Abstract
It is unclear whether 12‐lead ECG employing standard criteria for left ventricular hypertrophy (LVH) provides similar information with respect to long‐term cardiovascular risk as echocardiography. The authors performed a retrospective cohort study of 1376 individuals without cardiovascular disease, who underwent ECG (LVH defined using the Sokolow‐Lyon voltage combination (>35 mm) or the Cornell voltage‐duration product (>2440 mm × ms)) and echocardiography (LVH defined as LV mass index (LVMI) >95 g/m2 for women and >115 g/m2 for men). The prognostic ability of LVH was assessed in Cox regression models adjusted for age, sex, smoking, systolic blood pressure, total cholesterol, antihypertensive medication, and fasting glucose. The primary end point was the composite of coronary events, heart failure, stroke, or death. The main secondary end point was heart failure or cardiovascular death. Median age was 67 (range 56‐79) years, 68% were male. Eleven percent had ECG‐defined LVH, 17% had echocardiographic LVH. Over median 8.5 years, 29% experienced a primary event. Event rates were 29%/35% for persons without/with ECG‐defined LVH and 27%/39% for those without/with echocardiographic LVH. The Sokolow‐Lyon combination, Cornell product, and ECG‐defined LVH did not significantly predict the primary end point (P ≥ .05), but ECG‐defined LVH predicted heart failure or cardiovascular death (adjusted hazard ratio (HR), 1.86, 95% confidence interval (CI), 1.13‐3.08); P = .02). Conversely, LVMI was a significant, independent predictor of the primary end point (adjusted HR, 1.87, 95% CI, 1.13‐3.10; P = .01), as was echocardiographic LVH (adjusted HR, 1.27, 95% CI, 1.01‐1.61; P = .04). Echocardiographic LVH may be a better predictor of long‐term cardiovascular risk than ECG‐defined LVH in middle‐aged and older individuals.
Keywords: echocardiography; electrocardiography; hypertrophy, left ventricular; prognosis; risk assessment
1. INTRODUCTION
Left ventricular hypertrophy (LVH) is a well‐known risk factor for cardiovascular morbidity and mortality. 1 , 2 , 3 Early diagnosis is essential as regression of LVH is associated with a reduction in cardiovascular events. 4 , 5 , 6 Although several modalities are available for detection of LVH, ECG and echocardiography are most commonly used in daily clinical practice. ECG is easy to perform, but its specificity and, in particular, sensitivity are limited. On the other hand, echocardiography has a higher diagnostic accuracy, but is more expensive and time‐consuming. 7 , 8 , 9 , 10
Contemporary guidelines for arterial hypertension and appropriate use criteria for echocardiography do not support universal application of echocardiography in patients with hypertension, but recommend its use when the results are likely to influence management. 10 , 11 , 12 However, it is unclear whether 12‐lead ECG employing standard criteria for LVH provides similar information with respect to long‐term cardiovascular risk as echocardiography. Data on direct prognostic comparisons remain scarce, though some studies have suggested potential advantages of echocardiography compared with ECG depending on the setting. 13 , 14 , 15 Therefore, the aim of our study was to investigate the prognostic ability of LVH diagnosed by either ECG or echocardiography in older normotensive and hypertensive individuals without established cardiovascular disease.
2. MATERIAL AND METHODS
2.1. Study population
The Malmö Preventive Project (1974‐1992, n = 33 346) was a population‐based cohort study that included inhabitants of Malmö, Sweden, born 1921‐1949. In 2002‐2006, 18 238 of the remaining individuals attended a re‐screening, the Malmö Preventive Project Re‐Examination Study. Approximately one‐tenth of these participants (n = 1792), randomly chosen from groups defined by fasting plasma glucose (normal fasting glucose, impaired fasting glucose, or diabetes), underwent a 12‐lead ECG and echocardiography at a subsequent visit. 16 , 17 Both examinations were performed on the same day. The study was approved by the Regional Ethical Review Board of Lund University, Sweden, and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent.
For the present study, the authors excluded individuals with conditions thought to affect ECG analysis, including prior myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, heart failure, or left or right bundle branch block (n = 272). Participants with missing echocardiographic variables (n = 50), ECG variables (n = 12), pertinent explanatory variables, that is, smoking status, systolic blood pressure, plasma total cholesterol, or use of antihypertensive medication (n = 76), or those who emigrated (n = 6) were also excluded, leaving a final sample size of 1376 (Figure S1).
2.2. Baseline evaluation
A comprehensive, self‐administered, computer‐based form was used to obtain information on lifestyle (eg, smoking history), medical history, symptoms, and active medications. Details on prior cardiovascular disease were confirmed through national and local registries, using appropriate International Classification of Diseases (ICD‐9 and ICD‐10) codes. Height and weight were measured in light indoor clothing. Blood pressure was recorded twice in the supine position after 5 minutes of rest, and the average was used for analysis. Blood samples for analysis of plasma glucose and plasma cholesterol were drawn after an overnight fast.
2.3. Hypertension
Hypertension was defined per the 2018 European Society of Cardiology/European Society of Hypertension (ESC/ESH) guidelines (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg and/or use of antihypertensive medication) and per the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines (systolic blood pressure ≥130 mm Hg and/or diastolic blood pressure ≥80 mm Hg and/or use of antihypertensive medication). 10 , 11
2.4. Electrocardiogram
A 10‐second, 12‐lead ECG strip was recorded at 50 mm/s and 1 mV/cm, using MAC, MAC5K, or MAC8 devices (GE Healthcare, Milwaukee, WI). The manufacturer's automated Marquette 12SL algorithm was used for assessment of QRS duration. This algorithm uses signal‐averaging by creating a median QRS complex, that is, a representative QRS complex generated from the median voltages by aligning all QRS complexes of the same shape in time. QRS onset and offset are determined by analyzing the slopes in all 12 simultaneous leads, using the time‐aligned median complexes. The QRS duration is then measured as a global interval, from the earliest detection of depolarization in any lead (onset) to the latest detection of depolarization in any lead (offset). Experienced technicians with no knowledge of the clinical data manually measured R‐ and S‐wave amplitudes on signal‐averaged ECG complexes. The authors then calculated the Cornell voltage‐duration product (CP; males: (RaVL + SV3) × QRS duration; females: (RaVL + SV3 + 6 mm) × QRS duration) and the Sokolow‐Lyon voltage combination (SL; SV1 + (RV5 or RV6, whichever was taller)). Partitions defining LVH were >35 mm for SL and >2440 mm × ms for CP. 10 , 18
2.5. Echocardiography
All echocardiographic images were acquired by six experienced sonographers, using either an S3 transducer (Sonos 5500 Philips) or a 3V2c transducer (Acuson Sequoia). These technicians subsequently performed offline, guideline‐compliant analysis, without knowledge of the clinical data. 19 The thickness of the interventricular septum, LV internal diameter, and thickness of the posterior wall were obtained from end‐diastolic, 2‐dimensional images, in the parasternal long‐axis view. Left ventricular mass was computed with the Devereux equation and indexed for body surface area (DuBois formula) (conventional scaling; LVH: LVMI > 95 g/m2 [women] and >115 g/m2 [men]). Sensitivity analyses were performed by indexing for height1.7 (allometric scaling #1; LVH: LVMI > 60 g/m1.7 [women] and >80 g/m1.7 [men]) and for height2.7 (allometric scaling #2; LVH: LVMH > 47 g/m2.7 [women] >50 g/m2.7 [men]). 10 , 20 , 21 , 22 Relative wall thickness was calculated as 2 × posterior wall thickness/LV internal diameter (abnormal relative wall thickness: >0.42). 19 All measurements were averaged from 3‐5 cardiac cycles. Leósdóttir et al 16 previously reported intra‐ and inter‐observer variabilities (coefficients of variation) for measurements of the interventricular septum, LV inner diameter, and thickness of the posterior wall at 10.5% and 13.0%, 3.3%, and 4.1%, and 5.5% and 12.1%, respectively.
2.6. Outcomes
The primary outcome was the composite of coronary events (ie, myocardial infarction or invasively treated stable or unstable ischemic heart disease), heart failure, stroke, or death from any cause, whichever came first. This broad composite end point was chosen to maximize the total number of events. Sensitivity analyses were performed using (a) heart failure or death from cardiovascular causes and (b) myocardial infarction, stroke, or death from cardiovascular causes. These two secondary end points emphasized heart failure and ischemic outcomes, respectively. Diagnoses were obtained through national and local registries, using ICD‐9 and ICD‐10 codes (Table S1). Only primary diagnoses were considered. Moderate‐to‐high validities for all diagnoses were previously reported in the Swedish National Inpatient Register. 23 Mortality data were acquired using the National Registry on Causes of Mortality at the Swedish Central Bureau of Statistics. Follow‐up ended on December 31, 2014.
2.7. Statistical analyses
Summary statistics for continuous variables were plotted as means and standard deviations (clear or approximate normal distribution) or medians and interquartile ranges (clear non‐normal distribution). Categorical variables were presented as counts with corresponding percentages. Group‐wise comparisons were done using independent samples t test or one‐way analysis of variance, Mann‐Whitney U test or Kruskal‐Wallis test, or Pearson's chi‐square test, as appropriate.
Kaplan‐Meier analysis with the log‐rank test and Cox proportional‐hazards regression, assuming an uncensored policy for handling ties, were used to assess the risks associated with electrocardiographic and echocardiographic markers of LVH. 24 Hazard ratios (HR) were reported unadjusted and adjusted for age, sex, smoking status, systolic blood pressure, plasma total cholesterol, use of antihypertensive medication, and fasting plasma glucose status (ie, normal fasting glucose, impaired fasting glucose, or diabetes mellitus). A supplemental analysis was performed with further adjustment for LVH (conventional echocardiographic LVH for ECG measures, electrocardiographic LVH for echocardiographic measures). Standardized HRs were reported for continuous markers of LVH. Whether the prognostic implications of LVH were modified by baseline variables was examined using the likelihood‐ratio test for regression models with and without the relevant interaction term and displayed using Forest plots.
A two‐sided P‐value < .05 was considered statistically significant. No adjustments for multiple comparisons were made as the study was considered exploratory. The statistical packages IBM SPSS Statistics 24 (IBM) and Stata/IC 15 (StataCorp LP) were used for all computations.
3. RESULTS
3.1. Baseline characteristics
Table 1 summarizes baseline clinical, laboratory, electrocardiographic, and echocardiographic characteristics of the study participants. Median time from the first screening visit to ECG and echocardiography was 5.5 (interquartile range: 3.9‐7.4) months. One hundred and fifty‐one individuals (11%) had ECG‐defined LVH, and 240 (17%) had echocardiographic LVH. Individuals who experienced a primary composite event were older, had higher blood pressures, higher concentrations of fasting plasma glucose, and were more often on antihypertensive medications. In addition, the Cornell voltage‐duration product was on average greater, and markers of echocardiographic LVH more prevalent, in the group of participants with an incident event. Table S2 shows baseline characteristics according to LVH subgroup.
Table 1.
Baseline clinical, laboratory, electrocardiographic, and echocardiographic characteristics
| Coronary events, heart failure, stroke, or death from any cause | ||||
|---|---|---|---|---|
| All participants, n = 1376 | No events, n = 971 | Events, n = 405 | P‐value | |
| Demographics | ||||
| Age, y | 67 (61‐70) | 66 (59‐70) | 70 (65‐74) | <.001 a |
| Male sex | 942 (68%) | 657 (68%) | 285 (70%) | .32 b |
| Active smoking | 204 (15%) | 133 (14%) | 71 (18%) | .07 b |
| Anthropometrics and vital signs | ||||
| Height, cm | 172 ± 9 | 172 ± 9 | 171 ± 9 | .74 c |
| Weight, kg | 83 ± 14 | 82 ± 14 | 83 ± 14 | .75 c |
| Body mass index, kg/m2 | 28.1 ± 4.3 | 28.1 ± 4.2 | 28.1 ± 4.4 | .97 c |
| Overweight (body mass index 25‐29.9 kg/m2) | 680 (49%) | 493 (51%) | 187 (46%) | .12 b |
| Obese (body mass index ≥ 30 kg/m2) | 387 (28%) | 268 (28%) | 119 (29%) | .50 b |
| Systolic blood pressure, mm Hg | 148 ± 20 | 147 ± 19 | 150 ± 22 | .01 c |
| Diastolic blood pressure, mm Hg | 85 ± 10 | 85 ± 10 | 85 ± 11 | .75 c |
| Heart rate, bpm | 72 ± 12 | 72 ± 12 | 72 ± 13 | .63 c |
| Antihypertensive medication use | ||||
| Any antihypertensive medication | 565 (41%) | 356 (37%) | 209 (52%) | <.001 b |
| Angiotensin‐converting‐enzyme inhibitor | 264 (19%) | 168 (17%) | 96 (24%) | .006 b |
| Beta blocker | 258 (19%) | 172 (18%) | 86 (21%) | .13 b |
| Calcium channel blocker | 125 (9%) | 85 (9%) | 40 (10%) | .51 b |
| Diuretic | 78 (6%) | 37 (4%) | 41 (10%) | <.001 b |
| Hypertension (2018 ESC/ESH) | 1063 (77%) | 724 (75%) | 339 (84%) | <.001 b |
| Hypertension (2017 ACC/AHA) | 1255 (91%) | 877 (90%) | 378 (93%) | .07 b |
| Laboratory data | ||||
| Fasting plasma glucose, mmol/L | 6.2 (5.5‐7.2) | 6.2 (5.5‐7.1) | 6.3 (5.5‐7.6) | .02 a |
| Total cholesterol, mmol/L | 5.6 ± 1.1 | 5.6 ± 1.0 | 5.5 ± 1.1 | .16 c |
| Low‐density lipoprotein cholesterol, mmol/L | 3.6 ± 1.0 | 3.6 ± 0.9 | 3.5 ± 1.0 | .16 c |
| Electrocardiogram | ||||
| Sokolow‐Lyon combination, mm | 22 ± 7 | 21 ± 7 | 22 ± 7 | .40 c |
| Cornell product, mm × ms | 1535 ± 625 | 1512 ± 604 | 1590 ± 669 | .04 c |
| Left ventricular hypertrophy (either Sokolow‐Lyon or Cornell) | 151 (11%) | 98 (10%) | 53 (13%) | .11 b |
| Echocardiogram | ||||
| Left ventricular mass, g | 172 ± 48 | 169 ± 46 | 180 ± 52 | <.001 c |
| Left ventricular mass index, g/m2 | 88 ± 22 | 87 ± 20 | 93 ± 24 | <.001 c |
| Left ventricular hypertrophy (left ventricular mass index in g/m2) | 240 (17%) | 147 (15%) | 93 (23%) | <.001 b |
| Left ventricular mass index, g/m1.7 | 69 ± 18 | 67 ± 17 | 72 ± 19 | <.001 c |
| Left ventricular hypertrophy (left ventricular mass index in g/m1.7) | 493 (36%) | 316 (33%) | 177 (44%) | <.001 b |
| Left ventricular mass index, g/m2.7 | 40 ± 10 | 39 ± 10 | 42 ± 11 | <.001 c |
| Left ventricular hypertrophy (left ventricular mass index in g/m2.7) | 245 (18%) | 151 (16%) | 94 (23%) | <.001 b |
| Relative wall thickness | 0.41 ± 0.08 | 0.40 ± 0.08 | 0.42 ± 0.09 | .001 c |
| Ejection fraction, % | 61 ± 6 | 61 ± 6 | 61 ± 8 | .90 c |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; ESC/ESH, European Society of Cardiology/ European Society of Hypertension.
Mann‐Whitney U test.
Pearson's chi‐square test.
Independent samples t test.
3.2. Incident events
Median follow‐up time from ECG and echocardiography was 8.5 (interquartile range: 8.0‐9.1) years for all participants. A total of 405 (29%) individuals experienced a primary event, with an incidence density of 36.0 per 1000 person‐years. Heart failure or death from cardiovascular causes occurred in 92 (7%) participants (incidence density, 7.6 per 1000 years), and myocardial infarction, stroke, or death from cardiovascular causes in 238 participants (17%; incidence density, 21.0 per 1000 years). Primary event rates were 29%/35% for persons without/with ECG‐defined LVH and 27%/39% for those without/with echocardiographic LVH. Corresponding numbers in patients with ESC/ESH‐defined hypertension were 31%/36% for participants without/with ECG‐defined LVH and 30%/40% for those without/with echocardiographic LVH. Event rates for the primary outcome, stratified for ECG and echocardiographic findings in individuals fulfilling the 2017 ACC/AHA criteria for hypertension, are displayed in Figure 1.
Figure 1.
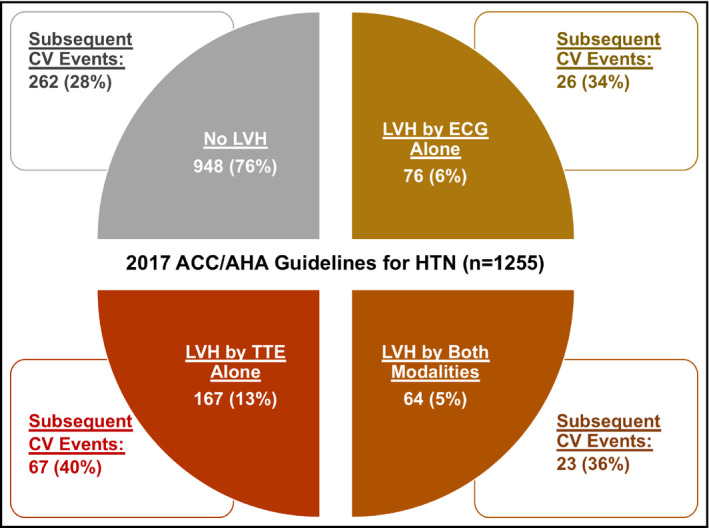
Rates of the primary outcome stratified for ECG and echocardiographic findings in participants fulfilling the 2017 ACC/AHA criteria for hypertension. ACC/AHA, American College of Cardiology/American Heart Association; CV, cardiovascular; HTN, hypertension; LVH, left ventricular hypertrophy; TTE, transthoracic echocardiography
3.3. Electrocardiography
Means ± standard deviations of the Sokolow‐Lyon voltage combination and Cornell voltage‐duration product were 22 ± 7 mm and 1535 ± 625 mm × ms, and 151 (11%) had electrocardiographic LVH. None of the ECG markers significantly predicted the primary outcome (Table 2 and Figure 2A). The Cornell voltage‐duration product and electrocardiographic LVH were significantly associated with heart failure or death from cardiovascular causes, albeit the former on unadjusted analysis only. Conversely, only the Sokolow‐Lyon voltage combination was associated with myocardial infarction, stroke, or death from cardiovascular causes. Additional adjustment for echocardiographic LVH resulted in attenuation of most of these associations (Table S3). Electrocardiography‐based LVH was not associated with the primary outcome across a broad range of subgroups, including baseline hypertension status (Figure S2A).
Table 2.
Hazard ratios for markers of left ventricular hypertrophy
| Unadjusted | Adjusted a | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| Coronary events, heart failure, stroke, or death from any cause | ||||
| ECG | ||||
| Sokolow‐Lyon combination | 1.33 (0.81‐2.21) | .26 | 1.32 (0.79‐2.20) | .29 |
| Cornell product | 1.34 (0.92‐1.94) | .13 | 1.18 (0.80‐1.74) | .42 |
| Left ventricular hypertrophy b | 1.23 (0.92‐1.64) | .16 | 1.18 (0.88‐1.58) | .26 |
| Echocardiography | ||||
| Left ventricular mass index, g/m2 | 2.64 (1.64‐4.26) | <.001 | 1.87 (1.13‐3.10) | .01 |
| Left ventricular hypertrophy, g/m2 | 1.45 (1.15‐1.83) | .002 | 1.27 (1.01‐1.61) | .04 |
| Relative wall thickness | 2.12 (1.32‐3.41) | .002 | 1.23 (0.75‐2.03) | .41 |
| Left ventricular mass index, g/m1.7 | 1.71 (1.19‐2.46) | .004 | 1.53 (1.02‐2.30) | .04 |
| Left ventricular hypertrophy, g/m1.7 | 1.36 (1.12‐1.66) | .002 | 1.30 (1.05‐1.61) | .01 |
| Left ventricular mass index, g/m2.7 | 2.36 (1.54‐3.62) | <.001 | 1.53 (0.97‐2.43) | .07 |
| Left ventricular hypertrophy, g/m2.7 | 1.43 (1.13‐1.80) | .003 | 1.16 (0.92‐1.47) | .21 |
| ECG or echocardiography | ||||
| Left ventricular hypertrophy, g/m2 | 1.45 (1.17‐1.79) | <.001 | 1.28 (1.03‐1.60) | .02 |
| Left ventricular hypertrophy, g/m1.7 | 1.40 (1.15‐1.70) | <.001 | 1.32 (1.07‐1.62) | .009 |
| Left ventricular hypertrophy, g/m2.7 | 1.42 (1.15‐1.75) | .001 | 1.19 (0.96‐1.48) | .12 |
| Heart failure or death from cardiovascular causes | ||||
| ECG | ||||
| Sokolow‐Lyon combination | 2.19 (0.79‐6.05) | .13 | 2.04 (0.75‐5.58) | .17 |
| Cornell product | 2.19 (1.06‐4.53) | .04 | 1.58 (0.75‐3.33) | .23 |
| Left ventricular hypertrophy b | 2.18 (1.33‐3.58) | .002 | 1.86 (1.13‐3.08) | .02 |
| Echocardiography | ||||
| Left ventricular mass index, g/m2 | 9.40 (3.78‐23.35) | <.001 | 5.73 (2.22‐14.81) | <.001 |
| Left ventricular hypertrophy, g/m2 | 2.53 (1.65‐3.90) | <.001 | 2.11 (1.36‐3.27) | <.001 |
| Relative wall thickness | 2.67 (1.02‐7.00) | .045 | 1.18 (0.44‐3.16) | .75 |
| Left ventricular mass index, g/m1.7 | 4.54 (2.32‐8.90) | <.001 | 4.16 (1.95‐8.88) | <.001 |
| Left ventricular hypertrophy, g/m1.7 | 2.05 (1.36‐3.09) | <.001 | 1.87 (1.20‐2.90) | .006 |
| Left ventricular mass index, g/m2.7 | 7.00 (3.17‐15.47) | <.001 | 4.50 (1.90‐10.65) | <.001 |
| Left ventricular hypertrophy, g/m2.7 | 2.34 (1.52‐3.61) | <.001 | 1.79 (1.15‐2.80) | .01 |
| ECG or echocardiography | ||||
| Left ventricular hypertrophy, g/m2 | 2.70 (1.79‐4.08) | <.001 | 2.21 (1.45‐3.36) | <.001 |
| Left ventricular hypertrophy, g/m1.7 | 2.38 (1.56‐3.63) | <.001 | 2.10 (1.34‐3.27) | .001 |
| Left ventricular hypertrophy, g/m2.7 | 2.67 (1.77‐4.02) | <.001 | 2.07 (1.36‐3.16) | <.001 |
| Myocardial infarction, stroke, or death from cardiovascular causes | ||||
| ECG | ||||
| Sokolow‐Lyon combination | 2.96 (1.61‐5.46) | <.001 | 2.67 (1.43‐5.01) | .002 |
| Cornell product | 1.47 (0.91‐2.37) | .12 | 1.39 (0.84‐2.28) | .20 |
| Left ventricular hypertrophy b | 1.42 (0.99‐2.03) | .06 | 1.39 (0.97‐2.00) | .07 |
| Echocardiography | ||||
| Left ventricular mass index, g/m2 | 3.08 (1.67‐5.71) | <.001 | 2.27 (1.19‐4.34) | .01 |
| Left ventricular hypertrophy, g/m2 | 1.53 (1.13‐2.06) | .005 | 1.36 (1.01‐1.85) | .04 |
| Relative wall thickness | 2.49 (1.36‐4.56) | .003 | 1.56 (0.82‐2.96) | .18 |
| Left ventricular mass index, g/m1.7 | 1.70 (1.06‐2.74) | .03 | 1.75 (1.03‐2.97) | .04 |
| Left ventricular hypertrophy, g/m1.7 | 1.25 (0.97‐1.62) | .09 | 1.23 (0.94‐1.62) | .13 |
| Left ventricular mass index, g/m2.7 | 2.76 (1.60‐4.78) | <.001 | 1.88 (1.04‐3.39) | .04 |
| Left ventricular hypertrophy, g/m2.7 | 1.51 (1.12‐2.03) | .006 | 1.23 (0.91‐1.67) | .18 |
| ECG or echocardiography | ||||
| Left ventricular hypertrophy, g/m2 | 1.56 (1.19‐2.05) | .001 | 1.41 (1.07‐1.86) | .02 |
| Left ventricular hypertrophy, g/m1.7 | 1.28 (0.99‐1.66) | .06 | 1.24 (0.95‐1.63) | .12 |
| Left ventricular hypertrophy, g/m2.7 | 1.55 (1.19‐2.03) | .001 | 1.31 (0.995‐1.73) | .054 |
Standardized HRs were reported for continuous markers of left ventricular hypertrophy (Sokolow‐Lyon voltage combination, Cornell voltage‐duration product, left ventricular mass index, relative wall thickness).
Abbreviations: CI, confidence interval; HR, hazard ratio.
Adjusted for age, sex, smoking status, systolic blood pressure, plasma total cholesterol, use of antihypertensive medication, and fasting plasma glucose category.
Left ventricular hypertrophy by either ECG definition.
Figure 2.
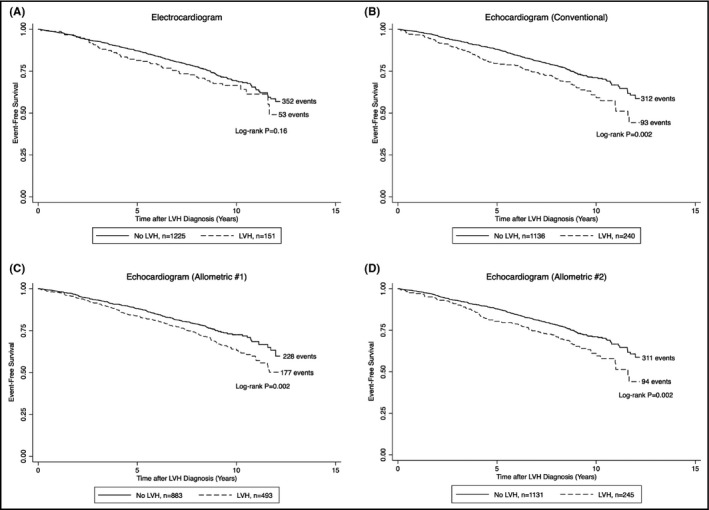
A, Kaplan‐Meier survival curves for the composite of coronary events, heart failure, stroke, or death from any cause, according to the absence or presence of electrocardiographic left ventricular hypertrophy. B, Kaplan‐Meier survival curves for the composite of coronary events, heart failure, stroke, or death from any cause, according to the absence or presence of echocardiographic left ventricular hypertrophy (conventional). C, Kaplan‐Meier survival curves for the composite of coronary events, heart failure, stroke, or death from any cause, according to the absence or presence of echocardiographic left ventricular hypertrophy (allometric #1). D, Kaplan‐Meier survival curves for the composite of coronary events, heart failure, stroke, or death from any cause, according to the absence or presence of echocardiographic left ventricular hypertrophy (allometric #2). LVH, left ventricular hypertrophy
3.4. Echocardiography
Mean LVMI was 88 ± 22 g/m2, with 240 (17%) participants fulfilling the conventional criteria for LVH. Both LVMI and LVH were significantly related to all three composite outcomes after multivariable adjustment, with effect sizes strongest for the association with heart failure or death from cardiovascular causes (Table 2 and Figure 2B). Similar findings, albeit with slightly weaker effect sizes, were obtained when using allometric scaling, that is, when indexing LVM for height1.7 and for height2.7, respectively (Table 2 and Figure 2C,D). Mild attenuation of these associations was seen with additional adjustment for electrocardiographic LVH (Table S3). However, the addition of electrocardiographic LVH to echocardiographic LVH did not significantly alter the discrimination ability or model performance for any of the outcomes as assessed by Harrell's concordance index and the likelihood‐ratio test, respectively (results not shown). LVH by either echocardiographic definition was consistently associated with the primary outcome across subgroups (Figure S2B‐D). Conversely, relative wall thickness was only associated with outcomes on univariable analysis (Table 2 and Table S3).
4. DISCUSSION
In this retrospective, population‐based cohort study of older individuals without established cardiovascular disease, the authors found that echocardiographic, but not electrocardiographic, markers of LVH independently predicted long‐term risk of composite coronary events, heart failure, stroke, or death from any cause. The presence of hypertension, irrespective of the definition used, did not significantly modify the association between LVH and the primary composite end point.
Only few studies have compared the prognostic utilities of ECG and echocardiographically determined LVH. The Uppsala Longitudinal Study of Adult Men investigators reported that electrocardiographic LVH (defined using the Cornell voltage‐duration product), and echocardiographic LVMI predicted total mortality independently of each other and were thus complementary. However, only LVMI was associated with cardiovascular mortality. 13 A correction factor for females as suggested by the Losartan Intervention for End point reduction (LIFE) study was not employed. 18 Similarly, a case‐control study using data from the Oregon Sudden Unexpected Death Study found both echocardiographic LVH and Sokolow‐Lyon‐based LVH to be linked with sudden cardiac arrest, though the latter association was marginal. 14 Finally, the prospective Cardiovascular Health Study showed that both echocardiographic and electrocardiographic LVH, the latter based on Cornell criteria, predicted incident congestive heart failure. 15 Although the authors were unable to demonstrate a significant association between LVH on ECG alone and the primary composite end point, our results did not significantly differ from prior studies as the authors found both echocardiographic and electrocardiographic LVH to predict heart failure or cardiovascular mortality, an end point that is mechanistically more clearly linked to adverse cardiac remodeling than ischemic events. 25 , 26 In fact, the point estimates for electrocardiographic and echocardiographic LVH in predicting this end point were virtually identical. Furthermore, the Sokolow‐Lyon combination displayed a strong association with ischemic end points while the Cornell product did not. This is supported by a recent meta‐analysis that suggested distinct predictive properties of these methods for LVH detection. 27 Finally, prior studies have reported a significantly greater proportion of individuals with echocardiographically versus ECG‐defined LVH, with considerable discordance between the two modalities. 13 , 14 , 15 , 28 A study including the present cohort found age, blood pressure, female sex, relative wall thickness, and use of antihypertensive medication to affect the probability of concordance between ECG and echocardiography, 28 but none of these variables significantly interacted with the association between LVH and clinical outcomes.
Blood pressure measurements are often based on a single or few visits and are not necessarily standardized, leading to variability and difficulty in diagnosing hypertension. 29 Hypertension‐mediated organ damage, for example, LVH, constitutes a potential intermediate state between uncomplicated hypertension and the development of clinical events, and its detection may potentially improve risk stratification. 10 , 11 Intuitively, the lower blood pressure threshold defined by the 2017 ACC/AHA guidelines would lead to a reduced prevalence of LVH among hypertensive patients. 30 Our overall prevalence of echocardiographic LVH agreed with prior studies having reported prevalences of 5%‐20% in the general population. 31 , 32 , 33 , 34 However, our prevalence estimates could have been spuriously lowered by the inclusion of rather healthy survivors from a cohort originating decades ago as well as the exclusion of individuals with known cardiovascular disease. In our subgroup of individuals with ACC/AHA defined hypertension, event rates were lowest in those without any signs of LVH and highest in those with echocardiographically detected LVH alone. This may in part be explained by the presence of undiagnosed infiltrative cardiomyopathy. 35 However, even those without LVH experienced high rates of cardiovascular events. Interestingly, electrocardiographic LVH was not a significantly stronger predictor of outcomes among non‐obese individuals. 36 , 37 Nevertheless, this was probably a result of the power limitations described below. Given the lower sensitivity of ECG and the added complexity and cost of broad application of echocardiography, the exact adjunctive role of detection and assessment of LVH in refining cardiovascular risk prediction thus remains unclear. Matters are further complicated by the difficulties in predicting which individual patients would benefit the most from antihypertensive therapy, 38 , 39 and by extension, which patients, particularly among those without any electrocardiographic abnormalities, would be most likely to benefit from echocardiographic assessment. 6 , 40 , 41 Further studies are required to determine whether an increased use of echocardiography, or potentially rapid point‐of‐care ultrasound, may improve contemporary risk stratification and inform treatment decisions for hypertension.
4.1. Strengths and limitations
Major strengths of our study include a stable, population‐based sample with long‐term follow‐up and limited emigration. Furthermore, multiple LVH definitions were assessed. However, the low event rate and related power to detect associations and interactions with LVH, particularly on ECG, is a limitation. This is evident from the inability of the association between electrocardiographic measures and certain end points to meet formal significance despite point estimates resembling those for echocardiographic measures. Our selection of components comprising the primary composite end point that may not be directly pathophysiologically related to the presence of LVH, thus obscuring potentially important relationships with certain individual end points, could also be questioned; however, this choice was consciously made to maximize the total number of events. Given the composition of the study population, the generalizability of our results to younger individuals, women, and ethnicities other than white Swedish patients may also be limited. Most study participants fulfilled the ACC/AHA criteria for hypertension, limiting the number of individuals with normotension. In addition, 24‐hour ambulatory blood pressure monitoring was not performed which could have strengthened the validity of our hypertension variable, detected resistant and masked hypertension, and provided a more precise estimate of cardiovascular risk. 10 , 11 , 42 Participant selection based on glycemic categories, with oversampling of those with impaired fasting glucose or diabetes, prevented us from using fasting plasma glucose as a continuous variable and may have been a source of selection bias; however, tests of heterogeneity for the prognostic implications of LVH were not statistically significant. Finally, the authors were unable to explore a purely manual or automated approach to ECG analysis and to test newer ECG criteria for LVH as only variables related to the Sokolow‐Lyon and Cornell approaches were measured. 43 , 44 , 45 On the other hand, the Sokolow‐Lyon and Cornell methods are also the ones traditionally endorsed by guidelines. 10
5. CONCLUSIONS AND PERSPECTIVES
In this population‐based cohort study of normotensive and hypertensive older individuals, traditional echocardiographic LVH was an independent predictor of the composite of coronary events, heart failure, stroke, or death from any cause, while electrocardiographic LVH was not. However, both echocardiographic and electrocardiographic LVH predicted heart failure or cardiovascular mortality. Further work is needed to determine if echocardiography should be employed more often to improve risk stratification and inform treatment decisions among patients with hypertension.
CONFLICT OF INTEREST
Dr Muthiah Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award UL 1TR002541), serves on advisory boards for Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, and Boehringer Ingelheim, Cytokinetics, and Relypsa, and participates on clinical end point committees for studies sponsored by Galmed, Novartis and the NIH. Dr Deepak L. Bhatt discloses the following relationships—Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE‐DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS‐II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co‐leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Novo Nordisk, PLx Pharma, Takeda. Dr Michael Hecht Olsen discloses that he has received a part‐time clinical research grant from the Novo Nordisk Foundation. Dr Manan Pareek discloses the following relationships—Advisory Board: AstraZeneca; Speaker's Fee: AstraZeneca, Bayer, Boehringer Ingelheim. The other authors have no disclosures to report.
AUTHOR CONTRIBUTIONS
LRP and AMDK conceptualized the hypothesis, interpreted the data, drafted the manuscript, and revised the manuscript critically for important intellectual content. SSP, MV, DLB, JJ, CBFA, ML, and MHO conceptualized the hypothesis, designed the work, interpreted the data, and revised the manuscript critically for important intellectual content. MP conceptualized the hypothesis, designed the work, analyzed and interpreted the data, drafted the manuscript, and revised the manuscript critically for important intellectual content. All authors have read and approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the paper are appropriately investigated and resolved.
Supporting information
Supplementary Material
Pedersen LR, Kristensen AMD, Petersen SS, et al. Prognostic implications of left ventricular hypertrophy diagnosed on electrocardiogram vs echocardiography. J Clin Hypertens. 2020;22:1647–1658. 10.1111/jch.13991
Line Reinholdt Pedersen and Anna Meta Dyrvig Kristensen shares equal contribution as co‐first authors.
REFERENCES
- 1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322(22):1561‐1566. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Salomon M, D'Agostino RB, Belanger AJ, Kannel WB. Prognostic implications of baseline electrocardiographic features and their serial changes in subjects with left ventricular hypertrophy. Circulation. 1994;90(4):1786‐1793. [DOI] [PubMed] [Google Scholar]
- 3. Verma S, Mazer CD, Bhatt DL, et al. Empagliflozin and cardiovascular outcomes in patients with type 2 diabetes and left ventricular hypertrophy: a subanalysis of the EMPA‐REG outcome trial. Diabetes Care. 2019;42(3):e42‐e44. [DOI] [PubMed] [Google Scholar]
- 4. Wachtell K, Okin PM, Olsen MH, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE study. Circulation. 2007;116(7):700‐705. [DOI] [PubMed] [Google Scholar]
- 5. Okin PM, Devereux RB, Harris KE, et al. Regression of electrocardiographic left ventricular hypertrophy is associated with less hospitalization for heart failure in hypertensive patients. Ann Intern Med. 2007;147(5):311‐319. [DOI] [PubMed] [Google Scholar]
- 6. Soliman EZ, Ambrosius WT, Cushman WC, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with hypertension: SPRINT (systolic blood pressure intervention trial). Circulation. 2017;136(5):440‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devereux RB, Casale PN, Wallerson DC, et al. Cost‐effectiveness of echocardiography and electrocardiography for detection of left ventricular hypertrophy in patients with systemic hypertension. Hypertension. 1987;9(2 pt 2):69‐76. [DOI] [PubMed] [Google Scholar]
- 8. Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81(3):815‐820. [DOI] [PubMed] [Google Scholar]
- 9. Pewsner D, Juni P, Egger M, Battaglia M, Sundstrom J, Bachmann LM. Accuracy of electrocardiography in diagnosis of left ventricular hypertrophy in arterial hypertension: systematic review. BMJ. 2007;335(7622):711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: The task force for the management of arterial hypertension of the european society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the european society of cardiology and the European society of hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 11. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 12. American College of Cardiology Foundation Appropriate Use Criteria Task Force , American Society of Echocardiography , American Heart Association , et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 appropriate use criteria for echocardiography. A report of the american college of cardiology foundation appropriate use criteria task force, american society of echocardiography, american heart association, american society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, society of critical care medicine, society of cardiovascular computed tomography, society for cardiovascular magnetic resonance American college of chest physicians. J Am Soc Echocardiogr. 2011;24(3):229‐267. [DOI] [PubMed] [Google Scholar]
- 13. Sundstrom J, Lind L, Arnlov J, Zethelius B, Andren B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103(19):2346‐2351. [DOI] [PubMed] [Google Scholar]
- 14. Narayanan K, Reinier K, Teodorescu C, et al. Electrocardiographic versus echocardiographic left ventricular hypertrophy and sudden cardiac arrest in the community. Heart Rhythm. 2014;11(6):1040‐1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Almahmoud MF, O'Neal WT, Qureshi W, Soliman EZ. Electrocardiographic versus echocardiographic left ventricular hypertrophy in prediction of congestive heart failure in the elderly. Clin Cardiol. 2015;38(6):365‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leosdottir M, Willenheimer R, Plehn J, et al. Myocardial structure and function by echocardiography in relation to glucometabolic status in elderly subjects from 2 population‐based cohorts: a cross‐sectional study. Am Heart J. 2010;159(3):414‐420.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pareek M, Vaduganathan M, Bhatt DL, Leosdottir M, Olsen MH. Prognostic implications of fasting plasma glucose in subjects with echocardiographic abnormalities. Int J Cardiol. 2017;241:423‐429. [DOI] [PubMed] [Google Scholar]
- 18. Dahlof B, Devereux RB, Julius S, et al. Characteristics of 9194 patients with left ventricular hypertrophy: the LIFE study. Losartan intervention for endpoint reduction in hypertension. Hypertension. 1998;32(6):989‐997. [DOI] [PubMed] [Google Scholar]
- 19. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233‐270. [DOI] [PubMed] [Google Scholar]
- 20. Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56(1):91‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marwick TH, Gillebert TC, Aurigemma G, et al. Recommendations on the use of echocardiography in adult hypertension: a report from the European association of cardiovascular imaging (EACVI) and the American society of echocardiography (ASE). J Am Soc Echocardiogr. 2015;28(7):727‐754. [DOI] [PubMed] [Google Scholar]
- 22. Kuznetsova T, Haddad F, Tikhonoff V, et al. Impact and pitfalls of scaling of left ventricular and atrial structure in population‐based studies. J Hypertens. 2016;34(6):1186‐1194. [DOI] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):11‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543‐2546. [PubMed] [Google Scholar]
- 25. Norton GR, Woodiwiss AJ, Gaasch WH, et al. Heart failure in pressure overload hypertrophy. The relative roles of ventricular remodeling and myocardial dysfunction. J Am Coll Cardiol. 2002;39(4):664‐671. [DOI] [PubMed] [Google Scholar]
- 26. Drazner MH, Rame JE, Marino EK, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the cardiovascular health study. J Am Coll Cardiol. 2004;43(12):2207‐2215. [DOI] [PubMed] [Google Scholar]
- 27. Zhang H, Hu L, Wei X. Prognostic value of left ventricular hypertrophy in hypertensive patients: a meta‐analysis of electrocardiographic studies. J Clin Hypertens. 2020;22(2):254‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petersen SS, Pedersen LR, Pareek M, et al. Factors associated with diagnostic discrepancy for left ventricular hypertrophy between electrocardiography and echocardiography. Blood Press. 2017;26(1):54‐63. [DOI] [PubMed] [Google Scholar]
- 29. Bliziotis IA, Destounis A, Stergiou GS. Home versus ambulatory and office blood pressure in predicting target organ damage in hypertension: a systematic review and meta‐analysis. J Hypertens. 2012;30(7):1289‐1299. [DOI] [PubMed] [Google Scholar]
- 30. Vaduganathan M, Pareek M, Qamar A, Pandey A, Olsen MH, Bhatt DL. Baseline blood pressure, the 2017 ACC/AHA high blood pressure guidelines, and long‐term cardiovascular risk in SPRINT. Am J Med. 2018;131(8):956‐960. [DOI] [PubMed] [Google Scholar]
- 31. Savage DD, Garrison RJ, Kannel WB, et al. The spectrum of left ventricular hypertrophy in a general population sample: the Framingham study. Circulation. 1987;75(1 Pt 2):I26‐I33. [PubMed] [Google Scholar]
- 32. Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham heart study. Ann Intern Med. 1988;108(1):7‐13. [DOI] [PubMed] [Google Scholar]
- 33. Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454‐1459. [DOI] [PubMed] [Google Scholar]
- 34. Schirmer H, Lunde P, Rasmussen K. Prevalence of left ventricular hypertrophy in a general population; the Tromso study. Eur Heart J. 1999;20(6):429‐438. [DOI] [PubMed] [Google Scholar]
- 35. Quarta CC, Buxbaum JN, Shah AM, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cuspidi C, Facchetti R, Bombelli M, Sala C, Grassi G, Mancia G. Accuracy and prognostic significance of electrocardiographic markers of left ventricular hypertrophy in a general population: findings from the pressioni arteriose monitorate E loro associazioni population. J Hypertens. 2014;32(4):921‐928. [DOI] [PubMed] [Google Scholar]
- 37. Muiesan ML, Salvetti M, Di Castelnuovo A, et al. Obesity and ECG left ventricular hypertrophy. J Hypertens. 2017;35(1):162‐169. [DOI] [PubMed] [Google Scholar]
- 38. Pareek M, Vaduganathan M, Biering‐Sørensen T, et al. Pulse pressure, cardiovascular events, and intensive blood pressure lowering in the systolic blood pressure intervention trial (SPRINT). Am J Med. 2019;132(6):733‐739. [DOI] [PubMed] [Google Scholar]
- 39. Oxlund CS, Pareek M, Rasmussen BSB, et al. Body mass index, intensive blood pressure management, and cardiovascular events in the SPRINT trial. Am J Med. 2019;132(7):840‐846. [DOI] [PubMed] [Google Scholar]
- 40. SPRINT Research Group , Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373(22):2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lonn EM, Bosch J, Lopez‐Jaramillo P, et al. Blood‐pressure lowering in intermediate‐risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2009–2020. [DOI] [PubMed] [Google Scholar]
- 42. Bhatt DL, James GD, Pickering TG, Devereux RB. Relation of arterial pressure level and variability to left ventricular geometry in normotensive and hypertensive adults. Blood Press Monit. 1996;1(5):415‐424. [PubMed] [Google Scholar]
- 43. Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic value of a new electrocardiographic method for diagnosis of left ventricular hypertrophy in essential hypertension. J Am Coll Cardiol. 1998;31(2):383‐390. [DOI] [PubMed] [Google Scholar]
- 44. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy by losartan versus atenolol: the losartan intervention for endpoint reduction in hypertension (LIFE) study. Circulation. 2003;108(6):684‐690. [DOI] [PubMed] [Google Scholar]
- 45. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292(19):2343‐2349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


