Abstract
This multicenter, randomized, double‐blind, parallel‐group phase III clinical trial aimed to investigate the efficacy and safety of a rosuvastatin + amlodipine combination compared with that of rosuvastatin or amlodipine monotherapy in hypertensive patients with dyslipidemia. A total of 106 patients of 15 institutions in Korea were randomly assigned to 1 of 3 treatment groups: rosuvastatin 20 mg + amlodipine 10 mg, amlodipine 10 mg, or rosuvastatin 20 mg. After 8 weeks of treatment, the mean ± SD of change in mean sitting systolic blood pressure (msSBP) was −22.82 ± 12.99 mm Hg in the rosuvastatin + amlodipine group, the most decreased among the treatment groups. The percentage of patients whose msSBP decreased ≥20 mm Hg or msDBP decreased ≥10 mm Hg was also highest in this group (74.29%). The mean ± SD percentage change in low‐density lipoprotein cholesterol (LDL‐C) level from baseline after 8 weeks was −52.53% ± 11.21% in the rosuvastatin + amlodipine group, the most decreased among the treatment groups. More patients in the rosuvastatin + amlodipine group achieved their target LDL‐C goal at 8 weeks, compared with the other treatment groups (97.14%). No serious adverse events or adverse drug reactions were observed in all groups. In hypertensive patients with dyslipidemia, combination treatment with rosuvastatin 20 mg + amlodipine 10 mg effectively reduced blood pressure and LDL‐C levels while maintaining safety.
Keywords: amlodipine, dyslipidemia, hypertension, rosuvastatin, single‐pill combination
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death and one of the major public health issues affecting approximately 200 million individuals worldwide.1, 2 The global cost of CVD is expected to increase to $1044 billion by 2030.3 To reduce the global burden of CVD and improve public health, it is important to screen and manage the modifiable risk factors of CVD. Hypertension and dyslipidemia are major risk factors for the development of CVD such as coronary artery disease (CHD).4, 5, 6 These two diseases frequently coexist. More than 60% of patients with hypertension have dyslipidemia, and inversely, about 50% of patients with dyslipidemia have hypertension.7 In the United States, the prevalence of combined hypertension and dyslipidemia has been reported to be approximately 18%.8 Patients with both hypertension and dyslipidemia had more than a 2‐fold increase in CHD prevalence compared to patients with either disease alone. A similar trend was also observed for stroke and peripheral artery disease.9, 10
Despite the clinical significance of hypertension and dyslipidemia, the global prevalence of each disease still remains high5 and is expected to increase continuously as the prevalence increases with age.11 Although lipid‐lowering agents and antihypertensive agents are effective treatment methods to achieve the target levels, the rate of well‐controlled patients is still low.12, 13, 14 In one study, only 9% of American hypertensive patients with dyslipidemia achieved the target levels for BP and lipid profile.8 This suboptimal situation may be partially due to poor compliance.15, 16 Since most patients require long‐term administration and are asymptomatic for long periods of time until complications from both diseases occur, low compliance is common in patients with both diseases. In addition, polypharmacy and complexity of treatment regimen are factors related to decreased compliance.17 Low compliance leads to increased CVD risk and mortality, resulting in an annual global economic burden of $3.96 billion to $792 million.16, 18, 19
Previous studies have shown that integrated management of blood pressure (BP) and lipid levels together tends to decrease the incidence of CVD‐related events rather than separated management.18, 20 Based on these results, the importance of multifactorial intervention with respect to CVD risk factors has been emphasized in clinical practice.21, 22, 23
Based on these previous studies, the efficacy and safety of a single‐pill combination of atorvastatin and amlodipine have been demonstrated in hypertensive patients with dyslipidemia,17, 24, 25, 26 and they are commercially available in the real clinical world. However, single‐pill combination consisting of rosuvastatin, one of the most potent statins in patients with dyslipidemia, has never been studied. In this study, we aimed to investigate the efficacy and safety of the rosuvastatin + amlodipine combination.
2. METHODS
2.1. Study population
This study was conducted in patients with hypertension and dyslipidemia over the age of 19 years who met the following criteria: (a) mean sitting systolic BP (msSBP) <180 mm Hg; (b) mean sitting diastolic BP (msDBP) <110 mm Hg; (c) low‐density lipoprotein cholesterol (LDL‐C) ≤250 mg/dL; (d) triglyceride <400 mg/dL; and (e) voluntary written consent for participation in this clinical trial. After screening, patients who fulfilled the criteria of adherence to medication ≥80% in the wash‐out/run‐in period, triglyceride <400 mg/dL, 140 mm Hg ≤ msSBP < 180 mm Hg, msDBP <110 mm Hg, and required treatment according to the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines were finally enrolled in the study and randomly assigned.
Patients who had differences in BP on both arms (msSBP ≥ 20 mm Hg or msDBP ≥ 10 mm Hg) during screening were excluded. Patients with the following history or laboratory abnormalities were also excluded: (a) history of hypersensitivity reaction to study drugs; (b) history of unstable angina, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass surgery within 24 weeks of screening; (c) history of transient ischemic attack, cerebrovascular disease within 24 weeks of screening; (d) history of severe ocular diseases such as retinal hemorrhage, visual disturbances within 24 weeks of screening; (e) rhabdomyolysis or myopathy; (f) history of autoimmune diseases such as rheumatoid arthritis or systemic lupus erythematosus; (g) history of alcohol or drug abuse; (h) history of major psychiatric illnesses such as schizophrenia, major depressive disorder, and bipolar disorder; (i) history of gastrointestinal surgery except for appendectomy or herniorrhaphy, or gastrointestinal diseases such as Crohn's disease, acute, or chronic pancreatitis that may affect the absorption of study drugs; (j) history of malignancy within the last 5 years; (k) history of galactose intolerance, Lapp lactose deficiency, or glucose‐galactose malabsorption; (l) hypertrophic obstructive cardiomyopathy, severe obstructive coronary artery disease, or hemodynamically significant aortic or mitral stenosis; (m) orthostatic hypotension with symptoms; (n) ventricular tachycardia, atrial fibrillation, atrial flutter, or arrhythmia that the investigator determined to be clinically meaningful; (o) diseases causing secondary hypertension such as coarctation of the aorta, hyperaldosteronemia, renal artery stenosis, Cushing's syndrome, pheochromocytoma, or polycystic kidney disease; (p) thyroid dysfunction (thyroid‐stimulating hormone ≥1.5 times the upper limit of normal); (q) uncontrolled diabetes mellitus (HbA1C ≥ 9%); (r) severe heart failure (NYHA III/IV); (s) renal insufficiency (CrCl < 30 mL/min); (t) aspartate aminotransferase or alanine aminotransferase levels ≥2 times the upper limit of normal; (u) creatinine phosphokinase ≥2 times the upper limit of normal; (v) all chronic inflammatory diseases requiring anti‐inflammatory therapy; or (w) HIV‐positive. In addition, patients who were expected to require drugs that may affect the study, patients who had participated in other clinical trials within 12 weeks prior to screening or within five times the half‐life of the study drug, and women who were pregnant or breastfeeding were excluded.
2.2. Study design
This multicenter, randomized, double‐blind, parallel‐group phase III clinical trial was conducted at 15 institutions in Korea from May 2017 to January 2018. Patients who met the inclusion/exclusion criteria were instructed to make therapeutic lifestyle changes for at least 4 weeks from the screening. The placebo run‐in period was 4 weeks in parallel with therapeutic lifestyle changes. After a wash‐out/run‐in period, patients eligible for randomization were finally enrolled and randomly assigned to 1 of 3 treatment groups: single‐pill combination of rosuvastatin (20 mg) plus amlodipine (10 mg), amlodipine (10 mg), and rosuvastatin (20 mg). Randomization was performed in a 1:1:1 ratio by using SAS version 9.4 (SAS Institute Inc) with the block randomization method. All patients were given three tablets consisting of one tablet for the test medicine and two tablets for placebos. During the placebo run‐in period, patients were orally administered a placebo for run‐in once a day at the same time (morning) as far as possible. During the 8‐week treatment period, the assigned drugs were administered once a day at the same time (morning) if possible. A double‐dummy technique was used to maintain a double‐blind study. All patients were asked to visit the institution at 4 and 8 weeks after randomization to assess the efficacy and safety and were also educated to maintain adherence to medication of 80% or more at each visit during the study.
This study complied with the Declaration of Helsinki and Good Clinical Practice Guideline defined by the International Council for Harmonization (ICH). It was approved by the Korea Food and Drug Administration and the institutional review board of each participating institution.
2.3. Efficacy and safety assessment
The primary end point was to investigate the efficacy of combination therapy with rosuvastatin and amlodipine in hypertensive patients with dyslipidemia via changes in msSBP and percentage changes in LDL‐C from baseline after 8 weeks of treatment, compared with each individual therapy. The secondary end points were the percentage change in LDL‐C from baseline after 4 weeks of treatment; the percentage change in total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), Apo B, Apo A‐I, high‐sensitivity C‐reactive protein (hsCRP) from baseline after 4 and 8 weeks of treatment; the change in msDBP from baseline after 4 and 8 weeks of treatment; and the percentage of patients who achieve target goal in LDL‐C and blood pressure (BP) after 8 weeks of treatment. We also compared the BP response rate defined as the percentage of patients whose msSBP decreased ≥20 mm Hg or msDBP ≥10 mm Hg after 8 weeks of treatment.
Patients were instructed to visit the institution of the study at 4 and 8 weeks after randomization to perform laboratory tests and BP measurements. During the study, the patients were instructed to measure BP daily. We assessed the safety by collecting the records of adverse events (AEs) and checking vital signs, laboratory tests, electrocardiograms, and physical examination results at each visit. The treatment‐emergent adverse events (TEAEs), adverse drug reactions (ADRs), and serious adverse events (SAEs) were compared among the treatment groups. In addition, the incidence of myopathies and the proportion of patients who had serum aspartate or alanine aminotransferase levels twice consecutively ≥3× the upper limit of normal were compared.
An AE was defined as any harmful and unintended sign, symptom, or disease that occurred in the patient, regardless of whether it was related to the study drug. TEAEs were defined as: (a) AEs that occurred after the first administration of the study drug; and (b) symptoms that occurred prior to the first administration of the study drug, with severity worse after the first administration of the study drug. ADRs were defined as all harmful and unintended reactions that occur with any dose of the study drug, which can be suspected to be causally related to the drug. SAEs were defined as any of the following AEs occurring at any dose of the study drug: (a) AE that resulted in death or life‐threatening condition; (b) AE that required the patient to be admitted or need to extend the length of hospitalization; (c) AE that lead to permanent or significant disability; and (d) other cases of medically important situations such as drug dependence, abuse, or blood diseases.
2.4. Statistical analysis
Continuous data were presented as mean ± standard deviation (SD), and categorical data were presented as number with percentages. When comparisons were made between groups, we stratified patients into groups I, II, and III, and performed analyses using the Cochran‐Mantel‐Haenszel (CMH) test. The CMH test results provided odds ratios, corresponding 95% confidence intervals, and bilateral P‐values. Efficacy was analyzed using an analysis of covariance (ANCOVA) model with the treatment group as the effect and the relevant baseline measurements as the covariate. The ANCOVA model results showed least‐square mean (LSM), standard deviation, LSM difference between treatment group (test group vs control group), corresponding 95% confidence interval, and bilateral P‐value. To demonstrate the difference between the groups by ANCOVA, the hypothesis of superiority was confirmed through a 95% confidence interval.
3. RESULTS
3.1. Study population
In 15 institutions, 246 patients were screened after written consent, and a total of 106 patients who were finally identified as eligible were randomly assigned to three groups (Figure 1). Among them, one participant had not taken the study drug, five participants dropped out, and a total of 100 patients completed the clinical trial.
Figure 1.

Study design of the Clinical Trial
Baseline demographic and clinical characteristics were similar among the three treatment groups (Table 1). The mean age was 63.07 ± 10.03 years and 81 (77.88%) were males. The mean duration of hypertension was 130.62 ± 98.21 months, and the mean duration of dyslipidemia was 74.48 ± 66.11 months. According to the LDL‐C risk criteria at baseline, eight participants (7.69%) were in group I (LDL‐C; 160‐250 mg/dL), 26 participants (25.00%) were in group II (LDL‐C; 130‐250 mg/dL), and 70 participants (67.31%) were in group III (LDL‐C; 100‐250 mg/dL).
Table 1.
Demographic and clinical baseline characteristics (full analysis set)
| Characteristics | RSV/AML | AML | RSV | Total |
|---|---|---|---|---|
| 35 | 34 | 35 | 104 | |
| Age, mean (SD), y | 61.63 (8.74) | 64.65 (10.97) | 62.97 (10.34) | 63.07 (10.03) |
| Sex, n (%) | ||||
| Male | 27 (77.14) | 23 (67.65) | 31 (88.57) | 81 (77.88) |
| Female | 8 (22.86) | 11 (32.35) | 4 (11.43) | 23 (22.12) |
| Height, mean (SD), cm | 163.40 (8.35) | 164.16 (7.98) | 165.02 (6.83) | 164.20 (7.70) |
| Weight, mean (SD), kg | 69.29 (11.90) | 71.05 (11.43) | 72.50 (10.75) | 70.95 (11.33) |
| BMI, mean (SD), kg/m2 | 25.87 (3.37) | 26.34 (3.59) | 26.51 (2.62) | 26.24 (3.20) |
| Smoking, n (%) | ||||
| Never | 10 (28.57) | 16 (47.06) | 10 (28.57) | 36 (34.62) |
| Current | 9 (25.71) | 7 (20.59) | 16 (45.71) | 32 (30.77) |
| Former | 16 (45.71) | 11 (32.35) | 9 (25.71) | 36 (34.62) |
| msSBP, mm Hg | 155.46 (10.56) | 151.58 (10.46) | 157.22 (9.48) | |
| msDBP, mm Hg | 92.45 (7.25) | 88.82 (9.73) | 94.65 (7.62) | |
| LDL‐C, mg/dL | 152.94 (28.18) | 145.18 (25.67) | 157.34 (25.19) | |
| TC, mg/dL | 217.60 (32.14) | 210.94 (31.26) | 224.37 (30.53) | |
| TG, mg/dL | 179.46 (78.47) | 148.59 (62.61) | 175.06 (87.17) | |
| HDL, mg/dL | 44.14 (10.57) | 48.06 (13.65) | 45.69 (9.80) | |
| APO B, mg/dL | 135.09 (24.28) | 122.09 (19.33) | 133.29 (20.62) | |
| APO A‐I, mg/dL | 133.34 (21.59) | 137.21 (26.21) | 135.60 (21.43) | |
| hsCRP, mg/dL | 2.45 (3.01) | 1.64 (1.74) | 1.31 (1.58) | |
| Duration of hypertension, mean (SD), mo | 122.57 (111.52) | 135.78 (103.47) | 133.66 (79.30) | 130.62 (98.21) |
| Duration of dyslipidemia, mean (SD), mo | 74.90 (76.16) | 80.65 (70.03) | 68.07 (50.98) | 74.48 (66.11) |
| Categories of risk at baseline, n (%) | ||||
| Group I | 5 (14.29) | 2 (5.88) | 1 (2.86) | 8 (7.69) |
| Group II | 9 (25.71) | 3 (8.82) | 14 (40.00) | 26 (25.00) |
| Group III | 21 (60.00) | 29 (85.29) | 20 (57.14) | 70 (67.31) |
Duration of hypertension (month) = (randomization date − diagnosis date of hypertension + 1) × 12/365.25.
Duration of dyslipidemia (month) = (randomization date − diagnosis date of dyslipidemia + 1) × 12/365.25.
Abbreviations: AML, amlodipine (10 mg); BMI, body mass index; RSV, rosuvastatin (20 mg); RSV/AML, rosuvastatin (20 mg) + amlodipine (10 mg); SD, standard deviation.
The mean drug adherence for rosuvastatin + amlodipine, amlodipine, and rosuvastatin was 98.29 ± 3.62%, 99.18 ± 5.17%, and 97.22 ± 5.72%, respectively, and 98.21 ± 4.94% for a total of 105 participants (Table S1).
3.2. Efficacy outcomes
Of the 106 randomized patients, 104 were included in the full analysis set, and 2 were excluded due to missing data. The mean changes in msSBP at 8 weeks were −22.82 ± 12.99 mm Hg in the rosuvastatin + amlodipine group, −15.89 ± 11.50 mm Hg in the amlodipine group, and −5.80 ± 17.37 mm Hg in the rosuvastatin group (Table S2). BP lowering was significantly greater in the rosuvastatin + amlodipine group than in the rosuvastatin group (LSM; −23.41% vs −5.22%, P < .0001; Figure 2A). The mean changes in msDBP at 4 and 8 weeks as well as in msSBP at 4 weeks were also significantly greater in the rosuvastatin + amlodipine group than in the rosuvastatin group. The proportion of patients who achieved target blood pressure for 8 weeks as well as the proportion of BP responders were significantly higher in the rosuvastatin + amlodipine group compared to the rosuvastatin group (Figure 2B, Table S3). Between the rosuvastatin + amlodipine group and amlodipine group, there were no significant differences in all parameters (mean changes in msSBP and msDBP, rate of target BP achievement, and rate of BP responder; Figure 2).
Figure 2.
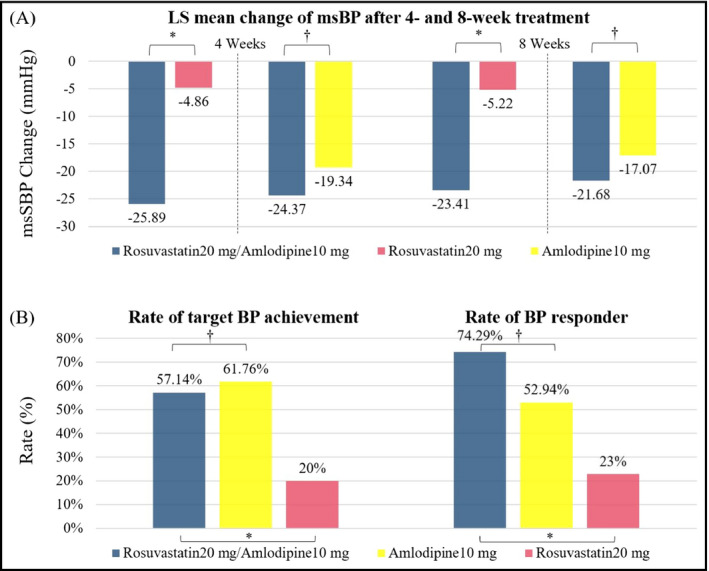
Comparison of changes in mean sitting systolic blood pressure after treatment. A, Least‐squares mean change from baseline in mean sitting systolic blood pressure. B, Rate of target goal achievement (left) and blood pressure responder (right). *P‐value < .0001, † P‐value > .05
The mean percentage changes in LDL‐C from baseline after 8 weeks were −52.53 ± 11.21% in the rosuvastatin + amlodipine group, −1.25 ± 17.90% in the amlodipine group, and −49.96 ± 15.60% in the rosuvastatin group (Table S4). LDL‐C lowering was significantly greater in the rosuvastatin + amlodipine group than in the amlodipine group (LSM; −52.00% vs −1.79%, P < .0001; Figure 3A, Table S4). The percentage changes in LDL‐C at 4 weeks, the secondary end point, was also significantly greater in the rosuvastatin + amlodipine group. The proportion of patients who achieve the target for LDL‐C at 8 weeks was significantly higher in the rosuvastatin + amlodipine group compared to the amlodipine group (Figure 3B, Table S5). Between the rosuvastatin + amlodipine group and rosuvastatin group, there were no significant differences in the percentage changes in LDL‐C and rate of target LDL‐C achievement (Figure 3).
Figure 3.
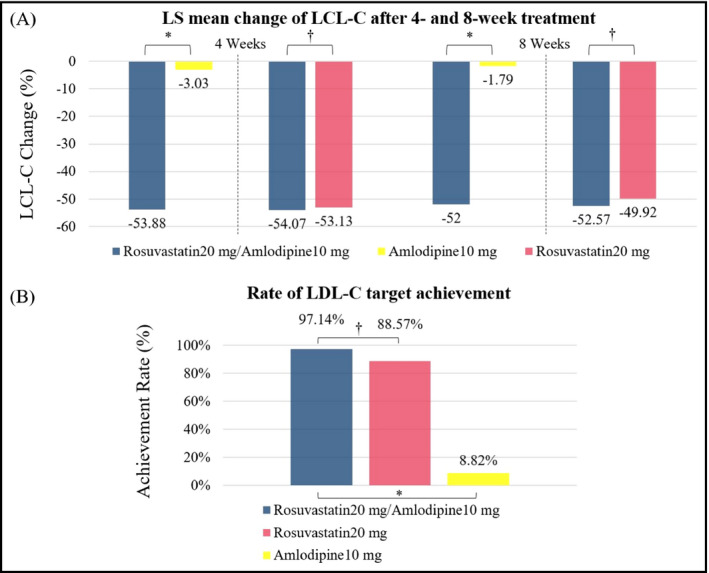
Comparison of changes in low‐density lipoprotein cholesterol (LDL‐C) after treatment. A, Least‐squares mean percent change from baseline in LDL‐C. B, Rate of target goal achievement. *P‐value < .0001, † P‐value > .05
Compared with the amlodipine group, the efficacy of rosuvastatin + amlodipine in terms of lipid lowering was also consistent with respect to changes in total cholesterol, triglyceride, and HDL‐C. Except for TG at 4 weeks, the changes of all lipid parameters at 4 weeks and 8 weeks in the rosuvastatin + amlodipine group were significantly greater than in the amlodipine group (P < .05; Tables S6‐S11). The percentage changes in non‐HDL‐C, Apo B, LDL‐C/HDL ratio, TC/HDL‐C ratio, non‐HDL‐C/HDL‐C ratio, and Apo B/Apo A‐I ratio were also significantly greater with rosuvastatin + amlodipine than with amlodipine, but there was no difference between the two groups in terms of Apo A‐I and hsCRP levels.
In terms of CVD risk reduction, the benefits of combination were also maintained. The Framingham Risk Score has not been proven to assess the response to treatment, but when all groups were scored under the same conditions, the combination group had the best results in terms of risk reduction (Figure S1).
3.3. Safety outcomes
Among 106 randomly assigned patients, 105 patients who received at least one drug were included in the Safety Set. TEAEs occurred in 5/35 (14.29%) patients treated with rosuvastatin/amlodipine, 3/34 (8.82%) patients treated with amlodipine, and 4/36 (11.11%) patients treated with rosuvastatin (Table S12). Of these, ADRs that were considered to be causally related to the study drug occurred in 2 (5.71%) patients in the rosuvastatin + amlodipine group, 1 (2.94%) patient in the amlodipine group, and 1 (2.78%) patient in the rosuvastatin group; peripheral edema in two patients in the rosuvastatin + amlodipine group, headache in one patient in the amlodipine group, and dizziness in one patient in the rosuvastatin group. During the study period, TEAEs or ADRs leading to study drug discontinuation, or leading to death did not occur in all treatment groups. No patients were found to have myopathy or serum aspartate or alanine aminotransferase levels twice consecutively ≥3× the upper limit of normal. No patients showed clinically significant changes in vital signs and on electrocardiography.
4. DISCUSSION
The main findings of this study are as follows. In hypertensive patients with dyslipidemia, (a) BP‐lowering and lipid‐modulating effects of the rosuvastatin + amlodipine combination were superior to those of rosuvastatin or amlodipine alone; (b) target level achievement rate of the combination was superior to that of each single drug while them also being tolerable in terms of safety.
Since the use of a combination pill rather than the use of a regimen with free‐drug components can improve the efficacy by increasing compliance, there have been many attempts to develop a single‐pill, fixed‐dose combination for the treatment of various diseases.27 A previous meta‐analysis reported that the fixed‐dose combination reduced the risk of non‐compliance by 26% compared with that of a regimen with free‐drug components.27 In hypertensive patients with dyslipidemia, the safety and efficacy of co‐administered amlodipine and atorvastatin have been demonstrated,17, 24, 26, 28 and single‐pill combinations of amlodipine and atorvastatin have been shown to improve compliance, thus resulting in a reduction in CVD events.15, 16, 28
Rosuvastatin, a 3‐hydroxy‐3‐methyl‐glutaryl‐CoA reductase inhibitor, is one of the highly potent statins that is quickly absorbed, reaches peak plasma concentrations in a short time, has a long half‐life, and is widely prescribed worldwide.29 In our knowledge, there has been no study of a two‐drug combination with rosuvastatin + amlodipine for hypertensive patients with dyslipidemia, unlike those for atorvastatin + amlodipine. Interestingly, the safety and efficacy of a triple combination including rosuvastatin + amlodipine have been recently reported.30, 31 However, it is still necessary to confirm the efficacy and safety of the dual combination with rosuvastatin + amlodipine since results regarding the efficacy and safety of a drug combination are not always positive.32
Beyond the improvement in compliance that can be achieved by reducing the pill burden, the exact mechanism by which BP lowering and lipid modulation are synergistic with each other in the combination group is unknown. Statins are thought to be able to slightly lower the blood pressure by contributing to restoring the endothelial dysfunction of the vessel wall through increasing the bioavailability of nitric oxide, reducing oxidative stress, and inhibiting inflammatory responses.33 Similarly, amlodipine has been reported to have anti‐inflammatory and antioxidative stress actions in addition to BP lowering, which may have a positive effect on lipid modulation.34 Although there was no statistical significance, it is interesting that patients treated with amlodipine + rosuvastatin had a greater decrease in BP at 4 and 8 weeks than those treated with amlodipine in this study. Similarly, patients treated with amlodipine + rosuvastatin had a greater reduction in LDL than patients treated with rosuvastatin.
There has been a continuing interest in the hypotensive effects of statin beyond the lipid‐lowering effect,35 and some studies have demonstrated a small but meaningful decrease in BP.36, 37 However, there is still a debate that does not show consistent results.38, 39 This is because most studies were designed with a small sample size and a relatively short period, and the concomitant antihypertensive therapy makes it difficult to clearly identify the BP‐lowering effects of statins. Additionally, there have been no studies where rosuvastatin was used for BP lowering, compared with other types of statins. In this study, the short duration of 8 weeks and the small number of patients did not result in significant changes in BP in both groups, and therefore, additional well‐designed studies are required.
There are also some limitations to this study. The small sample size and relatively short follow‐up period are certain limitations of this study. Ultimately, it is important to confirm that this single‐pill combination retains the CVD protective effect that was proven for each individual drug, and whether there is a more synergic effect. However, it was difficult to confirm this effect on CVD by this study design alone, and additional studies in this regard are needed.
5. CONCLUSIONS
In hypertensive patients with dyslipidemia, the single‐pill, fixed‐dose combination of rosuvastatin (20 mg)+amlodipine (10 mg) effectively reduced BP and LDL‐C levels while maintaining safety. Furthermore, this single‐pill combination is expected to help patients achieve the goals for hypertension and dyslipidemia through long‐term compliance.
AUTHOR CONTRIBUTIONS
W Kim and CG Park had access to the data and take responsibility for the accuracy of the data analysis. CG Park had the final responsibility for the decision to submit for publication. W Kim, M Kim, and CG Park involved in concept and design. W Kim, K Chang, EJ Cho, J‐C Ahn, CW Yu, K‐I Cho, Y‐J Kim, D‐H Kang, S‐Y Kim, S‐H Lee, U Kim, S‐J Kim, YK Ahn, CH Lee, JH Shin, M Kim, and CG Park involved in acquisition, analysis, or interpretation of data. W Kim drafted the manuscript. K Chang, EJ Cho, J‐C Ahn, CW Yu, K‐I Cho, Y‐J Kim, D‐H Kang, S‐Y Kim, S‐H Lee, U Kim, S‐J Kim, YK Ahn, CH Lee, and JH Shin involved in critical revision of the manuscript for important intellectual content. M Kim involved in technical or material support. CG Park involved in supervision.
Supporting information
Kim W, Chang K, Cho EJ, et al. A randomized, double‐blind clinical trial to evaluate the efficacy and safety of a fixed‐dose combination of amlodipine/rosuvastatin in patients with dyslipidemia and hypertension. J Clin Hypertens. 2020;22:261–269. 10.1111/jch.13774
ClinicalTrials.gov identifier: NCT03103256.
Funding information
This study was financially supported by Yuhan Corporation. The sponsor supported the supply of the investigational products, laboratory tests, and clinical research coordinator expenses.
REFERENCES
- 1. Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet . 2016;388:1545‐1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeemon P, Gupta R, Onen C, et al. Management of hypertension and dyslipidemia for primary prevention of cardiovascular disease. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, eds. Cardiovascular, Respiratory, and Related Disorders. Washington, DC: The International Bank for Reconstruction and Development/The World Bank (c) 2017 International Bank for Reconstruction and Development/The World Bank; 2017:389‐404. [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13‐e115. [DOI] [PubMed] [Google Scholar]
- 5. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990–2015. JAMA. 2017;317:165‐182. [DOI] [PubMed] [Google Scholar]
- 6. Kopin L, Dyslipidemia LC. Dyslipidemia. Ann Intern Med. 2017;167:Itc81–Itc96. [DOI] [PubMed] [Google Scholar]
- 7. Sica DA. Fixed‐dose combination therapy–is it time for this approach to hypertension and dyslipidemia management? J Clin Hypertens (Greenwich, Conn.). 2004;6:164‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong ND, Lopez V, Tang S, Williams GR. Prevalence, treatment, and control of combined hypertension and hypercholesterolemia in the united states. Am J Cardiol. 2006;98:204‐208. [DOI] [PubMed] [Google Scholar]
- 9. Johnson ML, Pietz K, Battleman DS, Beyth RJ. Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care. 2004;10:926‐932. [PubMed] [Google Scholar]
- 10. Borghi C. Interactions between hypercholesterolemia and hypertension: Implications for therapy. Curr Opin Nephrol Hypertens. 2002;11:489‐496. [DOI] [PubMed] [Google Scholar]
- 11. Kostis JB. The importance of managing hypertension and dyslipidemia to decrease cardiovascular disease. Cardiovasc Drugs Ther. 2007;21:297‐309. [DOI] [PubMed] [Google Scholar]
- 12. Turnbull F. Managing cardiovascular risk factors: the gap between evidence and practice. PLoS Med. 2005;2:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson ML, Pietz K, Battleman DS, Beyth RJ. Therapeutic goal attainment in patients with hypertension and dyslipidemia. Med Care. 2006;44:39‐46. [DOI] [PubMed] [Google Scholar]
- 14. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patel BV, Leslie RS, Thiebaud P, et al. Adherence with single‐pill amlodipine/atorvastatin vs a two‐pill regimen. Vasc Health Risk Manag. 2008;4:673‐681. [PMC free article] [PubMed] [Google Scholar]
- 16. Chapman RH, Yeaw J, Roberts CS. Association between adherence to calcium‐channel blocker and statin medications and likelihood of cardiovascular events among us managed care enrollees. BMC Cardiovasc Disord. 2010;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Messerli FH, Bakris GL, Ferrera D, et al. Efficacy and safety of coadministered amlodipine and atorvastatin in patients with hypertension and dyslipidemia: results of the AVALON trial. J Clin Hypertens (Greenwich, Conn.). 2006;8:571–581; quiz 582‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson R, Lawes CM, Bennett DA, Milne RJ, Rodgers A. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual's absolute cardiovascular risk. Lancet (London, England). 2005;365:434‐441. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta‐analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940‐2948. [DOI] [PubMed] [Google Scholar]
- 20. Wong ND, Pio JR, Franklin SS, L'Italien GJ, Kamath TV, Williams GR. Preventing coronary events by optimal control of blood pressure and lipids in patients with the metabolic syndrome. Am J Cardiol. 2003;91:1421‐1426. [DOI] [PubMed] [Google Scholar]
- 21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the national cholesterol education program (ncep) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel iii). JAMA. 2001;285:2486‐2497. [DOI] [PubMed] [Google Scholar]
- 22. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 2014;23:3‐16. [DOI] [PubMed] [Google Scholar]
- 23. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 24. Neutel JM, Bestermann WH, Dyess EM, et al. The use of a single‐pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo‐controlled, multicenter study. J Clin Hypertens (Greenwich, Conn.). 2009;11:22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oliver S, Jones J, Leonard D, Crabbe A, Delkhah Y, Nesbitt S. Improving adherence with amlodipine/atorvastatin therapy: IMPACT study. J Clin Hypertens (Greenwich, Conn.). 2011;13:598‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Preston RA, Harvey P, Herfert O, et al. A randomized, placebo‐controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the respond trial. J Clin Pharmacol. 2007;47:1555‐1569. [DOI] [PubMed] [Google Scholar]
- 27. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed‐dose combinations improve medication compliance: a meta‐analysis. Am J Med. 2007;120:713‐719. [DOI] [PubMed] [Google Scholar]
- 28. Curran MP. Amlodipine/atorvastatin: a review of its use in the treatment of hypertension and dyslipidaemia and the prevention of cardiovascular disease. Drugs. 2010;70:191‐213. [DOI] [PubMed] [Google Scholar]
- 29. Adams SP, Sekhon SS, Wright JM. Lipid‐lowering efficacy of rosuvastatin. Cochrane Database Syst Rev. 2014;(11):Cd010254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hong SJ, Jeong HS, Cho JM, et al. Efficacy and safety of triple therapy with telmisartan, amlodipine, and rosuvastatin in patients with dyslipidemia and hypertension: The jeil telmisartan, amlodipine, and rosuvastatin randomized clinical trial. Clin Ther. 2019;41(2):233‐248.e9. [DOI] [PubMed] [Google Scholar]
- 31. Lee HY, Kim SY, Choi KJ, et al. A randomized, multicenter, double‐blind, placebo‐controlled study to evaluate the efficacy and the tolerability of a triple combination of amlodipine/losartan/rosuvastatin in patients with comorbid essential hypertension and hyperlipidemia. Clin Ther. 2017;39:2366‐2379. [DOI] [PubMed] [Google Scholar]
- 32. Elley CR, Gupta AK, Webster R, et al. The efficacy and tolerability of 'polypills': meta‐analysis of randomised controlled trials. PLoS ONE. 2012;7:e52145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Devaraj S, Siegel D, Jialal I. Statin therapy in metabolic syndrome and hypertension post‐jupiter: what is the value of CRP? Curr Atheroscler Rep. 2011;13:31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshii T, Iwai M, Li Z, et al. Regression of atherosclerosis by amlodipine via anti‐inflammatory and anti‐oxidative stress actions. Hypertens Res. 2006;29:457‐466. [DOI] [PubMed] [Google Scholar]
- 35. Chopra V, Choksi PU, Cavusoglu E. Beyond lipid lowering: the anti‐hypertensive role of statins. Cardiovasc Drugs Ther. 2007;21:161‐169. [DOI] [PubMed] [Google Scholar]
- 36. Strazzullo P, Kerry SM, Barbato A, Versiero M, D'Elia L, Cappuccio FP. Do statins reduce blood pressure? A meta‐analysis of randomized, controlled trials. Hypertension. 2007;49:792‐798. [DOI] [PubMed] [Google Scholar]
- 37. Briasoulis A, Agarwal V, Valachis A, Messerli FH. Antihypertensive effects of statins: a meta‐analysis of prospective controlled studies. J Clin Hypertens (Greenwich, Conn.). 2013;15:310‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Feldstein CA. Statins in hypertension: are they a new class of antihypertensive agents? Am J Ther. 2010;17:255‐262. [DOI] [PubMed] [Google Scholar]
- 39. Banach M, Nikfar S, Rahimi R, et al. The effects of statins on blood pressure in normotensive or hypertensive subjects – a meta‐analysis of randomized controlled trials. Int J Cardiol. 2013;168:2816‐2824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


