| P value | ‐ | .005 | .001 | <.0001 |
| Left ventricular hypertrophy | ||||
| Number of study subjects (n = 766) | 205 | 173 | 171 | 217 |
| Prevalence of left ventricular hypertrophy (%) | 5.4 | 4.6 | 4.7 | 1.8 |
| Odds ratio (95% confidence interval)* | ‐ | 0.92 (0.35‐2.45) | 0.77 (0.29‐2.03) | 0.33 (0.10‐1.11) |
| P value | ‐ | .87 | .60 | .07 |
Adjusted for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, and heart rate.
Similar findings were observed for ECG‐left ventricular hypertrophy. Statistical significance, however, was not reached in either unadjusted analysis or analysis adjusted for the confounding factors (P ≥ .07).
3.3. Association between intensive blood pressure and glucose control and the prevalence of albuminuria and ECG‐left ventricular hypertrophy
In further adjusted analysis, we investigated association between intensive blood pressure and glucose control and the prevalence of albuminuria and ECG‐left ventricular hypertrophy, with or without adjusting one factor for another.
Table 4 shows that blood pressure control to a level of 130 mm Hg systolic and 80 mm Hg diastolic or below was associated with a significantly lower risk of albuminuria in all patients (odds ratio [OR] 0.32, 95% CI 0.16‐0.64, P = .001), as well as in patients with a HbA1c of <7.0% (OR 0.33, 95% CI 0.11‐0.99, P = .048). Similar trends were observed for ECG‐left ventricular hypertrophy, but statistical significance was not reached (P ≥ .14, Figure 1).
Table 4.
Prevalence of albuminuria and left ventricular hypertrophy according to blood pressure among all patients and patients with HbA1c <7.0%
| Blood pressure (mm Hg) | |||
|---|---|---|---|
| ≥140/90 | 130‐139/80‐89 | <130/80 | |
| All patients | |||
| Albuminuria | |||
| Number of patients (n = 428) | 66/207 | 23/130 | 12/91 |
| Prevalence of albuminuria (%) | 31.9% | 17.7% | 13.2% |
| Odds ratio (95% CI)* | ‐ | 0.44 (0.25‐0.76) | 0.32 (0.16‐0.64) |
| P value | ‐ | .003 | .001 |
| Left ventricular hypertrophy | |||
| Number of patients (n = 766) | 19/378 | 8/223 | 4/165 |
| Prevalence of left ventricular hypertrophy (%) | 5.0% | 3.6% | 2.4% |
| Odds ratio (95% CI)* | ‐ | 0.65 (0.27‐1.56) | 0.43 (0.14‐1.32) |
| P value | ‐ | .34 | .14 |
| Patients with HbA1c <7.0% | |||
| Albuminuria | |||
| Number of patients (n = 228) | 21/99 | 13/75 | 5/54 |
| Prevalence of albuminuria (%) | 21.2% | 17.3% | 9.3% |
| Odds ratio (95% CI)* | ‐ | 0.75 (0.33‐1.69) | 0.33 (0.11‐0.99) |
| P value | ‐ | .48 | .048 |
| Left ventricular hypertrophy | |||
| Number of patients (n = 390) | 8/173 | 3/124 | 1/93 |
| Prevalence of left ventricular hypertrophy (%) | 4.6% | 2.4% | 1.1% |
| Odds ratio (95% CI)* | ‐ | 0.34 (0.08‐1.54) | 0.20 (0.02‐1.79) |
| P value | ‐ | 0.16 | 0.15 |
Abbreviation: CI, confidence interval.
Adjusted for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, and heart rate.
Figure 1.
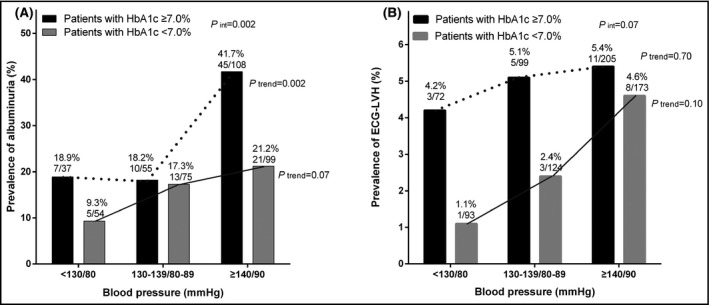
Prevalence of albuminuria (A) and ECG‐left ventricular hypertrophy (B) according to the less tight and tight control status of hypertension in patients with diabetes controlled (open bar) and uncontrolled (solid bar). The P value for interaction between the control status of hypertension and diabetes in relation to the prevalence of albuminuria and left ventricular hypertrophy, and the P values for trend and the number of patients are given
Table 5 shows that glucose control to a HbA1c level of <6.5% was associated with a significantly lower risk of albuminuria in all patients (OR 0.48, 95% CI 0.28‐0.84, P = .01) but not in patients with controlled hypertension (<140/90 mm Hg, OR 0.72,95% CI 0.31‐1.64, P = .43). Similar trends were observed for ECG‐left ventricular hypertrophy, but statistical significance was not reached either in all patients or in those with controlled hypertension (P ≥ .39, Figure 2).
Table 5.
Prevalence of albuminuria and left ventricular hypertrophy according to HbA1c among all patients and patients with controlled hypertension
| HbA1c | |||
|---|---|---|---|
| ≥7.0% | 6.5%‐6.9% | <6.5% | |
| All patients | |||
| Albuminuria | |||
| Number of patients(n = 428) | 62/200 | 14/84 | 25/144 |
| Prevalence of albuminuria (%) | 31.0% | 16.7% | 17.4% |
| Odds ratio (95% CI)* | ‐ | 0.46 (0.24‐0.89) | 0.48 (0.28‐0.84) |
| P value | ‐ | .02 | .01 |
| Left ventricular hypertrophy | |||
| Number of patients (n = 766) | 19/376 | 2/134 | 10/256 |
| Prevalence of left ventricular hypertrophy (%) | 5.1% | 1.5% | 3.9% |
| Odds ratio (95% CI)* | ‐ | 0.34 (0.08‐1.51) | 0.82(0.36‐1.87) |
| P value | ‐ | .15 | .63 |
| Patients with controlled hypertension | |||
| Albuminuria | |||
| Number of patients(n = 221) | 17/92 | 5/41 | 13/88 |
| Prevalence of albuminuria (%) | 18.5% | 12.2% | 14.8% |
| Odds ratio (95% CI)* | ‐ | 0.55 (0.18‐1.67) | 0.72 (0.31‐1.64) |
| P value | ‐ | .29 | .43 |
| Left ventricular hypertrophy | |||
| Number of patients (n = 388) | 8/171 | 5/69 | 6/148 |
| Prevalence of left ventricular hypertrophy (%) | 4.7% | 7.3% | 4.1% |
| Odds ratio (95% CI)* | ‐ | 0.53 (0.06‐4.78) | 0.53(0.13‐2.26) |
| P value | ‐ | .57 | .39 |
Abbreviation: CI, confidence interval.
Adjusted for age, sex, body mass index, current smoking and alcohol intake, serum total cholesterol and triglycerides, and heart rate.
Figure 2.
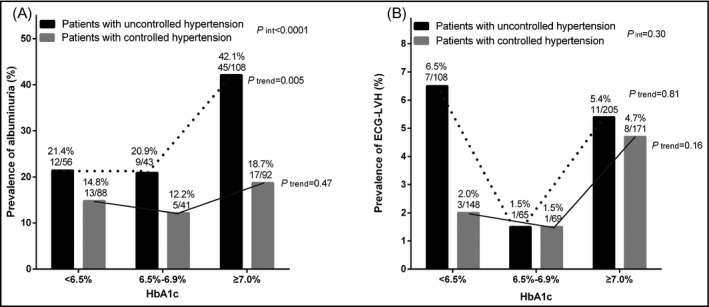
Prevalence of albuminuria (A) and ECG‐left ventricular hypertrophy (B) according to the less tight and tight control status of diabetes in patients with hypertension controlled (open bar) and uncontrolled (solid bar). The P value for interaction between the control status of diabetes mellitus and hypertension in relation to the prevalence of albuminuria and left ventricular hypertrophy, and the P values for trend and the number of patients are given
4. DISCUSSION
Our findings are fourfold. First, controlling both blood pressure and glucose is associated with lower prevalence of albuminuria and left ventricular hypertrophy. Second, intensive control of blood pressure, for instance, to <130/80 mm Hg, or glucose, for instance, to a HbA1c level <6.5%, is associated with even lower prevalence of albuminuria and left ventricular hypertrophy. Third, the association of blood pressure control with both albuminuria and ECG‐left ventricular hypertrophy tended to be greater than that of glucose control in those patients with both diseases. Fourth, the association of blood pressure control and glucose control with albuminuria tended to be greater than that with ECG‐left ventricular hypertrophy. Our study contributes to the current literature with the data from the real‐world setting of two clinical disciplines, that is, endocrinology and cardiovascular medicine.
Our observation on association between blood pressure and glucose control and the prevalence of albuminuria and ECG‐left ventricular hypertrophy is in line with the results of prospective observational studies17 and randomized controlled trials.7, 8, 9, 10 In a large Turkish cohort of treated hypertensive patients with diabetes (n = 1708), patients with controlled hypertension (< 140/85 mm Hg) and diabetes mellitus (blood glucose <126 mg/dL) had the lowest risk of albuminuria.17 In the UKPDS7, 8, 9, 18 as well as ADVANCE trials,10, 19, 20 which both investigated combined antihypertensive and antidiabetic therapy, patients on both antihypertensive and antidiabetic therapy had the lowest risk of most clinical outcomes, especially albuminuria in the ADVANCE trial.20
Our study provides evidence on association between intensive blood pressure and glucose control and the prevalence of albuminuria and ECG‐left ventricular hypertrophy and supports the recommendations of recent hypertension and diabetes guidelines.13, 14, 15 Nonetheless, because of the apparent harmful effects of blood pressure control to a level of 120 mm Hg or below11 and HbA1c to a level of 6.0%,12 we did not consider those levels of blood pressure and glucose control.
Our observation on stronger associations of blood pressure over glucose control with albuminuria and ECG‐left ventricular hypertrophy in patients with hypertension and diabetes mellitus is also in line with the results of prospective observational studies21 and the UKPDS7, 8, 9 and ADVANCE trials.10, 19 In the 1145 Framingham study participants with both hypertension and diabetes mellitus, the population‐attributable risk for all‐cause mortality and any cardiovascular event was 30% and 25%, respectively, from hypertension and 7% and 9%, respectively, from diabetes mellitus.21 In the UKPDS trial, patients with hypertension and type 2 diabetes assigned to tight blood pressure control achieved a significant 24% reduction in the risk of any diabetes‐related end points in comparison with those assigned to usual blood pressure control,7 whereas patients with type 2 diabetes assigned to tight glucose control only achieved a 12% reduction in the risk of any diabetes‐related end points in comparison with those assigned to usual glucose control.8, 9 In the ADVANCE trial, intensive blood pressure lowering in patients with diabetes mellitus reduced total and cardiovascular mortality,19 whereas intensive glucose lowering only reduced the risk of albuminuria.10 Experts therefore proposed that treatment of hypertension should be prioritized as the most important intervention for the average population with diabetes mellitus.22
In most of the instances, we observed significant associations with albuminuria but not ECG‐left ventricular hypertrophy. Because the prevalence of albuminuria in our study population was 2‐3 times that of ECG‐left ventricular hypertrophy, a straightforward explanation is therefore the inadequacy of power for the latter organ damage. ECG has low sensitivity in the detection of left ventricular hypertrophy.23 However, it is also possible that albuminuria is more sensitive to blood pressure24 or glucose control than ECG‐left ventricular hypertrophy. Indeed, in the ADVANCE trial, albuminuria was the only single end point that was significantly influenced by intensive glucose lowering.10
Our study should be interpreted within the context of its limitations. First, our study had a cross‐sectional design and does not allow any causal inference. In particular, it is unclear whether the findings are due to lesser duration or severity of disease, or due to more effective therapy. Second, our study had a relatively small sample size with few measurements of biological markers. Third, albuminuria was evaluated on a single spot urine sampling. However, albuminuria and creatinine were measured in a core laboratory. A stringent quality assurance program was implemented, including the exclusion of patients with suspected urinary tract infections.
In conclusion, in patients with both hypertension and diabetes mellitus, blood pressure and glucose control were associated with a lower prevalence of albuminuria and ECG‐left ventricular hypertrophy, especially when achieving a more stringent target.
CONFLICT OF INTEREST
Dr Wang reports receiving lecture and consulting fees from Astra‐Zeneca, Bayer, Daiichi‐Sankyo, MSD, Novartis, Pfizer, Sanofi, Servier, and Takeda. The other authors declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
Ji‐Guang Wang and Li‐Nong Ji contributed to the conception and design of the work. Wei Zhang performed data analysis and prepared the first draft of the manuscript together with Ji‐Guang Wang. All authors critically revised the manuscript and gave the final approval.
Data sharing
Qualified researchers may request access to patient‐level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient‐level data will be anonymized and study documents will be redacted to protect the privacy of our study participants. Further details on Sanofi's data‐sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.
ACKNOWLEDGEMENT
The registry was sponsored by Sanofi China (Shanghai) (DIREG_L_05728). The authors gratefully acknowledge the participation of the patients and the contribution of the investigators. The participating hospitals were listed in an online supplemental Appendix 1 (http://links.lww.com/HJH/ A634).
Appendix 1.
Participating hospitals of the China ATTEND Registry
The participating hospitals were listed in the alphabetical order of province and hospital, with departments, principal investigators and the number of enrolled patients in the parentheses.
Beijing: Anzhen Hospital (Cardiology, Chang‐Sheng Ma, n = 70; Endocrinology, Yi Zhao, n = 60), Chaoyang Hospital (Endocrinology, Yuan Xu n = 63), Tongren Hospital (Endocrinology, Jin‐Kui Yang, n = 60) and Xuanwu Hospital (Cardiology, Dong Xu, n = 50; Endocrinology, Li Wang, n = 53), Capital Medical University; Peking Union Medical College Hospital (Cardiology, Quan Fang, n = 90; Endocrinology, Xiao‐Ping Xing, n = 54); First Hospital (Cardiology, Jie Jiang, n = 75; Endocrinology, Xiao‐Hui Guo, n = 55) and People’s Hospital (Cardiology, Da‐Yi Hu, n = 70; Endocrinology, Li‐Nong Ji, n = 60), Peking University; Fujian: Fujian Medical University Union Hospital (Cardiology, Liang‐Long Chen, n = 76; Endocrinology, Li‐Bin Liu, n = 60); Guangdong: Guangdong Province People’s Hospital (Cardiology, Hua Yao, n = 76), Guangzhou; Hunan: The Third Xiangya Hospital (Cardiology, Kan Yang, n = 71; Endocrinology, Zhao‐Hui Mo, n = 60) and Xiangya Hospital (Cardiology, Tian‐Lun Yang, n = 71; Endocrinology, Min‐Xiang Lei, n = 51), Central South University, Changsha; Jiangsu: Jiangsu Province People’s Hospital (Cardiology, Ke‐Jiang Cao, n = 50; Endocrinology, Tao Yang, n = 60), Nanjing; General Hospital of Nanjing Military Command (Cardiology, Jian‐Bin Gong, n = 87; Endocrinology, Jian Wang, n = 60), Nanjing; Wuxi People’s Hospital (Cardiology, Zhen‐Yu Yang, n = 70; Endocrinology, Rui‐Fang Bu, n = 70); Jiangxi: The First Affiliated Hospital of Nanchang University (Cardiology, Meng‐Hong Wang, n = 70; Endocrinology, Jian‐Ying Liu, n = 51); Jilin: The First Affiliated Hospital (Cardiology, Yang Zheng, n = 70; Endocrinology, Gui‐Xia Wang, n = 70) and The Second Affiliated Hospital (Endocrinology, Yu Liu, n = 60), Jilin University, Changchun; Liaoning: The People’s Hospital of Liaoning Province (Cardiology, Zhan‐Quan Li, n = 60), Shenyang; Shanghai: Renji Hospital (Endocrinology, Wei Liu, n = 55), Ruijin Hospital (Cardiology, Ji‐Guang Wang, n = 71) and Shanghai First People’s Hospital (Cardiology, Shao‐Wen Liu, n = 77), Shanghai Jiaotong University School of Medicine; Sichuan: Sichuan Province People’s Hospital (Cardiology, Jian‐Hong Tao, n = 3; Endocrinology, Peng‐Qiu Li, n = 60), Chengdu; West China Hospital (Cardiology, Xiao‐Ping Chen, n = 39; Endocrinology, Hao‐Ming Tian, n = 58), Sichuan University, Chengdu; Zhejiang: The First Affiliated Hospital (Endocrinology, Cheng‐Jiang Li, n = 60) and The Second Affiliated Hospital (Cardiology, Jian‐An Wang, n = 84), Zhejiang University, Hangzhou.
Zhang W, Liu C‐Y, Ji L‐N, Wang J‐G; For the ATTEND investigators . Blood pressure and glucose control and the prevalence of albuminuria and left ventricular hypertrophy in patients with hypertension and diabetes. J Clin Hypertens. 2020;22:212–220. 10.1111/jch.13793
Funding Information
The registry was sponsored by Sanofi China (Shanghai) (DIREG_L_05728).
REFERENCES
- 1. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the China hypertension survey, 2012–2015. Circulation. 2018;137(22):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 2. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948‐959. [DOI] [PubMed] [Google Scholar]
- 3. Song J, Sheng CS, Huang QF, et al. Management of hypertension and diabetes mellitus by cardiovascular and endocrine physicians: a China registry. J Hypertens. 2016;34:1648‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Willey JZ, Moon YP, Kahn E, et al. Population attributable risks of hypertension and diabetes for cardiovascular disease and stroke in the northern Manhattan study. J Am Heart Assoc. 2014;3:e001106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mancia G, Grassi G, Zanchetti A. New‐onset diabetes and antihypertensive drugs. J Hypertens. 2006;24:3‐10. [DOI] [PubMed] [Google Scholar]
- 6. Zheng SL, Roddick AJ, Aghar‐Jaffar R, et al. Association between use of sodium‐glucose cotransporter 2 inhibitors, glucagon‐like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with all‐cause mortality in patients with type 2 diabetes. A systematic review and meta‐analysis. JAMA. 2018;319:1580‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UK Prospective Diabetes Study Group . Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703‐713. [PMC free article] [PubMed] [Google Scholar]
- 8. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854‐865. [PubMed] [Google Scholar]
- 9. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837‐853. [PubMed] [Google Scholar]
- 10. ADVANCE Collaborative Group , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 11. The ACCORD Study Group . Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ACCORD Study Group , Gerstein HC, Miller ME, et al. Long‐term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams B, Mancia G, Spiering W, Aboyans V, Desormais I, ESC Scientific Document Group . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018; 39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 14. American Diabetes Association . 10. Cardiovascular disease and risk management: standards of medical care in diabetes ‐ 2019. Diabetes Care. 2019; 42(Suppl 1):S103‐S123. [DOI] [PubMed] [Google Scholar]
- 15. Joint Committee for Guideline Revision . 2018 Chinese guidelines for prevention and treatment of hypertension‐a report of the revision committee of Chinese guidelines for prevention and treatment of hypertension. J Geriatr Cardiol. 2019;16:182‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joint Committee for Guideline Revision . 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fici F, Bakir EA, Beyaz S, Makel W, Robles NR. PAIT‐survey‐prevalence of albuminuria in patients with diabetes and hypertension in Turkey. Prim Care Diabetes. 2018;12:558‐564. [DOI] [PubMed] [Google Scholar]
- 18. Gray A, Clarke P, Farmer A, Holman R, United Kingdom Prospective Diabetes Study (UKPDS) Group . Implementing intensive control of blood glucose concentration and blood pressure in type 2 diabetes in England: cost analysis (UKPDS 63). BMJ. 2002;325:860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ADVANCE Collaborative Group , Patel A, MacMahon S, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the advance trial): a randomised controlled trial. Lancet. 2007;370:829‐840. [DOI] [PubMed] [Google Scholar]
- 20. Poulter NR. Blood pressure and glucose control in subjects with diabetes: new analyses from ADVANCE. J Hypertens. 2009;27(Suppl. 1):S3‐S8. [DOI] [PubMed] [Google Scholar]
- 21. Chen G, McAlister FA, Walker RL, Hemmelgarn BR, Campbell NR. Cardiovascular outcomes in Framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vijan S, Hayward RA. Treatment of hypertension in type 2 diabetes mellitus: blood pressure goals, choice of agents, and setting priorities in diabetes care. Ann Intern Med. 2003;138:593‐602. [DOI] [PubMed] [Google Scholar]
- 23. Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC, Tolentino A. Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;69:1694‐1703. [DOI] [PubMed] [Google Scholar]
- 24. Stenehjem AE, Bjørnerheim R, Os I. From treatment to organ damage; a 5‐year follow‐up study of ambulatory blood pressure in essential hypertension. Diversity between development of left ventricular hypertrophy and urinary albumin excretion. Blood Press. 2007;16:87‐94. [DOI] [PubMed] [Google Scholar]


