Abstract
Pulse pressure naturally increases over time as individuals’ age due to arteriosclerosis and diffuse vascular stiffening. However, the differential for widened pulse pressure is broad and includes causes of hyperdynamic circulation and high‐output heart failure, such as aortic regurgitation and hyperthyroidism. In the absence of an underlying cause, wide pulse pressure is a sign of deteriorating cardiovascular health and carries increased risk for mortality, disease progression, and adverse clinical outcomes in chronic diseases including cardiovascular disease and chronic kidney disease. Current emphasis of antihypertensive treatment on systolic and diastolic blood pressure does not always address pulse pressure, thus subjecting many patients to an independent risk factor for poor outcomes. Pulse pressure control is more successfully achieved with thiazide diuretics and long‐acting nitrates when compared to other antihypertensive agents, but further research is needed to quantify the additional benefits of pulse pressure control over conventional blood pressure therapy. This case review provides an overview of the pathogenesis, pathologic causes, and treatment of widened pulse pressure and evaluates current evidence for pulse pressure as a predictor of clinical outcomes.
Keywords: clinical management of high blood pressure, general, hypertension, vascular disease
1. INTRODUCTION
Pulse pressure (PP) is defined as the difference between systolic blood pressure (SBP) and diastolic blood pressure (DBP), which represent the maximal and minimal circulatory pressures during the cardiac cycle. 1 “Normal” values for SBP and DBP are estimated at 120 mm Hg and 80 mm Hg, respectively, producing an approximate value of 40 mm Hg for average PP. A “narrowed” PP is defined as <25% of the SBP while a “widened” PP is defined as >100 mm Hg, 1 although PP should always be considered in the context of the patient's absolute SBP and DBP and the values at which PP beings to confer increased risk of adverse clinical outcomes will vary according to specific disease states. 2 While the differential for a widened PP is broad, wide PP is most often seen in the context of extensive cardiovascular disease and is an independent predictor of disease progression and all‐cause mortality. 3 , 4 , 5 The aim of this article is to review the pathophysiology and causes of wide PP, its impact on disease outcomes, and effective strategies for treatment.
2. CASE
An 86‐year‐old man with a history of hypertension for 20 years, atrial fibrillation, hyperlipidemia, non‐insulin‐dependent diabetes mellitus, benign prostatic hypertrophy, and peripheral vascular disease presents to clinic. He denies acute complaints. Physical examination is notable for a gentleman in no acute distress with a pulse of 73 bpm, blood pressure (BP) of 201/66 mm Hg, and temperature of 98.1 F. Cardiopulmonary examination is notable for an irregularly irregular rhythm without murmur and clear lung fields to auscultation. No peripheral edema is present. His medications include diltiazem 240 mg daily, metoprolol succinate 50 mg daily, and lisinopril 40 mg daily. He reports excellent adherence to his medications, follows a low sodium diet, denies use of herbal medications and his only over‐the‐counter medication is acetaminophen. He asks if he should increase his antihypertensive regimen as he is concerned his DBP will be too low if his regimen is intensified.
3. PATHOPHYSIOLOGY & EPIDEMIOLOGY
The pathogenesis of wide PP may vary significantly between different populations. Importantly, a widening PP is a normal consequence of senescence. 6 Both SBP and DBP increase with age until age 55, after which they diverge as diastolic pressures decrease and systolic pressures continue to rise. 7 The result is a progressive widening of the PP beyond age 60, with isolated systolic hypertension—defined as an SBP ≥ 130 mm Hg with a DBP < 80 mm Hg—accounting for around 65% of hypertension diagnoses in elderly patients. 8 , 9
The hemodynamic physiology of age‐related wide PP and systolic hypertension may be succinctly attributed to arteriosclerosis—the gradual degenerative stiffening of arteries (Figure 1). 10 This process reduces the compliance of the central arterial system, leading to an increased proportion of flow occurring during systole and a consequent elevation in SBP. Sclerotic vasculopathies also increase the pulse wave velocity (PWV), defined as the ratio of the distance between two points (eg, between the carotid and femoral arteries) in the arterial system and the time it takes for the systole‐generated aortic pressure wave to travel from one point to the other. 11 , 12 This phenomenon may occur independently of any changes in MAP or systemic vascular resistance (SVR), 13 as peripheral arteries generally demonstrate less age‐related stiffening or increase in diameter to offload increased vascular hydrostatic pressure. 14 , 15 The most accurate readings of central blood pressure and resultant cardiovascular risk assessment are achieved by placing a sensor directly in the aorta, where it is easier to detect the PP amplification secondary to increased pulse wave reflection often resulting from increased SVR, decreased elasticity, and increased PWV. 11 Augmentation pressure (the absolute pressure increase from systolic ejection pressure due to pulse wave reflection) and its derivation, the aortic augmentation index 16 (defined as the ratio of central blood pressure to PP), are independent predictors of cardiovascular disease, 17 , 18 arterial stiffening, and all‐cause mortality. 6
Figure 1.
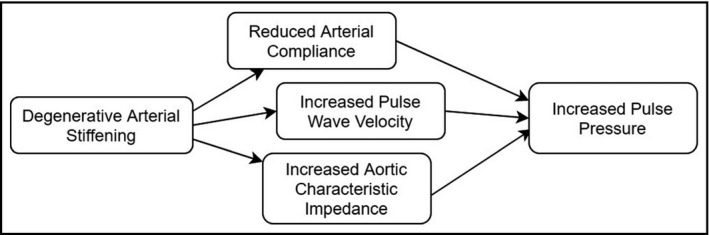
Pathophysiology of age‐related wide pulse pressure
In contrast to age‐related wide PP, wide PP in younger patients is most often seen in well‐conditioned athletes—rigorous exercise increases both stroke volume and cardiac output while decreasing SVR, leading to increased PP. 1 Pathologic variants of wide PP in patients under the age of 45 are less common. Several epidemiological studies have linked specific genetic loci to increased risk for developing wide PP, 19 , 20 but the mechanism and prevalence of these variants have yet to be confirmed. 6 More relevant are the rising rates of wide PP alongside increases in pediatric obesity and metabolic syndrome 21 ; thus a wide PP in the pediatric or young adult patient may constitute a telling marker of deteriorating cardiovascular health.
4. DIFFERENTIAL DIAGNOSIS
In a patient presenting with new‐onset hypertension with or without wide PP, BP values should first be confirmed with accurate measurement techniques before further workup for underlying causes can proceed (Figure 2). Auscultatory office blood pressure measurement is the traditional technique for diagnosing hypertension but is limited by its low reproducibility and vulnerability to confounding factors, including the white coat effect 22 , 23 and worse SBP measurement discrepancies in elderly patients. 24 The American Heart Association (AHA) considers 24‐hour ambulatory blood pressure monitoring (ABPM) the reference standard for blood pressure assessment, 25 although studies show automated office blood pressure monitoring (AOBPM) to be equivalent to ABPM while retaining the convenience of being an office measurement technique. 22 , 26 The diagnosis of hypertension and evaluation of subsequent response to treatment should be informed by at least one of these two measurement techniques based on an average of two or more readings on at least two occasions.
Figure 2.
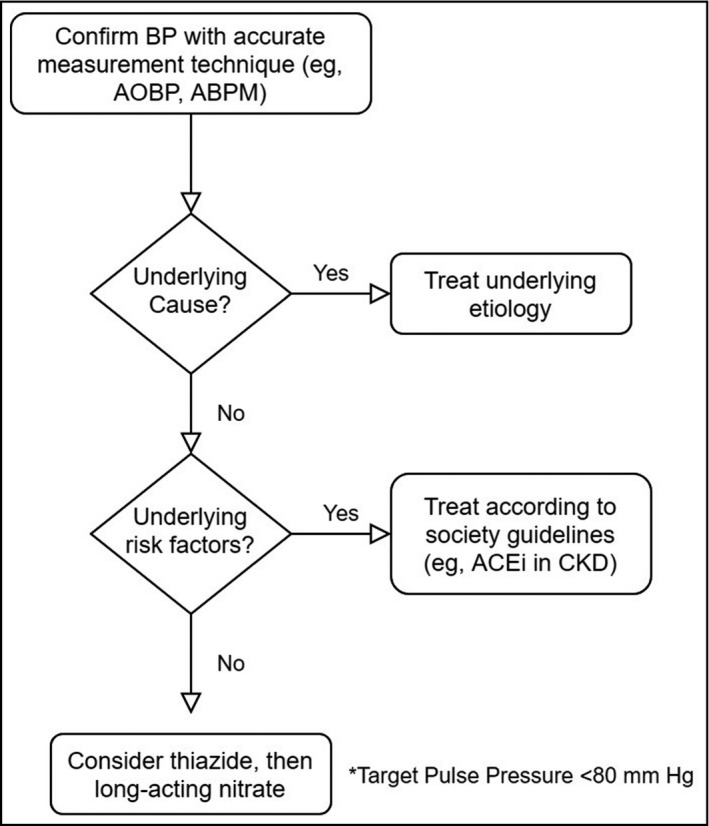
Management of wide pulse pressure
The astute clinician must be wary of various underlying pathologies that may include wide PP in their symptomatology (Table 1). In general, causes of a hyperdynamic circulation will often present with wide PP. Aortic regurgitation is a classic example and presents with a myriad of physical examination findings, including Corrigan's water hammer pulse, Quincke's pulse, and wide PP. 6 Research has also determined that increased PP in hypertensive patients with aortic regurgitation is independently associated with higher risk for development of clinically significant carotid artery stenosis. 27 Secondary causes of aortic insufficiency including aneurysm, 28 dissection, 29 , 30 endocarditis, 31 and connective tissue disorders 32 have also been shown to increase PP. Similarly, the formation of arteriovenous (AV) fistulas permanently increases both cardiac output and PP, placing patients at risk of high‐output heart failure. 33 , 34
Table 1.
Differential diagnosis for wide pulse pressure
| Diagnosis | |
|---|---|
| Physiologic | Advanced Age |
| Pregnancy | |
| Well‐Conditioned Athletes | |
| Pathologic | Atherosclerosis |
| Aortic Regurgitation | |
| Arteriovenous Fistula | |
| Wet Beriberi | |
| Distributive Shock | |
| Elevated Intracranial Pressure | |
| Hyperthyroidism/Thyrotoxicosis |
Other pathologic causes of wide PP span numerous organ systems and include diseases both common and uncommon. “Wet” Beriberi, defined as a severe thiamine deficiency, will present with peripheral neuropathy and high‐output heart failure. 35 While not universally present, wide PP is a common feature alongside Corrigan's pulse, Traube's sign, and Quincke's pulse. 36 , 37 A wide PP is a common feature of early distributive shock 38 , 39 and may even be a predictor of mortality and fluid responsiveness. 40 Elevated intracranial pressure (ICP) should also be on the differential, as wide PP may be a finding in the classical Cushing triad response. This triad is normally defined by bradycardia, respiratory depression, and hypertension. 41 , 42 The mechanism behind these physiologic responses is still unclear, but recent hypotheses theorize that they may be indicators of medullary compression by rising ICP and impending tonsillar herniation. 43 Finally, thyrotoxicosis induces an increase in PP through its effect on basal metabolic rate. 44 Conversely, PP has been shown to decrease following successful treatment with beta‐adrenergic blockade. 45
5. CLINICAL OUTCOMES
The effect of wide PP on clinical outcomes and overall morbidity and mortality is profound and far‐reaching (Table 2). The association of wide PP with adverse cardiovascular outcomes is the most well‐documented correlation in the literature, although there is debate around gender distribution and the relative utility of PP against SBP or DBP to predict cardiovascular risk. 2 , 3 , 4 , 5 , 50 Multiple trials over the last three decades have demonstrated the predictive value of wide PP for coronary heart disease, 5 congestive heart failure, 51 stroke, 48 and general all‐cause mortality, 3 , 4 with a statistically significant increase in risk for composite cardiovascular events starting at PP > 80 mm Hg. 52 The Framingham Heart Study concluded that each 10 mm Hg increase in PP independently conferred a 23% increased risk of developing coronary heart disease 5 and each 16 mm Hg increase independently associated with a 55% increased risk of developing congestive heart failure. 53 These associations were unrelated to age or initiation of antihypertensive treatment in the follow‐up period. Conversely, patients with a narrow PP are at lower risk, indicating a protective quality of narrow PP against cardiovascular‐related mortality. 4 Other trials have demonstrated an 11% increase in stroke risk and a 16% increase in all‐cause mortality for every 10 mm Hg increase in PP. 54 Wide PP is also independently associated with risk of developing atrial fribrillation. 1 , 55 For every 20 mm Hg increase in PP, there is an adjusted hazard ratio of 1.28 for developing atrial fibrillation. This relationship exists independent of other BP values such as SBP, DBP, and MAP.
Table 2.
Large trials examining the effect of wide Pulse Pressure (PP) on clinical outcomes
| Study | Population | Outcome |
|---|---|---|
| Framingham Heart Study (1999) 5 | 1924 patients aged 50‐79 | Wide PP and systolic hypertension had a significantly positive association with coronary heart disease. Notably, there was a negative association between prevalence of coronary heart disease and diastolic blood pressure (DBP). Wide PP was accurate in predicting risk. Each 10 mm Hg increase in PP was associated with a 23% increase in risk for development of cardiovascular disease. |
| MRFIT Trial (2002) 47 | 342 815 men aged 35‐57 without history of diabetes or myocardial infarction | Systolic blood pressure (SBP) and DBP were more strongly related to cardiovascular disease than PP. Concordant elevations in both SBP and DBP posed the greatest risk for cardiovascular disease‐related mortality. However, in men aged 45 y or older, PP was associated with increased mortality regardless of blood pressure control. |
| San Antonio Heart Study (2009) 4 | 3632 patients aged 25‐64 without history of diabetes or cardiovascular disease | Prehypertensive patients with PP in the lower tertile were at lower risk of all‐cause mortality compared to prehypertensive patients with PP in the upper tertile. |
| JPHC Study (2011) 48 | 33 372 Japanese patients aged 40‐69 without history of cardiovascular disease or cancer | Increased PP, SBP, and DBP were all positively associated with risk of stroke. Notably, increased PP in otherwise normotensive patients also increased stroke risk. |
| RENAAL Study (2003) 57 | 1513 patients with diabetic nephropathy and hypertension | Higher baseline PP was associated with the greatest risk of kidney disease progression but also with the greatest risk reduction following SBP control with losartan. Each 10 mm Hg increase in PP conferred a 17% increased risk of developing end‐stage renal disease. |
Wide PP is a predictor of worse outcomes in chronic kidney disease (CKD). In a study examining 329 patients with mild to moderate CKD, baseline PP and SBP correlated to declining effective glomerular filtration rate (eGFR). 56 A PP > 65 mm Hg was associated with worsening kidney function and may be a better predictor of adverse kidney outcomes than both systolic and diastolic BP. The RENAAL Study consisting of 1513 patients with diabetic nephropathy and hypertension found that higher baseline PP associated with the greatest risk of disease progression but also with the greatest risk reduction following BP control with losartan. 57 Data suggest that CKD and chronic hemodialysis are independent contributors to widened PP, with a positive correlation between wide PP and overall mortality. 58 , 59 , 60 Further study is needed, however, to clarify whether PP reduction improves prognosis for CKD and chronic hemodialysis patients.
There is some evidence supporting wide PP as a poor prognostic indicator for percutaneous coronary intervention (PCI) with higher rates of postoperative myocardial infarction and stroke, 61 , 62 although cases of idiopathic peri‐operative wide PP without adverse clinical outcomes have also been described. 63 In a study of 10 876 patients undergoing PCI, patients with wide PP were more often women with higher rates of hypercholesterolemia, kidney dysfunction, diabetes mellitus, and multiple vessel of left main coronary artery disease. 62 Therefore, it is likely that wide PP is instead an indicator of a higher‐risk patient population with a worse prognosis due to poor vascular health and other pre‐existing risk factors.
6. ANTIHYPERTENSIVE TREATMENT
Special care should be given to the treatment of wide PP in the setting of hypertension; surveys show lower diagnosis and treatment rates for isolated systolic hypertension in both the elderly and young adult populations, 64 even though it constitutes the majority subtype of uncontrolled hypertension in patients aged 50 or older. 9 Current hypertensive treatment is largely centered around reducing SBP and DBP, 65 but evidence suggests that successful treatment of these two measures does not always reduce PP. 66 Several studies have found that different antihypertensive medications have varying effects on PP (Table 3), with thiazide diuretics being largely superior to beta‐blockers in PP reduction. 54 , 67 , 68 , 69 Two double‐blind randomized controlled trials demonstrated efficacy of isosorbide dinitrate in decreasing SBP without significant change in DBP or heart rate, indicating that the drug may be an effective choice in controlling PP and treating isolated systolic hypertension. 70 , 71 A large retrospective analysis of the Veterans Affairs Single‐Drug Therapy for Hypertension Study examining six classes of hypertensive agents found that hydrochlorothiazide was more successful than clonidine, captopril, diltiazem, atenolol, and prazosin in treating to a PP goal of <50 mm Hg. 67 Furthermore, drug combinations including a thiazide diuretic were superior to combinations without a thiazide diuretic both in reducing PP and reaching goal systolic and diastolic BP. These findings suggest that multi‐drug regimens incorporating at least one thiazide diuretic‐class medication may optimize both overall BP and PP control.
Table 3.
Studies examining effects of different medications on Pulse Pressure (PP)
| Study | Conclusions |
|---|---|
| Cushman et al 67 |
Over 1 y of treatment, reductions in PP were greater in hydrochlorothiazide when compared to clonidine, captopril, diltiazem, atenolol, and prazosin. Hydrochlorothiazide decreased PP by 8.6 mm Hg, clonidine by 6.3 mm Hg, captopril by 4.1 mm Hg, diltiazem by 5.5 mm Hg, atenolol by 4.1 mm Hg, and prazosin by 5 mm Hg. |
| Chang et al 68 | In elderly patients receiving one or two antihypertensive drugs, mean PP was 7 mm Hg lower in those receiving diuretics alone or in combination with beta‐blockers when compared to those using beta‐blockers alone. |
| Starmans‐Kool et al 67 | Compared to placebo, isosorbide dinitrate produced a statistically significant reduction in office PP. Systolic blood pressure and mean arterial pressure decreased in the treatment group without a significant change in diastolic blood pressure. |
| Duchier et al 71 | In elderly patients with isolated systolic hypertension, isosorbide dinitrate more significantly reduced PP (27 mm Hg) when compared to placebo (13 mm Hg). This effect was seen without significant changes in diastolic blood pressure, heart rate, or side effects. |
| Williams et al 79 | Folic acid supplementation was superior to placebo in increasing systemic arterial compliance and reducing brachial PP without changing mean arterial pressure. |
Several studies including the TNT trial 72 have decried aggressive lowering of BP in accordance with a J‐curve effect, whereby the risk of cardiovascular events and adverse clinical outcomes paradoxically increases below a DBP of around 85 mm Hg. 73 , 74 This may be due to a parallel subclinical comorbidity or to insufficient coronary perfusion during diastole leading to cardiac myocyte ischemia. 75 However, the J‐curve phenomenon has been disputed by several other large randomized controlled trials including the HOT trial. 76 The San Antonio Heart Study found that low DBP only correlated with cardiovascular and all‐cause mortality if it occurred alongside widened PP, suggesting that low DBP by itself may not be a poor prognosticator and instead may reflect a wide PP secondary to underlying arterial stiffening and atherosclerosis. 4
However, both the ability to decrease PP and the benefits to long‐term all‐cause mortality of antihypertensive drugs have been largely attributed to their predominant effects on SBP. 77 , 78 As a result, current hypertension guidelines and goals for antihypertensive treatment are centered around SBP reduction and less so on PP control. 79 , 80 More recently, the SPRINT trial 81 and 2017 American College of Cardiology/AHA hypertension guidelines 65 , 79 , 80 further promoted the benefits of intensive SBP control. Given the data supporting wide PP as an independent risk factor for cardiovascular risk and mortality, 2 , 4 , 17 , 46 , 47 , 49 , 50 more research is needed to further explore PP reduction as a proxy for clinical response to antihypertensive treatment.
7. CASE REVISITED
Our patient presented with a markedly elevated PP of 135 mm Hg. Despite his geriatric age and history of extensive vasculopathy, presentation with a PP of 135 mm Hg in the context of significant elevation of SBP and no history of wide PP was a cause for legitimate concern. Further workup was unrevealing, indicating that an underlying pathology as discussed in previous sections was unlikely; it is probable that his abnormal BP was a direct result of long‐standing peripheral vascular disease and disseminated arteriosclerosis. A thiazide diuretic was added to the patient's medication regimen to decrease his systolic pressures and control PP.
8. CONCLUSION
The etiology of widened PP is often multifaceted and difficult to define. While advanced age will often play a role in arterial wall stiffness and systolic‐diastolic pressure discrepancy, consideration must also be given to underlying pathologies that may contribute to a hyperdynamic circulation and elevated PP. Wide PP has been demonstrated to be a poor prognostic factor in several chronic diseases including cardiovascular disease and CKD. Further study is needed to parse out the pathophysiology of associated adverse outcomes and the clinical value of PP control.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
KST contributed to writing and editing. EM contributed to editing. ADS conceived of the project and contributed to editing. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
DISCLAIMER
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the United States Department of Veterans Affairs or the United States government.
Tang KS, Medeiros ED, Shah AD. Wide pulse pressure: A clinical review. J. Clin. Hypertens. 2020;22:1960–1967. 10.1111/jch.14051
REFERENCES
- 1. Homan T, Cichowski E. Physiology, pulse pressure. In: StatPearls [Internet]: Treasure Island, FL: StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 2. Pastor‐Barriuso R, Banegas J, Damian J, Appel L, Guallar E. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med. 2003;139(9):731. [DOI] [PubMed] [Google Scholar]
- 3. Franklin SS, Gokhale SS, Chow VH, et al. Does low diastolic blood pressure contribute to the risk of recurrent hypertensive cardiovascular disease events? Hypertension. 2015;65(2):299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lorenzo C, Aung K, Stern M, Haffner S. Pulse pressure, prehypertension, and mortality: the San Antonio heart study. Am J Hypertens. 2009;22(11):1219. [DOI] [PubMed] [Google Scholar]
- 5. Franklin SS, Khan S, Wong ND, Larson M, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham Heart Study. Circulation. 1999;100(4):354‐360. [DOI] [PubMed] [Google Scholar]
- 6. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison's Principles of Internal Medicine. 19 ed.: McGraw‐Hill Professional; 2015:1611‐1627. [Google Scholar]
- 7. Folkow B. Structure and function of the arteries in hypertension. Am Heart J. 1987;114(4):938. [DOI] [PubMed] [Google Scholar]
- 8. Kannel W. Prevalence and implications of uncontrolled systolic hypertension. Drugs Aging. 2012;20:277‐286. [DOI] [PubMed] [Google Scholar]
- 9. Franklin SS, Jacobs M, Wong ND, L'Italien G, Lapuerta P. Predominance of isolated systolic hypertension among middle‐aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension. 2001;37(3):869‐874. [DOI] [PubMed] [Google Scholar]
- 10. Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19(4):341‐352. [DOI] [PubMed] [Google Scholar]
- 11. Mendes‐Pinto D, Rodrigues‐Machado M. Applications of arterial stiffness markers in peripheral arterial disease. J Vasc Bras. 2019;18:e20180093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blacher J, Asmar R, Djane S, London G, Safar M. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33(5):1111‐1117. [DOI] [PubMed] [Google Scholar]
- 13. Izzo JL Jr. Hypertension in the elderly: a pathophysiologic approach to therapy. J Am Geriatr Soc. 1982;30:352‐359. [DOI] [PubMed] [Google Scholar]
- 14. van der Heijden‐Spek JJ, Staessen JA, Fagard R, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent. Hypertension. 2000;35(2):637‐642. [DOI] [PubMed] [Google Scholar]
- 15. Kimoto E, Shoji T, Shinohara K, et al. Preferential stiffening of central over peripheral arteries in type 2 diabetes. Diabetes. 2003;52(2):448‐452. [DOI] [PubMed] [Google Scholar]
- 16. Husmann M, Jacomella V, Thalhammer C, Amann‐Vesti B. Markers of arterial stiffness in peripheral arterial disease. Vasa. 2015;44(5):341‐348. [DOI] [PubMed] [Google Scholar]
- 17. Nargesi A.A, Esteghamati S, Heidari B, et al. Nonlinear relation between pulse pressure and coronary heart disease in patients with type 2 diabetes or hypertension. J Hypertens. 2016;34(5):974‐980. [DOI] [PubMed] [Google Scholar]
- 18. Winston G, Palmas W, Lima J, et al. Pulse pressure and subclinical cardiovascular disease in the multi‐ethnic study of atherosclerosis. Am J Hypertens. 2013;26(5):636‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bielinski SJ, Lynch AI, Miller MB, et al. Genome‐wide linkage analysis for loci affecting pulse pressure: the family blood pressure program. Hypertension. 2005;46(6):1286‐1293. [DOI] [PubMed] [Google Scholar]
- 20. Wain LV, Verwoert GC, O'Reilly PF, et al. Genome‐wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nature Genet. 2011;43(10):1005‐1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zachariah JP, Graham DA, de Ferranti SD, Vasan RS, Newburger JW, Mitchell GF. Temporal trends in pulse pressure and mean arterial pressure during the rise of pediatric obesity in US children. J Am Heart Assoc. 2014;3(3):e000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pappaccogli M, Monaco S, Perlo E, et al. Comparison of automated office blood pressure with office and out‐off‐office measurement techniques. Hypertension. 2018;73(2):481‐490. [DOI] [PubMed] [Google Scholar]
- 23. Pickering T, James G, Boddie C. How common is white coat hypertension? JAMA. 1988;259(2):225‐228. [PubMed] [Google Scholar]
- 24. Miller S, Elam J, Graney M, Applegate W. Discrepancies in recording systolic blood pressure of elderly persons by ambulatory blood pressure monitor. Am J Hypertens. 1992;5(1):16‐21. [DOI] [PubMed] [Google Scholar]
- 25. Muntner P, Shimbo D, Carey RM, et al. Measurement of Blood Pressure in Humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35‐e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Myers MG. Automated office blood pressure measurement. Korean Circ J. 2018;48(4):241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Su T, Chien KL, Jeng J, et al. Pulse pressure, aortic regurgitation and carotid atherosclerosis: a comparison between hypertensives and normotensives. Int J Clin Prac. 2006;60(2):134‐140. [DOI] [PubMed] [Google Scholar]
- 28. Nataf P, Lansac E. Dilation of the thoracic aorta: medical and surgical management. Heart. 2006;92(9):1345‐1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parsa C, Williams J, Bhattacharya S, et al. Midterm results with thoracic endovascular aortic repair for chronic type B aortic dissection with associated aneurysm. J Thor Card Surg. 2010;141(2):322‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shingu Y, Shiiya N, Ooka T, et al. Augmentation index is elevated in aortic aneurysm and dissection. Ann Thor Surg. 2009;87(5):1373‐1377. [DOI] [PubMed] [Google Scholar]
- 31. Mann T, McLaurin L, Grossman W, Craige E. Assessing the hemodynamic severity of acute aortic regurgitation due to infective endocarditis. N Engl J Med. 1975;293(3):108‐113. [DOI] [PubMed] [Google Scholar]
- 32. Jondeau G, Boutouyrie P, Lacolley P, et al. Central pulse pressure is a major determinant of ascending aorta dilation in Marfan syndrome. Circulation. 1999;99(20):2677‐2681. [DOI] [PubMed] [Google Scholar]
- 33. Elkin D, Warren J. Arteriovenous fistulas: their effect on the circulation. JAMA. 1947;134(18):1524‐1528. [DOI] [PubMed] [Google Scholar]
- 34. Stern A, Klemmer P. High‐output heart failure secondary to arteriovenous fistula. Hemodial Int. 2011;15(1):104‐107. [DOI] [PubMed] [Google Scholar]
- 35. Sica D. Loop diuretic therapy, thiamine balance, and heart failure. Congest Heart Fail. 2007;13(4):244. [DOI] [PubMed] [Google Scholar]
- 36. The KC, Heart B. The beriberi heart. Arch Intern Med. 1930;45(1):1‐22. [Google Scholar]
- 37. Wagner P. Beriberi heart disease: physiologic data and difficulties in diagnosis. Am Heart J. 1964;69:200‐205. [DOI] [PubMed] [Google Scholar]
- 38. Brown S. The pathophysiology of shock in anaphylaxis. Immunol Allergy Clin North Am. 2007;27(2):165‐175. [DOI] [PubMed] [Google Scholar]
- 39. Houston M. Pathophysiology of shock. Crit Care Nurs Clinics. 1990;2(2):143‐149. [PubMed] [Google Scholar]
- 40. Al‐khalisy H, Nikiforov I, Jhajj M, Kodali N, Cheriyath P. A widened pulse pressure: a potential valuable prognostic indicator of mortality in patients with sepsis. J Community Hop Intern Med Perspect. 2015;5(6):29426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shalit M, Cotev S. Interrelationship between blood pressure and regional cerebral blood flow in experimental intracranial hypertension. J Neurosurg. 1974;40:594‐602. [DOI] [PubMed] [Google Scholar]
- 42. Cushing H. Some experimental and clinical observations concerning states of increased intracranial tension. Amer J Med Sci. 1902;124:375‐400. [Google Scholar]
- 43. Dunn L. Raised intracranial pressure. J Neurol Neurosurg Psychiatry. 2002;73(Suppl I):123‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klein I, Ojamaa K. Thyrotoxicosis and the heart. Endocrinol Metab Clin N Am. 1998;1(1):51‐62. [DOI] [PubMed] [Google Scholar]
- 45. Grossman W, Robin N, Johnson L, Brooks H, Selenkow H, Dexter L. The enhanced myocardial contractility of thyrotoxicosis: role of the beta adrenergic receptor. Ann Intern Med. 1971;74(6):869‐874. [DOI] [PubMed] [Google Scholar]
- 46. Thomas F, Blacher J, Benetos A, Safar M, Pannier B. Cardiovascular risk as defined in the 2003 European blood pressure classficiation: the assessment of an additional predictive value of pulse pressure on mortality. J Hypertens. 2008;26(6):1072. [DOI] [PubMed] [Google Scholar]
- 47. Domanski M, Mithcell G, Pfeffer M, et al. Pulse pressure and cardiovascular disease‐related mortality: follow‐up study of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA. 2002;287(20):2677. [DOI] [PubMed] [Google Scholar]
- 48. Okada K, Iso H, Cui R, Inoue M, Tsugane S. Pulse pressure is an independent risk factor for stroke among middle‐aged Japanese with normal systolic blood pressure: the JPHC study. J Hypertens. 2011;29(2):319. [DOI] [PubMed] [Google Scholar]
- 49. Panagiotakos D, Kromhout D, Menotti A, et al. The relation between pulse pressure and cardiovascular mortality in 12,763 middle‐aged men from various parts of the world: a 25‐year follow‐up of the seven countries study. Arch Intern Med. 2005;165(18):2142. [DOI] [PubMed] [Google Scholar]
- 50. Assmann G, Cullen P, Evers T, Petzinna D, Schulte H. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM. Eur Heart J. 2005;26(20):2120. [DOI] [PubMed] [Google Scholar]
- 51. Chae C, Pfeffer M, Glynn R. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281(7):634‐643. [DOI] [PubMed] [Google Scholar]
- 52. Myers MG, Kaczorowski J, Paterson J, Dolovich L, Tu K. Thresholds for diagnosing hypertension based on automated office blood pressure measurements and cardiovascular risk. Hypertension. 2015;66(3):489‐495. [DOI] [PubMed] [Google Scholar]
- 53. Haider A, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138(1):10‐16. [DOI] [PubMed] [Google Scholar]
- 54. Domanski M, Davis B, Pfeffer M, Kastantin M, Mitchell GF. Isolated systolic hypertension: prognostic information provided by pulse pressure. Hypertension. 1979;34(3):375‐380. [DOI] [PubMed] [Google Scholar]
- 55. Anstey D, Moise N, Kronish I, Abdalla M. Masked hypertension: whom and how to screen? Curr Hypertens Rep. 2019;21(4):26. [DOI] [PubMed] [Google Scholar]
- 56. Arulkumaran N, Diwakar R, Tahir Z, Mohamed M, Kaski J, Banerjee D. Pulse pressure and progression of chronic kidney disease. J Nephrol. 2010;23(2):189. [PubMed] [Google Scholar]
- 57. Bakris GL, Weir MR, Shanifar S, et al. Effects of blood pressure level on progression of diabetic nephropathy: results from the RENAAL study. Arch Intern Med. 2003;163(13):1555. [DOI] [PubMed] [Google Scholar]
- 58. Tozawa M, Iseki K, Oshiro S, et al. Evidence for elevated pulse pressure in patients on chronic haemodialysis: a case‐control study. Kidney Int. 2002;62:2195‐2201. [DOI] [PubMed] [Google Scholar]
- 59. Klassen P, Lowrie E, Reddan D, et al. Association between pulse pressure and mortality in patients undergoing maintenance haemodialysis. JAMA. 2002;287:1548‐1555. [DOI] [PubMed] [Google Scholar]
- 60. Blacher J, Guerin A, Pannier B, Marchais S, Safar M, London G. Impact of aortic stiffness on survival in end‐stage renal disease. Circulation. 1999;99:2434‐2439. [DOI] [PubMed] [Google Scholar]
- 61. Al‐Qamari A, Adeleke I, Kretzer A, Hogue CW. Pulse pressure and perioperative stroke. Curr Opin Anesthesiol. 2019;32(1):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Warren J, Nanayakkara S, Andrianopoulos N, et al. Impact of pre‐procedural blood pressure on long‐term outcomes following percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(22):2846‐2855. [DOI] [PubMed] [Google Scholar]
- 63. Low YH, Brudney CS, Pyati S. The significance (or the insignificance) of wide pulse pressure. Indian J Anesthes. 2016;60(11):864‐866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Johnson H, Bartels C, Thorpe C, Schumacher J, Pandhi N, Smith M. Differential diagnosis and treatment rates between systolic and diastolic hypertension in young adults: a multi‐disciplinary observational study. J Clin Hypertens. 2015;17(11):885‐894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Carey R, Whelton P. Prevention, detection, evaluation, and management of high blood pressure in adults: synopsis of the 2017 American College of Cardiology/American Heart Association hypertension guide. Ann Intern Med. 2018;168(5):351‐358. [DOI] [PubMed] [Google Scholar]
- 66. Safar M, Rudnichi A, Asmar R. Drug treatment of hypertension: the reduction of pulse pressure does not necessarily parallel that of systolic and diastolic blood pressure. J Hypertens. 2000;18(9):1159‐1163. [DOI] [PubMed] [Google Scholar]
- 67. Cushman WC, Materson BJ, Williams D, Reda D. Pulse pressure changes with six classes of antihypertensive agents in a randomized, controlled trial. Hypertension. 2001;38(4):953‐957. [DOI] [PubMed] [Google Scholar]
- 68. Chang J, Luchsinger J, Shea S. Antihypertensive medication class and pulse pressure in the elderly: analysis based on the third national health and nutrition examination survey. Am J Med. 2003;115(7):536‐542. [DOI] [PubMed] [Google Scholar]
- 69. Christensen K. Reducing pulse pressure in hypertension may normalize small artery structure. Hypertension. 1991;18(6):722‐727. [DOI] [PubMed] [Google Scholar]
- 70. Starmans‐Kool M, Klainjans H, Lustermans F, Kragten J, Breed J, Van Bortel L. Treatment of elderly patients with isolated systolic hypertension with isosorbide dinitrate in an asymmetric dosing schedule. J Human Hypertens. 1998;12:557‐561. [DOI] [PubMed] [Google Scholar]
- 71. Duchier J, Iannascoli F, Safar M. Antihypertensive effect of sustained‐release isosorbide dinitrate for isolated systolic systemic hypertension in the elderly. Am J Cardiol. 1987;60(1):99‐102. [DOI] [PubMed] [Google Scholar]
- 72. Bangalore S, Messerli F, Wun C, et al. J‐curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J. 2010;31(23):2897‐2908. [DOI] [PubMed] [Google Scholar]
- 73. Farnett L, Mulrow C, Linn W, Lucey C, Tuley M. The J‐curve phenomenon and the treatment of hypertension. JAMA. 1991;265(4):489‐494. [PubMed] [Google Scholar]
- 74. Alderman MH, Ooi WL, Shantha M, Cohen H. Treatment‐induced blood pressure reduction and the risk of myocardial infarction. JAMA. 1989;262(7):920‐924. [PubMed] [Google Scholar]
- 75. Owens P, O'Brien E. Hypotension in patients with coronary disease: can profound hypotensive events cause myocardial ischaemic events? Heart. 1999;82(4):477‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hansson L, Zanchetti A, Carruthers S, et al. Effects of intensive blood‐pressure lowering and low‐dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. Lancet. 1998;351(9118):1755‐1762. [DOI] [PubMed] [Google Scholar]
- 77. Wang J, Staessen JA, Franklin SS, Fagard R, Gueyffier F. Systolic and disatolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension. 2005;45(5):907. [DOI] [PubMed] [Google Scholar]
- 78. Knudsen S, Andersen N, Poulsen S, et al. Pulse pressure lowering effect of dual blockade with candesartan and lisinopril vs. high dose ACE inhibition in hypertensive type 2 diabetic subjects: A CALM II study post‐hoc analysis. Am J Hypertens. 2008;21(2):172‐176. [DOI] [PubMed] [Google Scholar]
- 79. Williams C, Kingwell BA, Burke K, McPherson J, Dart AM. Folic acid supplementation for 3 wk reduces pulse pressure and large artery stiffness independent of MTHFR genotype. Am J Clin Nutr. 2005;82(1):26‐31. 10.1093/ajcn.82.1.26 [DOI] [PubMed] [Google Scholar]
- 80. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507‐520. [DOI] [PubMed] [Google Scholar]
- 81. Group TSR . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]


