Abstract
Hypertension and chronic kidney disease (CKD) are serious interrelated public health problems. Despite the monitoring and control of high blood pressure, symptoms of CKD are not usually apparent in its early stages. Previously, we reported the utility of urinary vanin‐1 as an early biomarker of kidney injury in spontaneously hypertensive rats, but it remains unknown whether urinary vanin‐1 is associated with CKD in humans. In this study, we estimated associations between urinary vanin‐1 and parameters of kidney function in a cross‐sectional study of hypertensive patients. We measured concentrations of vanin‐1 using spot urine from 147 adult hypertensive patients (mean age, 72.8 years; 39.5% women). Patients were divided into 2 groups based on the median of the estimated glomerular filtration rate (eGFR). The group with eGFR < 60 mL/min per 1.73 m2 showed significantly higher levels of urinary vanin‐1 than those with eGFR ≥ 60 mL/min per 1.73 m2. On univariate analysis, urinary vanin‐1 as well as neutrophil gelatinase‐associated lipocalin (NGAL) showed significant negative correlations with eGFR; however, multivariate analysis revealed that urinary vanin‐1, but not NGAL, significantly correlated with eGFR. In addition, urinary vanin‐1 had a significant positive correlation with the urinary protein‐to‐creatinine ratio (UPCR) (r = 0.21; P = .021) and albumin‐to‐creatinine ratio (UACR) (r = 0.61; P < .01). In conclusion, urinary vanin‐1 is associated with lower eGFR and higher UPCR and UACR, and might be a potential marker of decreased kidney function in hypertensive patients. Further studies are needed to confirm these findings.
Keywords: biomarker, chronic kidney disease, hypertension
1. BACKGROUND
Hypertension and chronic kidney disease (CKD) are serious interrelated public health problems. 1 , 2 Hypertension causes cardio‐ and cerebrovascular disease as well as kidney disease 3 ; however, CKD also contributes to cardiovascular 4 , 5 and cerebrovascular 6 events and all‐cause mortality itself. 7 The symptoms of CKD are usually not apparent in the early stages, so most CKD patients are unaware of their condition. Early detection and appropriate intervention during the initial stages of CKD are necessary for the prevention of a further increase in the number of patients with end‐stage kidney disease (ESKD). The identification of novel biomarkers that enable screening for asymptomatic kidney disease or predict a decline in the estimated glomerular filtration rate (eGFR) is the primary aim of current CKD research. 8
To date, several biomarkers have been identified: kidney injury molecule‐1 (KIM‐1) and neutrophil gelatinase–associated lipocalin (NGAL). 9 KIM‐1 is a type I transmembrane glycoprotein, which is primarily expressed on the surface of T cells. 10 NGAL is a ubiquitous lipocalin‐carrying protein, highly expressed in the tubular epithelium, and released from tubular epithelial cells following damage. Previously, we reported that urinary vanin‐1 was a potential renal biomarker. Vanin‐1 is glycosylphosphatidylinositol (GPI)‐anchored pantetheinase, 11 , 12 and antioxidant response‐like elements within the promotor region of vanin‐1 (VNN1) act as stress‐regulated targets and enhance VNN1 expression in the presence of oxidative stress. We detected urinary vanin‐1 in an early stage of CKD using spontaneously hypertensive rats. However, it remains unknown whether vanin‐1 can be used as a marker of CKD in clinical studies. The aim of this study was to determine whether urinary vanin‐1 can be used a biomarker of CKD in hypertensive patients.
2. METHODS
2.1. Study population
This cross‐sectional pilot study was approved by the Ethics Committee of Osaka University of Pharmaceutical Sciences (No. 0035) and was conducted in accordance with the Declaration of Helsinki. Adult hypertensive patients were recruited from Kenwakai Hospital (Nagano, Japan) between 2017 and 2019. Eligible participants had a blood pressure (BP) of at least 140 mmHg systolic or 90 mmHg diastolic or used antihypertensive drugs. Major exclusion criteria included diabetes mellitus (DM), history of congestive heart failure, arterial fibrillation, angina, cardiovascular events in the past 6 months, polycystic kidney disease, immunoglobulin A (IgA) nephropathy, diabetic nephropathy, eGFR < 30 mL/min per 1.73 m2, dialysis, or transplantation. The reason that we excluded patients with glomerular injuries such as IgA nephropathy and diabetic nephropathy from our study was that urinary vanin‐1 reflects mainly tubular injury. In total, 147 patients participated after providing written informed consent (Figure 1).
FIGURE 1.
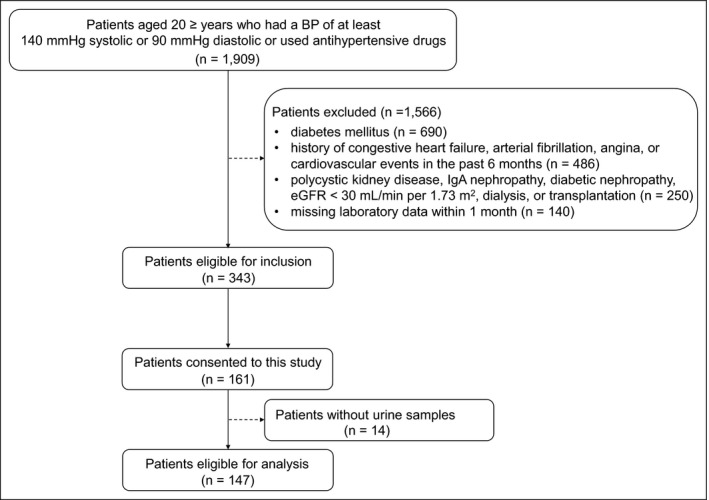
Flow chart describing the identification of the study cohort. BP, blood pressure; eGFR, estimated glomerular filtration rate; IgA, immunoglobulin A
2.2. Urine collection and biomarker analysis
Spot urine samples were collected from each participant and stored at –80°C until analysis. To determine the urinary protein‐to‐creatinine ratio (UPCR) and albumin‐to‐creatinine ratio (UACR), urinary concentrations of protein and albumin were measured in a central laboratory. Urinary and serum creatinine (Cr) were measured in a central laboratory by an enzymatic method. The biomarkers in the urine were measured using commercially available ELISA kits (vanin‐1, Cloud‐Clone Corp., Houston, TX, USA; KIM‐1 and NGAL, R&D Systems, Minneapolis, MN, USA), according to the manufacturers’ instructions. These values were normalized to the urinary Cr value. Other clinical data including eGFR were also collected from the clinical records. The equation calculates the eGFR from serum Cr, age, and sex using the following formula: (eGFR [mL/min/1.73 m2] = 194 × age−0.287 × serum Cr−1.094 × [0.739 for women]). 13 Analysis of the kidney outcome was based on the clinical definition (Kidney Disease Improving Global Outcomes, KDIGO, 2012). 14
2.3. Other measurements
Nurses were administered a questionnaire to collect detailed information on each participant’s medical history, smoking and drinking habits, and intake of medications. Height, weight, and BP were recorded by nurses. The body mass index (BMI) was the weight in kilograms divided by the square of the height in meters. DM was defined as a self‐reported diagnosis, a fasting glucose level of at least 126 mg/dL, or the use of antidiabetic agents.
2.4. Statistical analysis
Continuous variables are expressed as means ± SD or medians (interquartile ranges) and were analyzed using the Mann‐Whitney U test. Categorical variables are expressed as proportions and were compared using chi‐squared tests. Correlations between variables were determined by Spearman’s rank method. Multivariate analysis was performed to identify independent determinants of eGFR. P < .05 was considered significant. All statistical analyses were performed using SPSS for Windows software (ver. 19.0; SPSS Inc., Tokyo, Japan).
3. RESULTS
3.1. Patients’ characteristics
A total of 147 Japanese hypertensive patients were retrospectively reviewed. The mean age was 72.8 years, and 39.5% were women. The median (interquartile range) eGFR was 60.2 (42.3‐70.9) mL/min per 1.73 m2, indicating that approximately 50% of the participants had CKD. Correspondingly, 42.2% of patients with UACR ≥ 30 mg/g Cr, which is a possible variable contributing CKD. Based on the median eGFR, study participants were categorized into two groups: eGFR < 60 mL/min per 1.73 m2 and ≥ 60 mL/min per 1.73 m2. The lower eGFR (<60 mL/min per 1.73 m2) was significantly associated with decrease in hemoglobin (P = .019) and increases in HbA1c (P = .038), B‐type natriuretic peptide (BNP) (P = .03), proportion of patients with UACR ≥ 30 mg/g Cr (P = .002), and urinary vanin‐1 (P = .005) (Table 1).
TABLE 1.
Characteristics of participants by eGFR values
| Variable | Total (n = 147) | eGFR (mL/min per 1.73 m2) | ||
|---|---|---|---|---|
| <60 (n = 73) | ≥60 (n = 74) | P‐value | ||
| Age, years | 72.8 ± 8.3 | 73.0 ± 9.3 | 72.8 ± 7.3 | .88 |
| Women, n (%) | 58 (39.5) | 26 (35.6) | 32 (43.2) | .34 |
| Current smoker, n (%) | 26 (17.7) | 11 (15.1) | 15 (20.2) | .41 |
| Current drinker, n (%) | 74 (50.3) | 34 (46.6) | 40 (54.1) | .37 |
| BMI, kg/m2 | 24.2 ± 3.5 | 24.7 ± 3.8 | 23.7 ± 3.2 | .11 |
| SBP, mmHg | 133.1 ± 14.2 | 133.0 ± 14.6 | 133.2 ± 13.9 | .89 |
| DBP, mmHg | 74.2 ± 11.0 | 74.5 ± 12.6 | 74.0 ± 9.2 | .80 |
| Plasma glucose, mg/dL | 130.6 ± 32.9 | 136.6 ± 40.0 | 126.0 ± 25.6 | .09 |
| Hemoglobin, g/dL | 13.3 ± 1.5 | 12.9 ± 1.7 | 13.6 ± 1.2 | .019 |
| HbA1c, % | 6.0 ± 0.6 | 6.2 ± 0.8 | 5.9 ± 0.4 | .038 |
| LDL cholesterol, mg/dL | 104.0 ± 23.6 | 104.5 ± 24.5 | 103.5 ± 22.7 | .80 |
| HDL cholesterol, mg/dL | 60.7 ± 16.2 | 58.3 ± 14.2 | 63.1 ± 17.8 | .08 |
| LDL/HDL | 1.8 ± 0.6 | 1.9 ± 0.6 | 1.7 ± 0.6 | .23 |
| Triglycerides, mg/dL | 155.6 ± 109.2 | 165.4 ± 135.2 | 146.0 ± 75.2 | 0.28 |
| Estimated 24‐h urinary Na excretion, mEq/d | 164.9 ± 43.0 | 166.3 ± 45.3 | 164.0 ± 41.6 | .77 |
| BNP, pg/mL | 29.1 (16.4‐51.9) | 34.3 (17.5‐62.8) | 27.7 (13.1‐45.3) | .03 |
| UACR ≥ 30 mg/g Cr, n (%) | 62 (42.2) | 40 (54.8) | 22 (29.7) | .002 |
| Urinary vanin‐1, ng/mg Cr | 2.0 ± 3.9 | 2.9 ± 4.9 | 1.1 ± 1.9 | .005 |
| Urinary NGAL, ng/mg Cr | 13.0 ± 12.0 | 13.9 ± 13.0 | 12.2 ± 10.9 | .38 |
| Urinary KIM‐1, ng/mg Cr | 0.9 ± 1.3 | 1.0 ± 1.3 | 0.9 ± 1.3 | .45 |
All results are presented as mean ± SD, median (interquartile range), or n (%).
Abbreviations: BMI, body mass index; BNP, B‐type natriuretic peptide; Cr, creatinine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; KIM‐1, kidney injury molecule‐1; LDL, low‐density lipoprotein; NGAL, neutrophil gelatinase‐associated lipocalin; SBP, systolic blood pressure; UACR, urinary albumin‐to‐creatinine ratio.
3.2. Associations of BP with urinary biomarkers
Figure 2 shows correlation coefficients of urinary biomarkers with BP. In patients with lower eGFR (<60 mL/min per 1.73 m2), urinary vanin‐1 significantly correlated with systolic BP (SBP) (r = 0.33; P = .0039) and diastolic BP (DBP) (r = 0.24; P = .044); whereas urinary KIM‐1 showed significant correlation with SBP (r = 0.29; P = .012), but not DBP (r = 0.081; P = .48), and there were no significant correlations of urinary NGAL with SBP (r = 0.16; P = .19) and DBP (r = 0.090; P = .44). On the other hand, in patients with higher eGFR (≥60 mL/min per 1.73 m2), no significant correlations were found between urinary biomarkers and BP. Of note, urinary vanin‐1 and KIM‐1 tended to show at higher levels in patients with higher BP in patients with lower eGFR (<60 mL/min per 1.73 m2).
FIGURE 2.
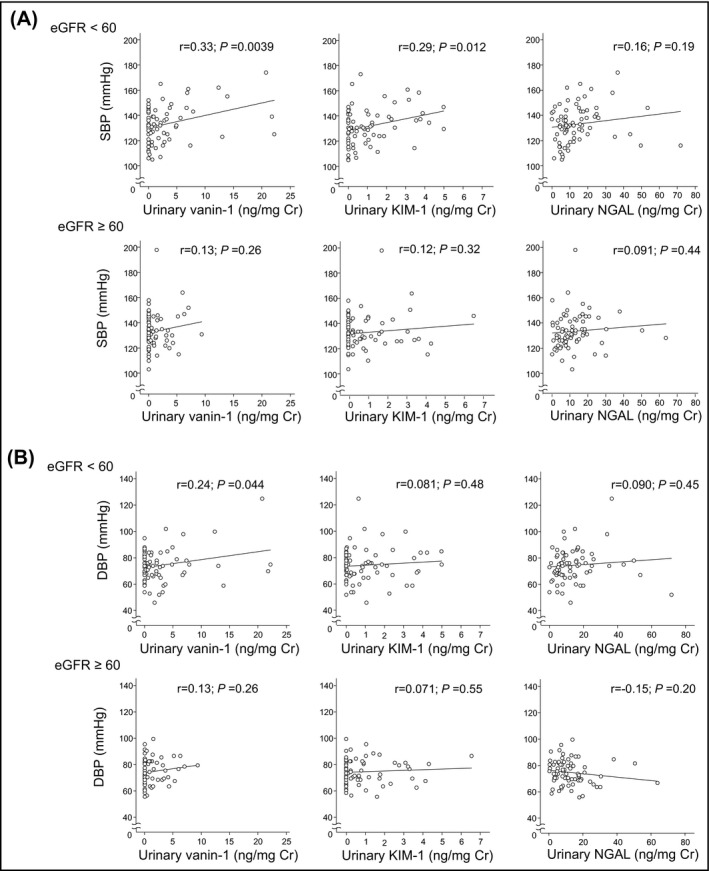
Scatterplot illustrating the relationship of SBP (A) and DBP (B) with urinary vanin‐1, KIM‐1, and NGAL, stratified with eGFR value (<60 mL/min per 1.73 m2; ≥60 mL/min per 1.73 m2). Cr, creatinine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; KIM‐1, kidney injury molecule‐1; NGAL, neutrophil gelatinase‐associated lipocalin; SBP, systolic blood pressure
3.3. Associations of eGFR with clinical parameters including urinary biomarkers
We investigated associations between eGFR and each variable in hypertensive patients using univariate analysis (Table 2). BMI tended to show negative correlation with eGFR (P = .08), and plasma glucose (r = −0.23; P = .01), hemoglobin (r = 0.34; P < .001), HbA1c (r = −0.30; P = .001), BNP (r = −0.34; P < .001), urinary vanin‐1 (r = −0.39; P < .001), and urinary NGAL (r = −0.21; P = .01) showed significant correlations with eGFR.
TABLE 2.
Univariate analysis of relationship between eGFR and variables in hypertensive patients
| Variable | r | P‐value |
|---|---|---|
| Age, years | −0.02 | .85 |
| BMI, kg/m2 | −0.15 | .08 |
| SBP, mmHg | −0.04 | .64 |
| DBP, mmHg | 0.02 | .85 |
| Plasma glucose, mg/dL | −0.23 | .01 |
| Hemoglobin, g/dL | 0.34 | <.001 |
| HbA1c, % | −0.30 | .001 |
| LDL cholesterol, mg/dL | −0.01 | .89 |
| HDL cholesterol, mg/dL | 0.12 | .16 |
| LDL/HDL | −0.06 | .48 |
| Triglyceride, mg/dL | −0.06 | .49 |
| BNP, pg/mL | −0.34 | <.001 |
| Urinary vanin‐1, ng/mg Cr | −0.39 | <.001 |
| Urinary NGAL, ng/ mg Cr | −0.21 | .01 |
| Urinary KIM‐1, ng/mg Cr | −0.05 | .55 |
Abbreviations: BMI, body mass index; BNP, B‐type natriuretic peptide; Cr, creatinine; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; KIM‐1, kidney injury molecule‐1; LDL, low‐density lipoprotein; NGAL, neutrophil gelatinase‐associated lipocalin;SBP, systolic blood pressure.
Next, we conducted multivariate analysis (Table 3). We selected the following variables: (a) The results of univariate analysis (plasma glucose, hemoglobin, HbA1c, BNP, urinary vanin‐1, and urinary NGAL) (Table 2); (b) variables related with BP when eGFR was lower (<60 mL/min per 1.73 m2) (urinary vanin‐1 and urinary KIM‐1) (Figure 2); and (c) potential variables related to the decrease in eGFR (smoker, BMI, and low‐density lipoprotein (LDL)/high‐density lipoprotein (HDL)). Multivariate analysis showed that BMI, BNP, and urinary vanin‐1 were independently associated with eGFR (Table 3).
TABLE 3.
Multivariate analysis of relationship between eGFR and novel renal biomarkers
| Variable | β | P‐value |
|---|---|---|
| BMI, kg/m2 | −0.18 | .041 |
| BNP, pg/mL | −0.31 | <.001 |
| Urinary vanin‐1, ng/mg Cr | −0.26 | .003 |
| R 2 | 0.23 | <.001 |
β indicates partial coefficient. The variables considered for entry into the models included BMI, smoker, LDL/HDL, plasma glucose, BNP, urinary vanin‐1, urinary NGAL, and urinary KIM‐1.
Abbreviations: BMI, body mass index; BNP, B‐type natriuretic peptide; Cr, creatinine; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
3.4. Associations of UPCR or UACR with urinary biomarkers
Figure 3 shows scatter plots of novel biomarkers with UPCR and UACR. Unlike UPCR, UACR was significantly correlated with each biomarker. Of note, urinary vanin‐1 was significantly correlated with UPCR (r = 0.21; P = .021) and UACR (r = 0.61; P < .01) and showed the highest correlation coefficient with UACR among novel biomarkers. Urinary KIM‐1 also showed significant correlation with UACR (r = 0.26; P < .01) and UPCR (r = 0.24; P < .01), whereas urinary NGAL showed a significant correlation with UACR (r = 0.23; P < .01), but not UPCR (r = 0.03; P = .75). Interestingly, significantly higher levels of urinary vanin‐1 were detected in patients with UACR ≥ 30 mg/g Cr and eGFR < 60 mL/min per 1.73 m2 (Figure S1).
FIGURE 3.
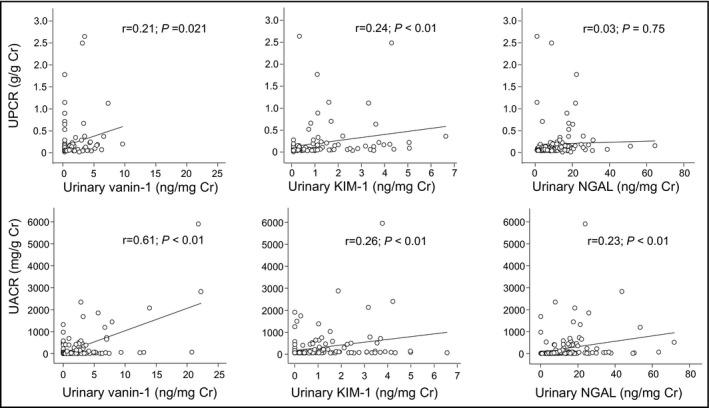
Scatter plot illustrating the relationship of UPCR (A) (n = 119) and UACR (B) (n = 146) with urinary vanin‐1, KIM‐1, and NGAL. Cr, creatinine; KIM‐1, kidney injury molecule‐1; NGAL, neutrophil gelatinase‐associated lipocalin; UACR, albumin‐to‐creatinine ratio; UPCR, urinary protein‐to‐creatinine ratio
4. DISCUSSION
In the present study, we demonstrated that elevation of the urinary vanin‐1 level was significantly correlated with lower eGFR in hypertensive patients treated with conventional therapy. In addition, after adjusting for each risk factor for CKD, urinary vanin‐1 was independently associated with eGFR. To the best of our knowledge, this is the first study to demonstrate that urinary vanin‐1 is a potential biomarker of CKD in humans.
CKD progression is characterized as glomerular as well as tubular injury. Especially, tubular injury leads to progressive tubulointerstitial fibrosis, which is a common pathway to ESKD. 15 Then, the detection of tubular injury could be more effective in therapeutic intervention using its biomarkers. Several studies reported urinary tubular markers (particularly NGAL and KIM‐1) as potential risk factors for CKD; however, the results were controversial. For example, a recent cross‐sectional study reported that higher levels of urinary KIM‐1 were associated with lower eGFR and greater albuminuria in patients with CKD. 16 On the other hand, a case‐control study showed that urinary KIM‐1 was associated with ESKD in univariate analysis, but there was no independent association with ESKD in multivariate analysis. 17 These findings are consistent with our results. As for NGAL, a cross‐sectional study demonstrated that urinary NGAL levels were correlated with the levels of eGFR 18 or severity of underlying renal parenchyma injury; 19 however, a matched case‐control study reported that urinary NGAL showed no significant correlation with ESKD in participants. 17 In our study, urinary NGAL was associated with eGFR in uni‐ but not multivariate analysis, although it was significantly correlated with UACR. On the other hand, urinary vanin‐1 was significantly correlated with eGFR in multivariate analysis, and it was best correlated with UACR compared with urinary KIM‐1 or NGAL. Thus, there is a greater merit of measuring vanin‐1 than NGAL and KIM‐1, even though they are established markers to assess renal injury.
Furthermore, we noted significantly higher levels of urinary vanin‐1 in patients with UACR ≥ 30 mg/g Cr and eGFR < 60 mL/min/1.73 m2 (Figure S1). The presence of albuminuria may be a marker of both alterations in glomerular permeability 20 and impaired proximal tubular uptake of filtered albumin which may be directly toxic to tubular cells. 21 Of course, the primary insult is glomerular, leading to injury of the proximal tubules due to exposure to abnormally filtered albumin. The damage to the proximal tubules then leads to a positive feedback mechanism resulting in interstitial fibrosis, vascular rarefaction, glomerular ischemia, and worsening of glomerulosclerosis. 22 Then, it is possible that kidney functions with high levels of urinary vanin‐1 and UACR despite eGFR ≥ 60 will decline in the future. We are following up the present patients to examine this possibility.
In this study, some patients showed lower eGFR in spite of SBP and DBP values within normal ranges, possibly because the patients received antihypertensive treatment. However, vanin‐1 was detected at higher levels in urine samples derived from patients with higher BP in patients whose eGFR was lower (<60 mL/min per 1.73 m2). On the other hand, patients with higher eGFR (≥60 mL/min per 1.73 m2) showed no significant correlation between urinary biomarkers and BP. Therefore, urinary vanin‐1 is a candidate of markers for the prediction of CKD as a result of hypertension.
Our cross‐sectional study could not provide information on whether vanin‐1 increased only under continuous renal dysfunction. However, our previous animal study 23 showed that urinary vanin‐1 was elevated and reached a plateau in spontaneously hypertensive rats with elevated BP and continuous renal dysfunction during high‐salt loading. If possible in the future, a prospective study will be conducted to examine whether a temporal change in the vanin‐1 level is associated with CKD progression.
Our observation of a cardiovascular marker, BNP, also reflected lower eGFR despite the exclusion of patients with a history of congestive heart failure, arterial fibrillation, angina, or cardiovascular events in the past 6 months. Individuals with CKD exhibit marked variation in the cardiovascular disease risk. 24 The decrease in kidney function leads to cardiovascular events, so the population in this study could have a latent risk of progression to heart disease. Furthermore, we found that levels of plasma glucose as well as HbA1c were higher in the lower eGFR group, and this group showed a higher urinary vanin‐1 level than the other group. This suggests that they show impaired glucose tolerance even if DM patients are excluded, and urinary vanin‐1 may be a better marker for the early phase or potential diabetic kidney disease. Further studies are needed to clarify this.
This study had some limitations. First, the study population was small. Second, this study was limited to non‐dialysis patients. Third, normal individuals were not recruited in this study, so we were unable to determine urinary vanin‐1 in these patients; however, our previous study revealed that urinary vanin‐1 was not detected in nondiabetic and diabetic patients without albuminuria. 25 Finally, the results of a cross‐sectional study also cannot be used to infer causality, so prospective studies are warranted.
In conclusion, the present study demonstrated that urinary vanin‐1 is closely and independently associated with CKD in hypertensive patients. Further studies are needed with larger populations to confirm our findings and to assess the utility of urinary vanin‐1 as a marker of CKD.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
KH supervised the conduct of the study and data analysis and had the primary responsibility of writing the manuscript. HM and KI conducted data interpretation and reviewed/edited the manuscript. EK supervised the conduct of the study, collected the data, and conducted data analysis and data interpretation.
Supporting information
Fig S1
Supplementary Material
Hosohata K, Matsuoka H, Iwanaga K, Kumagai E. Urinary vanin‐1 associated with chronic kidney disease in hypertensive patients: A pilot study. J Clin Hypertens. 2020;22:1458–1465. 10.1111/jch.13959
REFERENCES
- 1. Robinson BM, Tong L, Zhang J, et al. Blood pressure levels and mortality risk among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2012;82(5):570‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boggia J, Silvarino R, Luzardo L, Noboa O. Significance of white‐coat and masked hypertension in chronic kidney disease and end‐stage renal disease. Hypertens Res. 2014;37(10):882‐889. [DOI] [PubMed] [Google Scholar]
- 3. Kario K. Evidence and perspectives on the 24‐hour management of hypertension: Hemodynamic biomarker‐initiated 'anticipation medicine' for zero cardiovascular event. Prog Cardiovas Dis. 2016;59(3):262‐281. [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296‐1305. [DOI] [PubMed] [Google Scholar]
- 5. Alcober‐Morte L, Barrio‐Ruiz C, Parellada‐Esquius N, et al. Heart failure admission across glomerular filtration rate categories in a community cohort of 125,053 individuals over 60 years of age. Hypertens Res. 2019;42(12):2013‐2020. [DOI] [PubMed] [Google Scholar]
- 6. Kajitani N, Uchida HA, Suminoe I, et al. Chronic kidney disease is associated with carotid atherosclerosis and symptomatic ischaemic stroke. J Int Med Res. 2018;46(9):3873‐3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nakayama M, Sato T, Miyazaki M, et al. Increased risk of cardiovascular events and mortality among non‐diabetic chronic kidney disease patients with hypertensive nephropathy: The gonryo study. Hypertens Res. 2011;34(10):1106‐1110. [DOI] [PubMed] [Google Scholar]
- 8. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the united states. JAMA. 2007;298(17):2038‐2047. [DOI] [PubMed] [Google Scholar]
- 9. Fassett RG, Venuthurupalli SK, Gobe GC, Coombes JS, Cooper MA, Hoy WE. Biomarkers in chronic kidney disease: A review. Kidney Int. 2011;80(8):806‐821. [DOI] [PubMed] [Google Scholar]
- 10. Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule‐1 (kim‐1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up‐regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135‐4142. [DOI] [PubMed] [Google Scholar]
- 11. Aurrand‐Lions M, Galland F, Bazin H, Zakharyev VM, Imhof BA, Naquet P. Vanin‐1, a novel gpi‐linked perivascular molecule involved in thymus homing. Immunity. 1996;5(5):391‐405. [DOI] [PubMed] [Google Scholar]
- 12. Pitari G, Malergue F, Martin F, et al. Pantetheinase activity of membrane‐bound vanin‐1: Lack of free cysteamine in tissues of vanin‐1 deficient mice. FEBS Lett. 2000;483(2‐3):149‐154. [DOI] [PubMed] [Google Scholar]
- 13. Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated gfr from serum creatinine in japan. Am J Kidney Dis. 2009;53(6):982‐992. [DOI] [PubMed] [Google Scholar]
- 14. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease . Chapter 3: Management of progression and complications of ckd. Kidney Int Suppl. 2013;3(1):73‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grgic I, Campanholle G, Bijol V, et al. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82(2):172‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waikar SS, Sabbisetti V, Arnlov J, et al. Chronic Kidney Disease Biomarkers Consortium I. Relationship of proximal tubular injury to chronic kidney disease as assessed by urinary kidney injury molecule‐1 in five cohort studies. Nephrol Dialysis Transpl. 2016;31(9):1460‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foster MC, Coresh J, Bonventre JV, et al. Urinary Biomarkers and Risk of ESRD in the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol. 2015;10(11):1956‐1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bolignano D, Coppolino G, Campo S, et al. Neutrophil gelatinase‐associated lipocalin in patients with autosomal‐dominant polycystic kidney disease. Am J Nephrol. 2007;27(4):373‐378. [DOI] [PubMed] [Google Scholar]
- 19. Nickolas TL, Forster CS, Sise ME, et al. Ngal (lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82(6):718‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol. 1992;263(4 Pt 2):F601‐606. [DOI] [PubMed] [Google Scholar]
- 21. Sandoval RM, Wagner MC, Patel M, et al. Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol. 2012;23(3):447‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: Retrieval is disrupted in nephrotic states. Kidney Int. 2007;71(6):504‐513. [DOI] [PubMed] [Google Scholar]
- 23. Hosohata K, Yoshioka D, Tanaka A, Ando H, Fujimura A. Early urinary biomarkers for renal tubular damage in spontaneously hypertensive rats on a high salt intake. Hypertens Res. 2016;39(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 24. Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the american heart association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003;108(17):2154‐2169. [DOI] [PubMed] [Google Scholar]
- 25. Hosohata K, Ando H, Takeshita Y, et al. Urinary kim‐1 is a sensitive biomarker for the early stage of diabetic nephropathy in otsuka long‐evans tokushima fatty rats. Diabet Vascular Dis Res. 2014;11(4):243‐250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Supplementary Material


