Abstract
Cardiovascular diseases (CVD) are leading causes of mortality and morbidity in the Americas, resulting in substantial negative economic and social impacts. This study describes the trends and inequalities of CVD burden in the Americas to guide programmatic interventions and health system responses. We examined the CVD burden trends by age, sex, and countries between 1990 and 2017 and quantified social inequalities in CVD burden across countries. In 2017, CVD accounted for 2 million deaths in the Americas, 29% of total deaths. Age‐standardized DALY rates caused by CVD declined by −1.9% (95% uncertainty interval, −2.0 to −1.7) annually from 1990 to 2017. This trend varied with a striking decreasing trend over the interval 1994‐2003 (annual percent change (APC) −2.4% [−2.5 to 2.2]) and 2003‐2007 (APC −2.8% [−3.4 to −2.2]). This was followed by a slowdown in the rate of decline over 2007‐2013 (APC −1.83% [−2.1 to −1.6]) and a stagnation during the most recent period 2013‐2017 (APC −0.1% [−0.5 to 0.3]). The social inequality in CVD burden along the socio‐demographic gradient across countries decreased 2.75‐fold. The CVD burden and related social inequality have both substantially decreased in the Americas since 1990, driven by the reduction in premature mortality. This trend occurred in parallel with the improvement in the socioeconomic development and health care of the region. The deceleration and stagnation in the rate of improvement of CVD burden and persistent social inequality pose major challenges to reduce the CVD burden and the achievement of the United Nations’ Sustainable Development Goals Target 3.4.
Keywords: cardiovascular diseases, epidemiology, global burden of disease, health inequalities, trend analysis
1. INTRODUCTION
Cardiovascular diseases (CVD) are leading causes of mortality and morbidity worldwide including the World Health Organization (WHO) Region of the Americas, producing substantial economic and social impacts. 1 Although CVD age‐standardized disability‐adjusted life years (DALY) rate per population has decreased remarkably from 1990 to 2015, one‐third of the 1.9 million annual CVD deaths in the region occurred in people aged less than 70 years. 2 , 3 Moreover, more than 80% the CVD burden could be averted since IHD, stroke, and hypertensive heart disease (HHD) can be prevented through effective population‐based interventions and policies to tackle unhealthy diets (including sodium reduction and trans‐fat elimination), tobacco use, physical inactivity, and alcohol use and are amenable to health care (eg, hypertension management, CVD secondary prevention, and treatment of acute cardiovascular events). 4 , 5 The Sustainable Development Goals Agenda 2030 (SDGs) 6 and the WHO NCD Global Action Plan 2013‐2020 7 have recognized the importance of CVD by targeting a one‐third reduction in premature mortality due to NCDs by 2030 while leaving no one behind.
In response, global initiatives, such as the Global HEARTS Initiative, 8 led by the WHO and the US Centers for Disease Control and Prevention (CDC) and other partners, and the Resolve To Save Lives (RTSL) hypertension program, 9 are supporting national CVD programs by implementing science‐based and cost‐effective strategies for accelerating the reduction of the CVD burden. These strategies require comprehensive analyses of the CVD burden and inequalities to guide programmatic interventions and the health system response.
This study aims to examine the current level and trends, and cross‐country social inequalities of CVD and major cause‐specific CVD categories (eg, IHD, stroke, and HHD) burden in the Region of the Americas and discuss its implication for health policies.
2. METHODS
2.1. The global burden of disease 2017 study
We conducted a secondary analysis to explore the level and trends of CVD burden using estimates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2017. 10 The GBD provides a systematic, comparable method of quantifying health loss by disease, age, sex, year, and location to provide information on more than 350 causes of disease and injury. A wide range of all available up‐to‐date data sources and standardized methods are applied to produce health metrics estimates with 95% uncertainty intervals (95% UI) for age, sex, and country for the years 1990‐2017. Results are updated in every GBD study iteration for the entire time series, and new results supersede previous versions. GBD uses various health loss outcome metrics, including deaths, incidence, prevalence, years of life lost (YLL) due to premature mortality, years lived with disability (YLD), and DALY. The methods used in the GBD have been described in detail elsewhere 11 , 12 , 13 , 14 and summarized in the Appendix S1, pp 5‐8.
2.2. Definition of cardiovascular diseases
Cardiovascular diseases was defined according to the International Classification of Diseases, 10th revision (ICD‐10) with the codes I00‐I99. 1 The GBD 2017 cause list includes all‐CVD, and CVD cause categories: rheumatic heart disease (ICD‐10: I01, 102.0, I05‐109), IHD (I20‐I25), stroke (G45‐G46.8, I60‐I69), HHD (I11), non‐rheumatic valvular heart disease (I34‐I37.8), cardiomyopathy, and myocarditis (B33.2, I40‐I41, I42.1‐I42.8, I43, I51.4), atrial fibrillation and flutter (I48), aortic aneurysm (I71), peripheral arterial disease (I70.2‐I70.8, I73), endocarditis (I33, I38‐I39), and a category for others CVD and circulatory conditions (I28‐I28.8, I30‐I31.1, I31.8‐I32.8, I47, I51.0‐I51.3, I68.0, I72, I77‐I83, I86‐I89.0, I89.9, I98, K75.1). The underlying cause of death approach was used for mortality estimates. From CVD cause categories, we focused on IHD, stroke, and HHD as they account for more than 80% of all‐CVD burden.
2.3. Data sources
Data sources and methods relevant to all‐CVD and CVD cause categories from the GBD 2017 have been previously described 1 , 15 , 16 and summarized in the Appendix S1, pp 9‐10. For this report, we extracted estimates and 95% UI for deaths, incidence, prevalence, DALY, YLD, and YLL for all‐CVD and the 11 CVD cause categories by sex and age group for the Region of the Americas, 6 subregions, and 37 countries and territories (Appendix S1, pp 11) from 1990 to 2017 using the GBD Results Tool (permalink: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2017-permalink/0f96da90892fd04e5c469b7646cec568) at the Global Health Data Exchange.
2.4. Socio‐demographic Index (SDI)
SDI is a composite index of the overall development that positions all locations on a spectrum of socioeconomic development, using average educational attainment over age 15 years, lagged distributed income, and total fertility rate under age 25 years generated by the GBD study. The SDI has a value that ranges from 0 to 1, where 0 represents the lowest income per capita, lowest educational attainment and highest fertility observed and 1 represents the highest income per capita, highest educational attainment and lowest fertility observed, across all GBD geographies from 1980 to 2017 (Appendix S1, pp 12‐13). 17
2.5. Exploratory data analysis
We examined number, age‐ and sex‐specific rates, all‐ages crude and age‐standardized rates of incidence, prevalence, death, DALY, YLL, and YLD from all‐CVD and 11 cause categories of CVD on a country‐specific basis for 37 countries and territories of the Americas from 1990 to 2017.
2.6. Time trends analysis
We performed joinpoint regression analysis 18 to assess trends in all‐CVD, IHD, stroke, and HHD burden from 1990 to 2017. We estimated inflection points (joinpoints) from trends, annual percent change (APC) for time segments between two adjacent joinpoints, and the average annual percentage change (AAPC) by regressing a log‐linear function of the age‐standardized YLD, YLL, and DALY rates per 100 000 population on year. We configured the model to detect a maximum of 5 joinpoints and avoiding time segments comprising only two data points to ensure rate changes in outcome measures were due to consistent changes over time and not year‐to‐year variations. We calculated AAPCs with 95% UI for 1990‐2017 and sub‐periods (1990‐1999, 2000‐2009, and 2010‐2017), by sex at regional and country levels. The AAPC is a summary measure of the trend over a specified time interval, computed as a weighted average of the APC of each time segment from the Joinpoint model, with weights equal to the length of each segment over time. A Monte Carlo method 19 with 4499 randomly permuted data sets was used to estimate P‐value of each permutation test and the AAPC 95% UI, while the overall asymptotic level of P‐value is maintained through a Bonferroni adjustment. We assumed constant variance in rates over time. However, these tests also consider situations with non‐constant variance, Poisson variation, and possible autocorrelation errors. AAPC is considered significant when it is different from "zero" at alpha = 0.05. A constant trend is considered when the zero value is within both AAPC 95% UI limits, an increasing trend when both 95% UI limits are positive, and a decreasing trend when both 95% UI limits are negative.
2.7. Social inequality analysis
We measured cross‐country CVD burden inequalities along a social gradient defined by the SDI in 1990, 2000, 2010, and 2017, as well as by the average years of educational attainment by sex, to quantify CVD inequalities and assess its progress over time. Distributive inequality of CVD, IHD, stroke, and HHD burden across countries was measured with the slope index of inequality (SII) and the health inequality concentration index (CIx). 20 The SSI was computed by regressing country‐level age‐standardized DALY, YLL, and YLD rates due to CVD, IHD, stroke, and HHD in all‐ages population on a relative social position scale, defined by the midpoint of the cumulative class interval of the population ranked by SDI, and education attainment. We used a weighted regression model to account for heteroscedasticity, and a logarithmic transformation of the CVD burden measure to account for non‐linearity. The CIx was computed by fitting a Lorenz concentration curve to the observed cumulative relative distributions of the population ranked by SDI and disease burden measures, and numerically integrating the area under the curve. 21
2.8. GATHER compliance
This study complies with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) statement (Appendix S1, pp 14‐17). 22
3. RESULTS
3.1. The current level of CVD burden, 2017
GBD 2017 estimates for 1990‐2017 are available for download from the GBD Results Tool at the Global Health Data Exchange. Estimates produced by our study are available for download from the CVD Burden Results Tool. In the Americas, a total of 14.0 million [95% UI] 13.5‐14.5) new cases from CVD were estimated in 2017, an age‐standardized incidence rate of 1,134.3 cases (1096.1‐1171.2) per 100 000 population, 1.2‐fold higher than 992 cases (893.1‐954.3) per 100 000 population worldwide. A total of 79.9 million prevalent cases (76.9‐83.1) and a prevalence rate of 6572.8 cases (6331.7‐6822.9) per 100 000 population were estimated, which is slightly higher than 6081.6 (5860.8‐6320.8) per 100 000 population globally. In 2017, CVD was the leading cause of death in the Americas, accounting for 2.0 million (2.0‐2.0) deaths, and age‐standardized mortality of 157.8 deaths (155.8‐159.9) per 100 000 population, which is two‐thirds of the mortality rate worldwide (233.1 deaths [229.7‐236.4]) per 100 000 population. The CVD burden in the Americas is lower than globally, accounting for 3159.4 DALYs (3047.1‐3280.4) per 100 000 population, including 2761.9 YLLs (2723.4‐2801.7) per 100 000 population (87% of total DALY), and 397.6 YLD (292.3‐511.8) (Table 1).
Table 1.
Incidence, prevalence, mortality, disability‐adjusted life years (DALY), year lived with disabilities (YLD), and years of life lost (YLL) due to premature mortality from Cardiovascular Diseases in the World, Region of the Americas, and Latin America and the Caribbean region in 2017 and average annual percent change (AAPC) from 1990 to 2017
| Measures | Global | Region of the Americas | Latin America and the Caribbean (LAC) |
|---|---|---|---|
| Incidence | |||
| Number of cases 2017 |
72 721 167 (70 388 093 to 75 264 106) |
13 993 132 (13 5270 09 to 14 461 164) |
3 851 702 (3 714 275 to 4 002 281) |
| Incidence rate 2017 (age‐standardized rate per 100 000 population) | 922.3 (893.1 to 954.3) |
1134.3 (1096.1 to 1171.2) |
674.9 (650.9 to 701) |
| Prevalence | |||
| Number of cases 2017 |
485 620 950 (468 031 728 to 504 964 407) |
79 935 863 (76 962 688 to 83 098 914) |
33 920 858 (32 482 364 to 35 392 977) |
| Prevalence rate 2017 (age‐standardized rate per 100 000 population) |
6081.6 (5860.8 to 6320.8) |
6572.8 (6331.7 to 6822.9) |
5892.5 (5642 to 6151.4) |
| Mortality | |||
| Number of deaths 2017 |
17 790 949 (17 527 068 to 18 042 674) |
2 031 122 (2 005 589 to 2 057 266) |
915 517 (901 344 to 929 859) |
| Age‐standardized death rates 2017 (per 100 000 population) | 233.1 (229.7 to 236.4) | 157.8 (155.8 to 159.9) | 166.6 (164 to 169.2) |
| AAPC 1990‐2017 | −1.3 (−1.5 to −1.1) | −2.0 (−2.1 to −1.8) | −1.9 (−2.1 to −1.8) |
| Burden of disease | |||
| Age‐standardized DALY rates per 100 000 population | 4597.9 (4463.7 to 4734.2) | 3159.4 (3047.1 to 3280.4) | 3359.8 (3250.6 to 3477.6) |
| AAPC 1990‐2017 | −1.2 (−1.4 to −1.0) | −1.9 (−2.0 to −1.7) | −1.9 (−2.0 to −1.7) |
| Age‐standardized YLL rates per 100 000 population |
4148 (4082 to 4210.8) (90% of DALYs) |
2761.9 (2723.4 to 2801.7) (87% of DALYs) |
3015.4 (2965.8 to 3065.7) (90% of DALYs) |
| AAPC 1990‐2017 | −1.3 (−1.5 to −1.1) | −2.0 (−2.2 to −1.9) | −2.0 (−2.2 to −1.9) |
| Age‐standardized YLD rates per 100 000 population |
449.9 (333.4 to 574.1) (10% of DALYs) |
397.6 (292.3 to 511.8) (13% of DALYs) |
344.3 (249.4 to 454.1) (10% of DALYs) |
| AAPC 1990‐2017 | 0.0 (−0.1 to 0.0) | −0.3 (−0.3 to −0.3) | −0.2 (−0.2 to −0.1) |
3.2. Changes in CVD burden, 1990‐2017
The age‐standardized rate of CVD DALY decreased substantially in the Region of the Americas at an AAPC of −1.9% (95% UI −2.0 to −1.7) from 5263.7 years (5143.1‐5390.0) per 100 000 population in 1990 to 3159.4 years (3047.1‐3280.4) in 2017. This reduction rate varied within the full period, with a striking decreasing trend in 1994‐2003 (APC −2.4% [−2.5 to −2.2]) and 2003‐2007 (APC −2.8% [−3.4 to −2.2]). A slowdown in the decline in 2007‐2013 (APC −1.83% [−2.1 to −1.6]) and a stagnation in the most recent segment 2013‐2017 (APC −0.1% [−0.5 to 0.3]) (Figure 1A). Similar reduction patterns occurred in females and males (Figure 1B, 1C).
Figure 1.
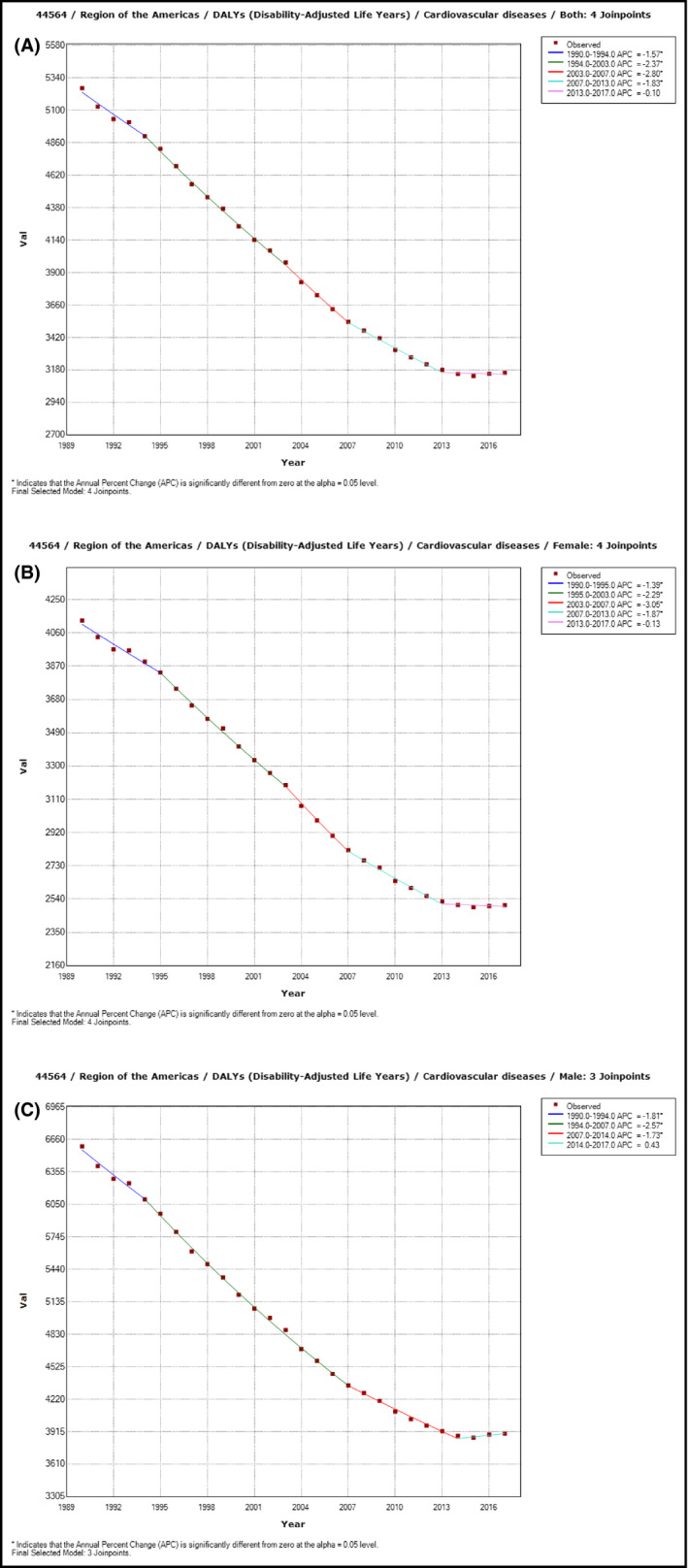
Trends in age‐standardized rates of cardiovascular disease disability‐adjusted life years per 100 000 populations by sex in the Region of the Americas, 1990‐2017. A: Both sexes combined. B: Female. C: Male. Dots represent estimates of the age‐standardized Cardiovascular diseases DALYs rate per 100 000 population, and the lines represent time series trend segments which slope, summarized by the annual percentage change (APC), are statistically different
Significant declines in age‐standardized DALY rates for CVD from 1990 to 2017 have been observed in all countries except the Dominican Republic with a constant trend (AAPC, 0.1% [−0.5 to 0.6]) (Table 2). However, from 2010 to 2017, the reduction rate in DALY improved in Peru and Paraguay, decelerated in almost all countries (eg, Argentina, Brazil, Canada, Colombia), stagnated in Belize, Cuba, El Salvador, Guatemala, Guyana, Mexico, Saint Lucia, Suriname, Trinidad and Tobago, the United States of Americas, and Venezuela; and conversely increased in Barbados, Costa Rica, Dominica, Dominican Republic, Jamaica, Saint Vincent and the Grenadines, and the US Virgin Islands (Table 2, Figure 2). Detailed time trends results of CVD burden measures (age‐standardized DALY, YLL, and YLD rates) by country and sex are in the Appendix S1, pp 18‐27.
Table 2.
Age‐standardized disability‐adjusted life years (DALY) rates per 100 000 population in 2017, and average (mean) annual percentage change in 1990‐2017 and 2010‐2017 in both sexes combines by country in the Region of the Americas
| Age‐standardized DALY rates per 100 000 population | |||
|---|---|---|---|
| Location Name | 2017 | Average annual percent change 1990‐2017 (%) | Average annual percent change 2010‐2017 (%) |
| Region of the Americas | 3159.4 (3047.1 to 3280.4) | −1.8 (−2 to −1.6) | −0.7 (−1.3 to −0.1) |
| Antigua and Barbuda | 3669 (3460 to 3891.5) | −1.8 (−2 to −1.5) | −0.6 (−0.8 to −0.4) |
| Argentina | 3561.9 (3233.1 to 3924.2) | −2.8 (−3.3 to −2.4) | −1.2 (−1.7 to −0.6) |
| Barbados | 3217.8 (2979.2 to 3463) | −1.8 (−2 to −1.5) | 0.3 (0 to 0.5) |
| Belize | 3709.5 (3536.8 to 3885.3) | −1.3 (−1.5 to −1.1) | −0.3 (−0.5 to 0) |
| Bermuda | 2518.5 (2357.1 to 2683.5) | −2.7 (−3 to −2.4) | −1.4 (−1.7 to −1.1) |
| Bolivia | 3766.2 (3160.2 to 4372.4) | −2.8 (−2.9 to −2.7) | −1.6 (−1.8 to −1.4) |
| Brazil | 3735 (3621.2 to 3849) | −2.8 (−3.1 to −2.5) | −1.8 (−2.4 to −1.1) |
| Canada | 1977.8 (1835 to 2125.3) | −1.8 (−1.9 to −1.7) | −0.1 (−0.4 to 0.2) |
| Chile | 2384.5 (2164.5 to 2618.1) | −2.6 (−3 to −2.3) | −1.5 (−1.9 to −1.2) |
| Colombia | 2423.2 (2207.1 to 2636.9) | −3.2 (−3.6 to −2.8) | −2.3 (−3.5 to −1.1) |
| Costa Rica | 2690 (2522.4 to 2880.7) | −1.7 (−2.1 to −1.3) | 1.6 (1 to 2.3) |
| Cuba | 3541.6 (3225.8 to 3854.5) | −1.3 (−1.7 to −1) | −0.5 (−1.3 to 0.4) |
| Dominica | 4406.7 (4154.4 to 4667.9) | −0.6 (−0.8 to −0.4) | 0.5 (0 to 1) |
| Dominican Republic | 5396.7 (4780.7 to 6024.8) | 0.1 (−0.5 to 0.6) | 1.9 (0.6 to 3.3) |
| Ecuador | 2704.2 (2485.2 to 2936.4) | −2.1 (−2.5 to −1.6) | −1.9 (−2.1 to −1.7) |
| El Salvador | 3265.5 (2844.8 to 3759.2) | −3 (−3.4 to −2.6) | 0.5 (−0.3 to 1.3) |
| Grenada | 4866.6 (4628.9 to 5112.5) | −2.4 (−2.7 to −2) | −0.4 (−0.5 to −0.2) |
| Guatemala | 2965.7 (2713.9 to 3225.5) | −1.1 (−2 to −0.1) | 0.6 (−0.4 to 1.6) |
| Guyana | 7727 (6998.9 to 8475.9) | −2.1 (−2.6 to −1.6) | −0.7 (−1.5 to 0.2) |
| Haiti | 8661.8 (7403.9 to 10 029.2) | −1.7 (−1.8 to −1.6) | −1.3 (−1.4 to −1.2) |
| Honduras | 4642 (3935.6 to 5391.1) | −1.7 (−1.9 to −1.5) | −0.8 (−1.1 to −0.4) |
| Jamaica | 4048.5 (3596.3 to 4567.7) | −0.7 (−1.3 to −0.2) | 2.4 (1.9 to 3) |
| Mexico | 2862 (2755.7 to 2986.5) | −1.5 (−2 to −1) | −0.5 (−1.3 to 0.2) |
| Nicaragua | 2579.6 (2322.4 to 2842) | −2.9 (−3.4 to −2.3) | −2.3 (−2.9 to −1.6) |
| Panama | 2479.1 (2324.8 to 2639.1) | −2.1 (−2.4 to −1.7) | −1.5 (−1.9 to −1.1) |
| Paraguay | 3832.5 (3345.8 to 4419.1) | −1.7 (−2.5 to −0.9) | −2.2 (−2.5 to −1.9) |
| Peru | 1812.5 (1594 to 2032.9) | −2.9 (−4 to −1.8) | −4.1 (−6.5 to −1.7) |
| Puerto Rico | 2214.6 (2078 to 2356) | −1.8 (−2.2 to −1.3) | −1.7 (−3.1 to −0.2) |
| Saint Lucia | 4005 (3761.3 to 4244.9) | −2.3 (−2.6 to −2) | 0.2 (−0.1 to 0.6) |
| Saint Vincent and the Grenadines | 5102.3 (4832.2 to 5372.1) | −0.7 (−1 to −0.4) | 0.7 (0.4 to 1) |
| Suriname | 5335.2 (4854.9 to 5791) | −0.6 (−0.9 to −0.3) | −0.5 (−1.2 to 0.2) |
| The Bahamas | 4882.4 (4527.4 to 5265.7) | −1 (−1.2 to −0.9) | −0.2 (−0.3 to −0.1) |
| Trinidad and Tobago | 4707.2 (4012.2 to 5489.5) | −2.1 (−2.4 to −1.8) | 0 (−0.9 to 0.9) |
| United States | 3029.7 (2900.9 to 3168) | −1 (−1.2 to −0.8) | 0.3 (−0.4 to 1) |
| Uruguay | 2988.3 (2726.8 to 3275.9) | −2 (−2.5 to −1.5) | −1.9 (−3.5 to −0.2) |
| Venezuela | 3870.9 (3428.3 to 4395.3) | −1.6 (−2 to −1.1) | 0 (−0.7 to 0.8) |
| Virgin Islands, US | 5102.2 (4434.9 to 5640.9) | −0.8 (−1 to −0.6) | 0.7 (0.4 to 1) |
Countries are sort alphabetically. AAPC is considered statistically significant when its value is different from "zero" at alpha = 0.05, or zero value is not within the AAPC 95 UI limits. There is a constant trend when the zero value is within both 95% UI limits for the AAPC. There is an increasing or upward trend when both AAPC 95% UI limits are positive, and a decreasing or downward trend when both AAPC 95% UI limits are negative.
Figure 2.
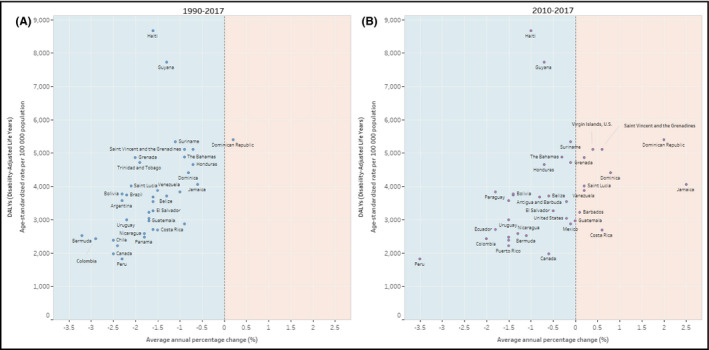
Age‐standardized rates of cardiovascular disease disability‐adjusted life years per 100 000 population in 2017 and average (mean) annual percentage change in 1990‐2017 (A) and 2010‐2017 (B) in both sexes combined by country. A: 1990‐2017. B: 2010‐2017. The light‐blue area represents a reduction in the average annual percentage change (downward trend), and the light‐orange area represents an increase in the average annual percentage change (upward trend)
3.3. Country variation in CVD burden in 2017
In 2017, the age‐standardized rates of CVD DALYs per 100 population vary importantly across countries of the Americas. The highest rates were observed in Haiti (8662 years [7404‐10 029]) and Guyana (7727 years [6998.9‐8475.9]); both countries are notable outliers (Figures 2 and 3). These high rates are followed by Dominican Republic, Suriname, Saint Vincent, and the Grenadines, and the US Virgin Islands. The lowest rates of CVD DALYs per 100 000 population were in Peru (1812 years; [1594‐2033]), and Canada (1978 years; [1835‐2125]), followed by Puerto Rico, Chile, Colombia, and Panama (Figure 2 and Table 2). IHD and stroke are the top two leading causes of the CVD burden, which combined share of the CVD DALY ranges from 75% to 85% across countries (Figure 3).
Figure 3.
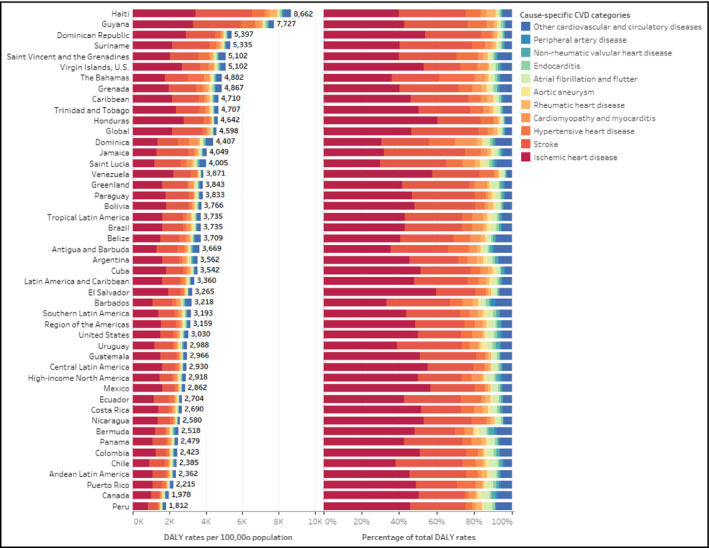
Age‐standardized rate and percentage of disability‐adjusted life years per 100 000 population for cardiovascular disease causes by subregion, and country in 2017
3.4. Age and sex disparities in CVD burden in 2017
In 2017 in the Americas, the CVD DALY increased with age exponentially starting at age 30‐34 years, being higher in men than women at every age group but 95 years and over. The largest cause of CVD in the first year of life was cardiomyopathy and myocarditis. IHD and stroke are by far the top two leading causes of CVD DALY in every age group from 30‐34 years to 95 years and over. The percent of total CVD DALY due to IHD and stroke increases from about 52% at 15‐19 years to more than 75% at 50‐54 years, leveling off around 75% at 95 years and over. (Figure 4).
Figure 4.
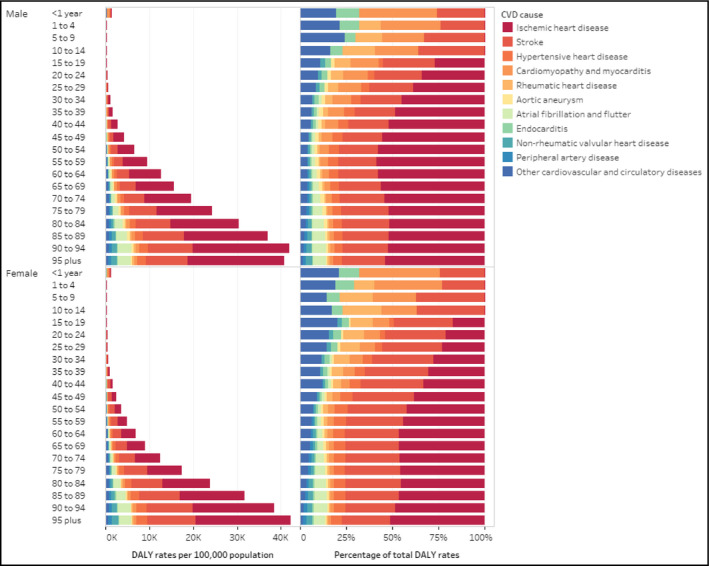
Age‐standardized rates and percentage of disability‐adjusted life years per 100 000 population for cardiovascular disease causes by age and sex in the Region of the Americas, 2017
3.5. Trends in cause‐specific CVD burden
From 1990 to 2017, the age‐standardized DALY rates due to cause‐specific CVD in the Americas decreased, except for peripheral artery disease and atrial fibrillation and flutter. The highest decrease in was observed in rheumatic heart disease (−2.3% [−2.4 to −2.2] per year) followed by IHD (−2.3% [−2.5 to −2.2]) and stroke (−1.8% [−2.0 to −1.6]) (Table 3, Figure 5). These cause‐specific CVD reduction rates decelerated from 2010 to 2017, while peripheral artery disease and atrial fibrillation and flutter continue having an upward trend (Table 3). Similar trends were observed in male and female, and in YLL, and YLD (Appendix S1, pp 28‐32).
Table 3.
Age‐standardized disability‐adjusted life years (DALY) rates per 100 000 population, in 1990 and 2017, and the average (mean) annual percentage change in 1990‐2017 and 2010‐2017 by cause‐specific cardiovascular diseases in both sexes combined in the Region of the Americas
| Age‐standardized DALY rates per 100 000 population | ||||
|---|---|---|---|---|
| Cardiovascular disease cause | 1990 | 2017 |
Average annual percentage change 1990‐2017 (%) |
Average annual percentage change 2010‐2017 (%) |
| Cardiovascular diseases | 5264 (5143 to 5390) | 3159 (3047 to 3280) | −1.9 (−2 to −1.7) | −0.8 (−1.1 to −0.6) |
| Ischemic heart disease | 2909 (2873 to 2962) | 1534 (1499 to 1572) | −2.3 (−2.5 to −2.2) | −1.1 (−1.4 to −0.8) |
| Stroke | 1355 (1302 to 1405) | 827 (782 to 873) | −1.8 (−2 to −1.6) | −0.7 (−1.3 to −0.1) |
| Cardiomyopathy and myocarditis | 196 (170 to 204) | 141 (134 to 156) | −1.2 (−1.4 to −1.1) | −0.6 (−1.1 to −0.2) |
| Hypertensive heart disease | 191 (158 to 215) | 168 (134 to 186) | −0.5 (−0.6 to −0.4) | −0.3 (−0.5 to 0) |
| Atrial fibrillation and flutter | 104 (85 to 126) | 108 (90 to 130) | 0.2 (0.1 to 0.2) | 0.1 (0.1 to 0.2) |
| Rheumatic heart disease | 106 (100 to 114) | 56 (50 to 64) | −2.3 (−2.4 to −2.2) | −1.6 (−1.8 to −1.3) |
| Aortic aneurysm | 82 (81 to 84) | 52 (50 to 53) | −1.7 (−1.8 to −1.6) | −0.5 (−0.8 to −0.2) |
| Non‐rheumatic valvular heart disease | 52 (45 to 57) | 46 (40 to 50) | −0.5 (−0.5 to −0.4) | 0 (−0.1 to 0.1) |
| Endocarditis | 39 (33 to 46) | 36 (32 to 45) | −0.2 (−0.4 to −0.1) | −0.1 (−0.4 to 0.3) |
| Peripheral artery disease | 20 (12 to 29) | 31 (19 to 54) | 1.7 (1.6 to 1.8) | 1.2 (1.1 to 1.3) |
| Other cardiovascular and circulatory diseases | 210 (190 to 236) | 161 (143 to 185) | −1 (−1 to −0.9) | −0.3 (−0.4 to −0.2) |
Cause‐specific cardiovascular diseases categories are sort ranked by magnitude of the burden measure in 2017. AAPC is considered statistically significant when its value is different from "zero" at alpha = 0.05, or zero value is not within the AAPC 95 UI limits. There is a constant trend when the zero value is within both 95% UI limits for the AAPC. There is an increasing or upward trend when both AAPC 95% UI limits are positive, and a decreasing or downward trend when both AAPC 95% UI limits are negative.
Figure 5.
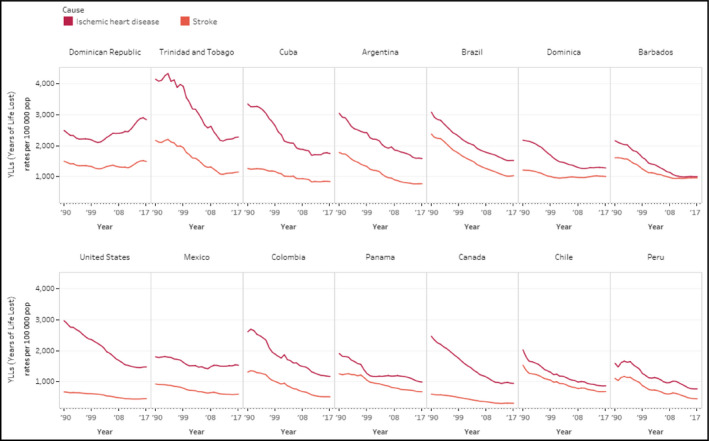
Age‐standardized years of life lost rates per 100 000 population from ischemic heart disease and stroke, both sexes combined in selected countries, 1990‐2017. This figure includes the 12 countries participating in the HEARTS initiative in the Region of the Americas as of the date of this study. United States and Canada are included as a reference for comparison
3.6. Social inequalities in CVD burden across countries
There were noticeable absolute and relative inequalities in the CVD burden across the 37 countries of the Americas; however, there were reductions in those inequalities during the period studied. The high CVD burden in both sexes combined was disproportionally concentrated among the most socially disadvantaged countries, as defined by the SDI. The SII shows an excess of −1443.3 (−2117.8 to −112.2) DALYs per 100 000 between the lowest and highest ends of the SDI gradient across countries in 1990; which went down to −525.1 (−1020.8 to 412.2) DALYs per 100 000 in 2017. Figure 6 shows the cross‐country health gradients of DALYs for both sexes combined in the SDI by all‐CVD, IHD, stroke, and HHD in 1990 and 2017. Only the IHD gradient in 1990 showed a mild pro‐poor distribution (ie, a disproportionate CVD burden concentration among the most socially advantaged); this pattern was reversed by 2017. For all‐CVD, and each four CVD cause categories were leveled down in 2017, attenuating their pro‐rich distribution observed in 1990.
Figure 6.
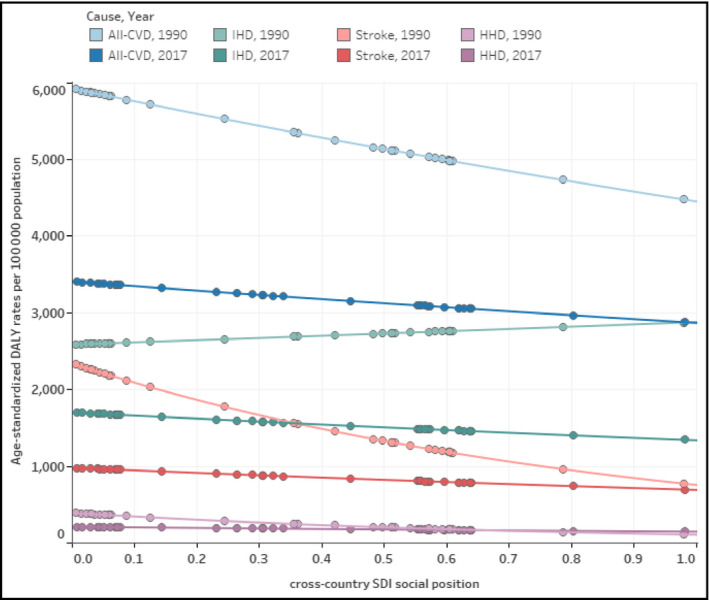
Health gradients of cross‐country inequality in the burden of cardiovascular disease, by cause category, both sexes combined in the Region of the Americas, 1990 and 2017. Dots represent countries for DALY rate and the SDI social position for a year (1990 or 2017) and cause‐specific cardiovascular diseases (CVD) category. Lines represent the best fit exponential regression for DALY rates on SDI social position, which visually approximate the slope of inequality. Cause‐specific CVD categories are label as follows: All‐CVD = all cardiovascular diseases combined; IHD = ischaemic heart disease; HHD = hypertensive heart disease. SDI: socio‐demographic index. DALY: disability‐adjusted life years
The stratification by education and sex revealed, in general, higher inequalities among women in all‐CVD burden: in 1990, there were an excess of −2626.7 (−2825.1 to −2150.7) DALYs per 100 000 among countries with the lowest educational attainment in women, compared to −1065.1 (−2115.9 to 1034.5) in men, accounting for a female‐to‐male relative gap of 2.47. In 2017 the SII for women showed an excess of −757.3 (−1082.0 to −81.1) DALYs per 100 000, whereas for men the SII was −432.7 (−1085.7 to 768.7), with a female‐to‐male gap of 1.75. Table 4 expands the results of the stratification by education and sex between 1990 and 2017 to IHD, stroke, and HHD as well. The mild pro‐poor distribution of the IHD gradient in the cross‐country SDI gradient in 1990 depicted in Figure 6 was only apparent among men, not women. The observed reductions in both absolute and relative cross‐country inequality in age‐standardized DALY rates between 1990 and 2017 were similar in both males and females for all‐CVD cause categories—except for IHD: its reduction was barely apparent in women and nonexistent in men. The complete set of results of exploratory analyses of cross‐country social inequalities in CVD burden by cause category, sex, and year, with age‐standardized deaths, YLD, and YLL rates is in the Appendix S1, pp 33‐52.
Table 4.
Summary measures of cross‐country social a inequality in age‐standardized DALY rates from cardiovascular diseases by sex in the Region of the Americas, 1990 and 2017
| Cause category | Sex group | year | Slope index of inequality | Concentration index of inequality | ||
|---|---|---|---|---|---|---|
|
Overall Cardiovascular diseases |
Both | 1990 | −1443.3 | (−2117.8 to −112.2) | −5.4 | (−15.2 to 4.4) |
| 2017 | −525.1 | (−1020.8 to 412.2) | −3.4 | (−13.2 to 6.4) | ||
| Female | 1990 | −2626.7 | (−2825.1 to −2150.7) | −11.2 | (−21.3 to −1.2) | |
| 2017 | −757.3 | (−1082 to −81.1) | −6.2 | (−16.2 to 3.7) | ||
| Male | 1990 | −1065.1 | (−2115.9 to 1034.5) | −3.5 | (−13.3 to 6.3) | |
| 2017 | −432.7 | (−1085.7 to 768.7) | −2.2 | (−11.9 to 7.5) | ||
|
Ischemic Heart disease |
Both | 1990 | 287.8 | (−300.4 to 1283) | 2.3 | (−7.2 to 11.9) |
| 2017 | −356.6 | (−567.6 to 48) | −4.1 | (−13.9 to 5.7) | ||
| Female | 1990 | −554.4 | (−793.8 to −112.7) | −4.8 | (−14.7 to 5) | |
| 2017 | −415.1 | (−533.4 to −144.1) | −7.7 | (−17.7 to 2.3) | ||
| Male | 1990 | 827.2 | (−138.5 to 2535.3) | 4.3 | (−5.2 to 13.9) | |
| 2017 | −258.6 | (−587.9 to 343.9) | −1.6 | (−11.3 to 8.1) | ||
| Stroke | Both | 1990 | −1548.4 | (−1439.6 to −1514.4) | −20.3 | (−30.5 to −10.1) |
| 2017 | −283.4 | (−395.9 to 12.5) | −7.3 | (−17.2 to 2.6) | ||
| Female | 1990 | −1575.4 | (−1478.9 to −1604.5) | −21.4 | (−31.7 to −11.2) | |
| 2017 | −231.7 | (−343.6 to 46.9) | −6.4 | (−16.3 to 3.5) | ||
| Male | 1990 | −1881.3 | (−1717.6 to −1920.8) | −22.5 | (−32.8 to −12.2) | |
| 2017 | −395.5 | (−492.5 to −103.4) | −9.2 | (−19.1 to 0.7) | ||
|
Hypertensive heart disease |
Both | 1990 | −276.6 | (−235.6 to −280.1) | −19.7 | (−29.9 to −9.5) |
| 2017 | −63.5 | (−89.5 to 58) | −3.9 | (−13.7 to 5.8) | ||
| Female | 1990 | −352.3 | (−290.7 to −397.5) | −21.7 | (−31.8 to −11.5) | |
| 2017 | −126.1 | (−123.6 to −69.2) | −9.4 | (−19.4 to 0.5) | ||
| Male | 1990 | −255.3 | (−221 to −245.6) | −15.9 | (−25.9 to −5.9) | |
| 2017 | 1.6 | (−56.5 to 210.3) | 2.6 | (−6.9 to 12.1) | ||
The negative sign of the slope index of inequality indicates a downward path from countries with the lowest social position (lowest SDI or education, the most socially disadvantaged) to the highest social position (highest SDI or education, the most socially advantage). The lower the burden, therefore, a negative sign of the slope. A negative sign of the concentration index of inequality indicates a concentration curve above the equidistribution line (the main diagonal), signaling that the burden of disease is disproportionally concentrated toward the most socially disadvantaged.
Sociodemographic index (SDI), for both sexes combined, and years of education attained, for females and males, were used as equity stratifiers.
4. DISCUSSION
Cardiovascular diseases is the leading cause of premature mortality in the Americas. However, our study reveals a substantial decline in the time trends of CVD burden in most of its 37 countries and territories from 1990 to 2017, driven by a significant reduction in premature CVD mortality, mainly IHD and stroke. This decline occurred in parallel with a better regional socioeconomic performance and better health care access and quality, fueled by the health system reform in most countries over the same interval. 23 It can be explained by the successful implementation of population health policies to reduce tobacco use, particularly in high‐income countries 24 and other risk factors such as cholesterol, 25 combined with a decline in raised blood pressure prevalence. These favorable risk factor trends were in turn driven by upstream improved socioeconomic conditions, behavioral risk factor profile and improved access and quality health care, 26 as well as more effective management of hypertension. 25 , 27
After decades of a favorable trend in decreasing age‐standardized CVD premature mortality rate, in the latest years (2007‐2017), a significant slowdown in the decline of the CVD burden was observed in many countries in the Americas, and 11 countries showed stagnation or a reversal in trends leading to increases. This trend shift, already documented for the USA 28 and high‐income countries, 29 might be explained by the high and increasing levels of prevalence in cardiometabolic risk factors driven by obesity and diabetes, documented in the Americas 25 and changes in other socioeconomic determinants. Indeed, the period 2007‐2013, and particularly 2007‐2009, coincided with the global financial crisis and the resulting social setbacks and austerity policies imposed on many countries. For example, in the USA, the health impacts of the 2007‐2009 economic crisis were more evident among men and racial/ethnic minorities. 30 Likewise, in Brazil, the effect of the 2014‐2016 economic recession showed 31 , 32 that an increase in the unemployment rate was associated with an increase in all‐cause mortality, mainly due to cancer and CVD. That impact was greater among black or mixed race, men, and individuals aged 30‐59 years. Both cases, the USA and Brazil, indicate the urgent need to identify potential factors impacting the capacity of current CVD prevention and control strategies to reduce CVD premature mortality, particularly among the most vulnerable population.
Our study shows a sustained and significant reduction of the social and gender inequalities in the burden of CVD across countries in the time frame studied. There are some relevant contentions to consider in these findings. Firstly, this reduction in inequality has been observed in one of the most inequitable regions in the world. 33 , 34 Secondly, this inequality reduction is coming from a distribution where the CVD burden is disproportionately concentrated among the countries most socially disadvantaged. This means the observed inequality reduction can be explained by reductions of the CVD burden in the most socially disadvantaged, and not due to the effect generated by the migration of the CVD burden from the affluent to the poor (ie, the so‐called pauperization of CVD burden). Our data show only one exception, in 1990 the IHD burden was still concentrated in males of the more affluent countries only to reverse that situation by 2017. Thirdly, this inequality reduction occurs in a regional scenario of a substantial decline in the average CVD burden: on one hand, this means that the inequality reduction is being achieved by leveling down the gradient (and not by leveling it up) and, on the other hand, this double‐trend 35 (that is, a reduction of CVD burden along with a reduction of CVD distributive inequality) signals, therefore, the right path to the ultimate goal of improving population health while leaving no one behind, as enshrined in the 2030 SDG Agenda.
Our finding that, after many decades of sustained reduction, premature mortality has stagnated or even increased in a third of the countries of the Region raise some compelling questions. What fraction of that stagnation or increase could be attributed to the persistence of unjustified social inequalities both across or within the countries? For instance, the PURE study 36 documented that people with a low level of education in low‐income and middle‐income countries, even with better overall CVD risk factor profiles than people in high‐income countries, have higher incidence and mortality from CVD because they have markedly poorer health care. Likewise, from 1999 through 2017, death rates for heart disease decreased for all racial and ethnic groups in the USA. However, non‐Hispanic black people were more than twice as likely as Asian or Pacific Island and non‐Hispanics to die of heart disease both in 1999 and 2017. 37
While the methods of this study can be applied in assessing the magnitude, trends, and social inequalities of the CVD burden, the results can be used for monitoring and performance evaluation at several levels. Additionally, the key finding of the study—the slowdown and stagnation of CVD mortality—serves as a powerful call for urgent actions.
This study has several strengths. It used the most recent estimates of CVD burden, which are based on all available data sources from countries and territories of the Americas, and a set of standard methods and procedures to produce comparable estimates of the CVD burden. The application of the joinpoint regression analysis 19 allows the following: (a) estimation of the AAPC and their 95% UI, as a summary measure of change for assessing trends; (b) identification of the inflection points (joinpoints) along the time series. This is an indication of trend changes where there is no evidence to believe that it is produced randomly, so it is expected to be a result of health interventions, population exposure to risk factors or determinants associated with the outcome. The quantification of the social inequality, namely the SII and the CIx, in CVD burden is also a strength and an innovation.
This study has several limitations, and those related to the GBD 2017 methodology and specifically for estimating the CVD burden are extensively described elsewhere. 1 , 11 , 12 , 16 A limitation is the level of under‐registration and incomplete medically certified cause of death in the vital statistics system from Haiti, Honduras, and Bolivia. Moreover, population‐based data for morbidity and sequelae due to CVD are generally poorly or not available for many countries. Indeed, estimates for countries with missing or limited data are modeled in the GBD and are of lower reliability than observed data collected via a robust surveillance and vital registration system. Data incompleteness leads to modeling, resulting in increased uncertainty, and potential under‐ and over‐estimations. Further data quality assessment is needed, particularly estimating the completeness of death registration and quantifying the proportion of cause‐of‐death coded as “garbage codes”; and applying standard data corrections and adjustments to overcome data incompleteness, and death distribution methods to improve the value of underlying cause of death for public health. Another limitation of our study is the exploratory nature of the inequality analysis, restricted to cross‐country inequalities, an analytical approach that cannot account for within‐country inequalities.
Cardiovascular diseases remains the leading cause of the disease burden in all countries of the Americas, accounting for 29% of all NCDs and 43% of the four major NCDs (CVD, cancer, diabetes, and chronic respiratory diseases). Moreover, of the four major NCDs, CVD has the highest decline rate, cancers have a moderate rate of decline, which has been stagnating in recent years, while diabetes rates have been increasing since 2013. Although to contain and reverse the recently observed slowdown and stagnation of CVD burden must be the highest priority for the entire Region, the challenge is even greater for the LMIC because they do not have enough resources neither the health system prepared to resist this enormous burden. This challenge requires a more innovative, socially inclusive, and strategic effort to implement evidence‐based low‐cost interventions with potential to safe millions of deaths, such as salt reduction through the replacement of regular salt with potassium‐enriched substitutes which can reduce blood pressure and hypertension incidence, 38 elimination of artificial trans‐fat, and improvement of hypertension management in primary care through simple standardized treatment protocols, a core set of medications, team‐based care and closely monitor patient progress and system performance. 39 Along with theses key interventions, this Region and particularly its most vulnerable economies can benefit significantly with more aggressive measures to reduce tobacco use and to overturn the obesity epidemic. Indeed, 12 countries of the Americas, supported by PAHO/WHO, CDC, RTSL, and World Hypertension League among other partners, are already implementing HEARTS, an innovative model which seeks to integrate effective public policies and interventions smoothly and progressively into already existing health delivery services to promote the adoption of global best practices in the prevention and control of CVD and improve the performance of the services through better control of hypertension and the promotion of CVD secondary prevention with emphasis on primary health care. 40 , 41
This study presents a robust methodological approach to monitor and evaluate the impact of such public policies and programs, especially for countries implementing HEARTS. We envision some future researches: (a) locally contextualized analyses to elucidating causes and drivers of its slowdown and stagnation; (b) measurement of social inequalities of CVD burden at subnational level; (c) quantification of the CVD burden impact on the life expectancy; and (d) evaluation of the potential impact of the premature CVD mortality reduction deceleration on reaching the SDG target 3.4 at country level.
5. CONCLUSIONS
The CVD burden and the related social inequality have substantially decreased in the Region of the Americas since 1990, driven by the accelerated reduction in premature CVD mortality, mainly in IHD and stroke. This positive trend has been occurring in parallel with the improvement in the socioeconomic development of the region including better access and quality of health care in most countries. However, the slowdown in the decline rate of CVD premature mortality in many countries, and the increasing trends in some countries in the most recent years, together with the remained socio‐demographic development inequalities across and within countries pose a significant challenge for programmatic efforts to reduce the CVD burden and to reach the SDG target 3.4.
CONFLICT OF INTEREST
All authors declare no conflicts of interest with the content of this manuscript.
AUTHOR CONTRIBUTIONS
R Martinez and P Ordunez contributed to the research idea and study design. R Martinez, PN Soliz, OJ Mujica, and P Ordunez had full access to all of the data in the study, conducted the data analyses, and take responsibility for the integrity of the data and the accuracy of the data analysis. R Martinez and P Ordunez were involved in drafting the manuscript. All authors contributed in interpretation of findings, and important intellectual content during the manuscript revisions. All authors accept accountability for the overall work.
DISCLOSURE STATEMENT
RM, PS, OJM, LR, and PO are staff members of the Pan American Health Organization. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the support of the Department of Noncommunicable Diseases and Mental Health, Pan American Health Organization, and Dr Anselm Hennis for the review of the manuscript and providing valuable suggestions.We would also like to acknowledge the support of the US Centers for Disease Control and Prevention, Resolve to Save Lives, World Hypertension League, and Lancet Commission on Hypertension Group and its commissioner J Patricio Lopez‐Jaramillo. This analysis was supported by Bloomberg Philanthropies and Resolve to Save Lives, an initiative of Vital Strategies, through a grant to the National Foundation for the Centers for Disease Control and Prevention Inc (CDC Foundation). Resolve to Save Lives is funded by grants from Bloomberg Philanthropies; the Bill and Melinda Gates Foundation; and Gates Philanthropy Partners, which is funded with support from the Chan Zuckerberg Foundation.
Martinez R, Soliz P, Mujica OJ, Reveiz L, Campbell NRC, Ordunez P. The slowdown in the reduction rate of premature mortality from cardiovascular diseases puts the Americas at risk of achieving SDG 3.4: A population trend analysis of 37 countries from 1990 to 2017. J Clin Hypertens. 2020;22:1296–1309. 10.1111/jch.13922
REFERENCES
- 1. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70(1):1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez R, Soliz P, Caixeta R, Ordunez P. Reflection on modern methods: Years of life lost due to premature mortality ‐ A versatile and comprehensive measure for monitoring non‐communicable disease mortality. Int J Epidemiol. 2019;48(4):1367‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lloyd‐Sherlock P, Ebrahim S, Martinez R, McKee M, Ordunez P. Reducing the cardiovascular disease burden for people of all ages in the Americas region: analysis of mortality data, 2000–15. Lancet Global Health. 2019;7(5):e604‐e612. [DOI] [PubMed] [Google Scholar]
- 4. Hachinski V, Azarpazhooh MR. Stroke is a burdensome but preventable brain disorder. Lancet Neurol. 2016;15(9):892‐893. [DOI] [PubMed] [Google Scholar]
- 5. Martinez R, Lloyd‐Sherlock P, Soliz P, et al. Trends in premature avertable mortality from non‐communicable diseases for 195 countries and territories, 1990–2017: a population‐based study. Lancet Global Health. 2020;8(4):e511‐e523. [DOI] [PubMed] [Google Scholar]
- 6. The sustainable development agenda ‐ United Nations sustainable development. https://www.un.org/sustainabledevelopment/development-agenda/. Accessed January 12, 2020.
- 7. World Health Organization . Global action plan for the prevention and control of noncommunicable diseases: 2013–2020.
- 8. World Health Organization . WHO; Global Hearts Initiative, working together to promote cardiovascular health. https://www.who.int/cardiovascular_diseases/global-hearts/en/. Accessed March 27, 2020.
- 9. Frieden TR, Bloomberg MR. Saving an additional 100 million lives. Lancet. 2018;391(10121):709‐712. [DOI] [PubMed] [Google Scholar]
- 10. Global Burden of Disease Study 2017 (GBD 2017) Data Resources. GHDx. http://ghdx.healthdata.org/gbd-2017. Accessed January 12, 2020.
- 11. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age‐sex‐specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dicker D, Nguyen G, Abate D, et al. Global, regional, and national age‐sex‐specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1684‐1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kyu HH, Abate D, Abate KH, et al. Global, regional, and national disability‐adjusted life‐years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roth GA, Johnson CO, Nguyen G, et al. Methods for estimating the global burden of cerebrovascular diseases. Neuroepidemiology. 2015;45(3):146‐151. [DOI] [PubMed] [Google Scholar]
- 17. Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2017 (GBD 2017) Socio‐Demographic Index (SDI) 1950–2017. GHDx. http://ghdx.healthdata.org/record/ihme-data/gbd-2017-socio-demographic-index-sdi-1950%E2%80%932017. Published 2018. Accessed January 12, 2020.
- 18. National Cancer Institute . Joinpoint regression program. https://surveillance.cancer.gov/joinpoint/. Accessed January 12, 2020.
- 19. Kim H‐J, Fay MP, Feuer EJ, Midthune DN. Permutation tests for Joinpoint Regression with applications to Cancer rates. Statist Med. 2000;19:351. [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization . Handbook on Health Inequality Monitoring, with a Special Focus on Low‐ and Middle‐Income Countries. Geneva, Switzerland: World Health Organization; 2013. [Google Scholar]
- 21. Mújica ÓJ, Moreno CM. From words to action: measuring health inequalities to “leave no one behind”. Pan Am J Public Health. 2019;43:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevens GA, Alkema L, Black RE, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. 2016;388(10062):e19‐e23. [DOI] [PubMed] [Google Scholar]
- 23. Atun R, de Andrade LOM, Almeida G, et al. Health‐system reform and universal health coverage in Latin America. Lancet. 2015;385(9974):1230‐1247. [DOI] [PubMed] [Google Scholar]
- 24. Bilano V, Gilmour S, Moffiet T, et al. Global trends and projections for tobacco use, 1990–2025: An analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385(9972):966‐976. [DOI] [PubMed] [Google Scholar]
- 25. NCD Risk Factor Collaboration (NCD‐RisC)—Americas Working Group . Trends in cardiometabolic risk factors in the Americas between 1980 and 2014: a pooled analysis of population‐based surveys. Lancet Global Health. 2020;8(1):e123‐e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee ES, Vedanthan R, Jeemon P, et al. Quality improvement for cardiovascular disease care in low‐ and middle‐income countries: a systematic review. PLoS One. 2016;11(6):e0157036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. NCD Risk Factor Collaboration (NCD‐RisC) . Contributions of mean and shape of blood pressure distribution to worldwide trends and variations in raised blood pressure: a pooled analysis of 1018 population‐based measurement studies with 88.6 million participants. Int J Epidemiol. 2018;47(3):872‐883i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roth GA, Johnson CO, Abate KH, et al. The burden of cardiovascular diseases among us states, 1990–2016. JAMA Cardiol. 2018;3(5):375‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopez AD, Adair T. Is the long‐term decline in cardiovascular‐disease mortality in high‐income countries over? Evidence from national vital statistics. Int J Epidemiol. 2019;48(6):1815‐1823. [DOI] [PubMed] [Google Scholar]
- 30. Margerison‐Zilko C, Goldman‐Mellor S, Falconi A, Downing J. Health impacts of the great recession: a critical review. Curr Epidemiol Rep. 2016;3(1):81‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hone T, Mirelman AJ, Rasella D, et al. Effect of economic recession and impact of health and social protection expenditures on adult mortality: a longitudinal analysis of 5565 Brazilian municipalities. Lancet Global Health. 2019;7(11):e1575‐e1583. [DOI] [PubMed] [Google Scholar]
- 32. Malta DC, Duncan BB, de Barros MBA,, et al. Fiscal austerity measures hamper noncommunicable disease control goals in Brazil. Ciencia e Saude Coletiva. 2018;23(10):3115‐3122. [DOI] [PubMed] [Google Scholar]
- 33. Pan American Health Organization . Health in the Americas+, 2017 Edition. Summary: Regional Outlook and Country Profiles. 2017. https://www.paho.org/salud-en-las-americas-2017/wp-content/uploads/2017/09/Print-Version-Spanish.pdf. Published 2017. Accessed March 11, 2020.
- 34. Economic Commission for Latin America and the Caribbean (ECLAC) . Social Panorama of Latin America 2018. LC/PUB.2019/3‐P. Santiago de Chile; 2019. http://repositorio.cepal.org/bitstream/handle/11362/44396/4/S1900050_en.pdf. Accessed March 11, 2020.
- 35. Minujin A, Delamonica E. Mind the Gap! Widening child mortality disparities. J Hum Dev. 2003;4(3):397‐418. [Google Scholar]
- 36. Yusuf S, Joseph P, Rangarajan S, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high‐income, middle‐income, and low‐income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. U.S Centers for Disease Prevention and Control . Health, United States Spotlight Racial and Ethnic Disparities in Heart Disease. 2019:2. https://www.cdc.gov/nchs/hus/spotlight/2019-heart-disease-disparities.htm
- 38. Bernabe‐Ortiz A, Sal y Rosas VG, Ponce‐Lucero V, et al. Effect of salt substitution on community‐wide blood pressure and hypertension incidence. Nat Med. 2020;26(3):374‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kontis V, Cobb LK, Mathers CD, Frieden TR, Ezzati M, Danaei G. Three public health interventions could save 94 million lives in 25 years. Circulation. 2019;140(9):715‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. PAHO/WHO; HEARTS in the Americas. https://www.paho.org/hq/index.php?option=com_content&view=article&id=15056:hearts-in-the-americas&Itemid=3465&lang=en. Accessed March 23, 2020.
- 41. Valdés González Y, Campbell NRC, Pons Barrera E, et al. Implementation of a community‐based hypertension control program in Matanzas, Cuba. J Clin Hypertens. 2020;22(2):142‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


