Abstract
Although electrocardiography (ECG) is a cost‐effective and convenient tool for routine screening of left ventricular hypertrophy (LVH), its performance has been shown to be poor. The Peguero‐Lo Presti, a novel voltage criterion, was found to be potentially better than the most commonly used criteria. We conducted a systematic review and meta‐analysis of its diagnostic accuracy compared to Cornell and Sokolow‐Lyon voltage criteria. Bibliographic databases were searched to identify relevant articles. Pooled sensitivity, specificity, diagnostic odds ratio (DOR), and summary receiver operating characteristic (ROC) curves were performed for comparison. Ten studies reporting data from 5984 individuals were included in the meta‐analysis. Peguero‐Lo Presti had the highest pooled sensitivity (43.0%, 95% confidence interval [CI]: 30.2‐56.9) followed by Cornell (26.1%; 95% CI: 16.9‐37.9) and Sokolow Lyon (22.0%; 95% CI: 14.1‐32.7). However, Peguero‐Lo Presti had the lesser pooled specificity (90.5%; 95% CI: 86.3‐93.5) and Cornell the highest (94.9%; 95% CI: 90.3‐97.3). The pooled DOR was 6.63 (95% CI: 3.95‐11.13), 5.50 (95% CI: 3.64‐8.30), and 2.94 (95% CI: 2.20‐3.92) for Peguero‐Lo Presti, Cornell, and Sokolow‐Lyon, respectively. Peguero‐Lo Presti had the best accuracy according to summary ROC curves, with an area under the curve of 0.827 compared to 0.715 for Cornell, and 0.623 for Sokolow‐Lyon. In conclusion, according to this meta‐analysis, Peguero‐Lo Presti has a better diagnostic performance than Cornell and Sokolow‐Lyon and might be more useful in routine clinical practice as a screening tool for LVH.
Keywords: Cornell, electrocardiography, left ventricular hypertrophy, Peguero‐Lo Presti, Sokolow‐Lyon
1. INTRODUCTION
Left ventricular hypertrophy (LVH) is a marker of subclinical cardiac disease, and modifiable predictor of incident cardiovascular disease and mortality. 1 , 2 Early and appropriate treatment can reverse LVH and its adverse clinical outcomes. 3 Therefore, early detection of LVH is a key component of primary and secondary cardiovascular prevention. Electrocardiography (ECG) is the most cost‐effective and convenient tool in routine clinical practice for the screening of LVH, which could be further assessed by cardiac imaging including echocardiography or cardiac MRI. 4
More than 30 ECG criteria have been developed for the screening of LVH and are endorsed by the American Heart Association. 4 However, all these criteria have low sensitivity, even despite improvement after adjustment for some extracardiac factors such as age and body habitus. 5 , 6 In 2017, Peguero and Lo‐Presti proposed a novel ECG voltage criterion for ECG screening of LVH. They showed that their proposed criterion has better diagnostic accuracy than the Cornell and Sokolow‐Lyon indices which were the most commonly used criteria. 7 However, following the release of the Peguero‐Lo Presti criterion, several studies evaluating its performance in various populations have been published, showing conflicting results. 8 , 9 , 10 Hence, we conducted a systematic review and meta‐analysis of diagnostic accuracy studies comparing the Peguero‐Lo Presti to the Cornell and Sokolow‐Lyon voltage criteria.
2. METHODS
This review was conducted and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines. 11 No protocol was published for this review.
2.1. Literature search
A systematic search in PubMed/MEDLINE, Excerpta Medica Database (EMBASE), and Google Scholar was performed to identify relevant studies published from inception through January 1, 2020, without language restriction. To have the highest sensitivity, the search strategy was only on the term “Peguero‐Lo Presti” and potential variants, such as “Peguero Lo‐Presti” and “Peguero Lo Presti,” without any other combination. The reference lists of all eligible articles and relevant reviews were reviewed to identify potential additional data sources.
2.2. Selection of studies to include in the review
We included all studies, irrespective of the study design, reporting data on the sensitivity and specificity of the Peguero‐Lo Presti criteria compared to the Cornell and Sokolow‐Lyon voltage criteria for the ECG diagnosis of LVH, or enough data to compute these estimates. These studies had to have used either echocardiography (or cardiac MRI) as the reference standard, with echocardiographic LVH defined as a left ventricular mass index (to body surface area) >115 g/m2 in male subjects and >95 g/m2 in female subjects. 12 We excluded studies for which we could not obtain raw data to calculate the sensitivity and specificity of the ECG criteria of interest, from both the published article and by contacting the authors. We included abstracts from conference proceedings if they had the required information.
Two investigators (JJN and UFN) independently screened the titles and abstracts of all retrieved records, then the full texts to determine eligibility according to the abovementioned selection criteria, and consensually retained studies to be included. Disagreements were solved through a discussion.
2.3. Data extraction and management
Data were extracted by one investigator (JJN) and cross‐checked by another investigator (UFN). These data included the following: name of the first author, year of publication, study design, country, total number of participants, mean or median age, proportion of males, and number of true positives, true negatives, false positives, and false negatives with respect to the each index criterion (Peguero‐Lo Presti, Sokolow‐Lyon, and Cornell) and the reference standard (echocardiography or cardiac MRI). For articles in which the true positives, true negatives, false positives, and false negatives were not provided, we requested them from the authors or derived them from the information available in the article.
The Peguero‐Lo Presti criterion is defined as the summation of the amplitude of the deepest S wave in any lead (SD) with the S wave in lead V4 (SV4), with a cutoff of ≥2.8 mV in males and ≥2.3 mV in females. 7 Sokolow‐Lyon voltage is obtained by adding the amplitude of S in V1 and the amplitude of R in V5 or V6 (SV1 + RV5 or RV6) with a cutoff of >3.5 mV, while the Cornell is computed as the amplitude of R in aVL plus the amplitude of S or QS complex in V3 (RaVL + SV3) with a cutoff of >2.8 mV in males and >2.0 mV in females. 4
We used the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) to assess the risk of bias in included studies. 13 Two investigators (JJN and UFN) independently ran the assessment. Discrepancies were discussed and resolved by these investigators through discussion and consensus.
2.4. Statistical analysis
We extracted the number of patients who were true positive (TP), false positive (FP), true negative (TN), and false negative (FN) for LVH, respectively. Where some of these data were missing, we calculated them from the specificity, sensitivity, and sample size provided by the included studies, according to the 2 × 2 table. 14 , 15 We performed descriptive statistics for sensitivity, specificity, and diagnostic odds ratio (DOR), as well as positive and negative likelihood ratios (LR+, LR−). Both pooled sensitivity and specificity were computed by the univariate analysis model using the metaprop function of meta package (R version 3.5.3, R Core Team). This function uses the number of events (TP or TN) and samples (TP + FN or TN + FP) and uses the Restricted Estimator of Maximum Likelihood method. DOR was computed using the metabin function of meta in R. Results were summarized in the forms of forest plots. To obtain a summary receiver operator characteristic (SROC) curve, we used the bivariate linear mixed model approach in mada package in R. 16 We used the reitsma function of mada to compute logit‐transformations of pairs of sensitivity and false‐positive rate (1‐specificity), before plotting the results as a ROC curve. We also computed the overall area under the SROC curve (AUC). The heterogeneity of included studies was evaluated by the Higgins' I 2‐statistic. 17 Statistical significance was defined as a two‐tailed P‐value < .05.
2.5. Ethical approval
This study is a systematic review and meta‐analysis using published data. No data were collected directly from patients. Therefore, an ethical approval is not needed.
2.6. Patient and Public involvement
As this is not a primary research, patients and the public were not involved.
3. RESULTS
3.1. Studies selection and characteristics
In total, we identified 128 records among which 10 studies reporting data from 5984 individuals were finally included in the meta‐analysis (Figure S1). 7 , 9 , 10 , 18 , 19 , 20 , 21 , 22 , 23 , 24 Additionally, four studies were included for a narrative summary only, as they did not provide enough data for inclusion in the meta‐analysis. 8 , 25 , 26 , 27 Study characteristics are summarized in Table 1 and Table S1. All the included studies had a low risk of bias (Figure S2). Eight studies used echocardiography as the reference standard for the diagnosis of LVH, while two studies used cardiac MRI. Six of these studies were conducted mostly in people with European ancestry, with one study each done participants of Chinese, Indian, Japanese, and Arab ancestries, respectively.
Table 1.
Characteristics of prevalence studies
| First author | Year of publication | Country | Design | Population | Patients included | Male (%) | Mean age (y) | Reference standard | Patients with LVH (%) | Sensitivity (%) | Specificity (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peguero‐Lo Presti | Cornell | Sokolow‐Lyon | Peguero‐Lo Presti | Cornell | Sokolow‐Lyon | ||||||||||
| Keskin | 2019 | Turkey | Prospective | Patients referred for echocardiography | 767 | 42.7 | 51.9 | Echocardiography | 154 (20.1) | 17.5 | 9.7 | 3.9 | 94.4 | 98.2 | 97.5 |
| Rodrigues | 2019 | Australia | Prospective | Patients with end‐stage kidney disease | 33 | Not reported | 49 | Echocardiography and cardiac MRI | 11 (33.3) | 55.0 | 46.0 | 18.0 | NR | NR | NR |
| Moustafa | 2019 | Egypt | Prospective | Patients referred for echocardiography | 200 | 79.5 | 59.8 | Echocardiography | 83 (41.5) | 55.4 | 32.5 | 26.5 | 92.3 | 97.4 | 82.9 |
| Ricciardi | 2019 | Italy | Retrospective | Patients who had echocardiography and ECG | 2134 | 48 | 69 | Echocardiography | 1251 (58.6) | 41.1 | 36.7 | 24.7 | 78.3 | 85.5 | 91.6 |
| Sparapani | 2019 | USA | Prospective | Participants from a population‐based study who had ECG and cardiac MRI data | 940 | 46.6 | 61.0 | Cardiac MRI | 69 (7.3) | 14.5 | 5.8 | 21.7 | 93.8 | 97.2 | 94.1 |
| Azevedo | 2019 | Portugal | Retrospective | Patients with hypertrophic cardiomyopathy | 88 | 63 | 56.7 | Cardiac MRI | 34 (38.6) | 58.8 | 38.2 | 41.2 | 96.3 | 98.1 | 94.4 |
| Narita | 2019 | Japan | Retrospective | Participants in a population‐based study | 866 | 37 | 57 | Echocardiography | 156 (18.0) | 20.5 | 11.5 | 14.7 | 94.1 | 99.0 | 94.2 |
| Patted | 2018 | India | Prospective | Hypertensive patients who had echocardiography and ECG | 400 | 73.5 | 63.8 | Echocardiography | 192 (48) | 54.2 | 39.6 | 29.2 | 91.3 | 89.4 | 86.5 |
| Shao | 2018 | China | Prospective | Hospitalized hypertensive patients | 235 | 49.4 | 65.3 | Echocardiography | 116 (49.4) | 73.3 | 56.0 | 57.8 | 75.6 | 84.9 | 58.0 |
| Lim | 2018 | Australia | Retrospective | Patients who had echocardiography and ECG | 144 | 59 | 74 | Echocardiography | 16.0 | 7.6 | 4.2 | NR | NR | NR | |
| Rodrigues | 2018 | Australia | Prospective | Patients with type 2 diabetes | 88 | 64 | 68 | Echocardiography | 26 (29) | 15.3 | 7.7 | 3.8 | NR | NR | NR |
| Sun | 2018 | China | Prospective | Participants in a population‐based study | 10 614 | 45.4 | 53.7 | Echocardiography | NR | NR | NR | NR | NR | NR | NR |
| Peguero | 2017 | USA | Prospective | Patients referred for echocardiography | 216 | 49.1 | 61.9 | Echocardiography | 81 (37.5) | 61.7 | 34.6 | 17.3 | 90.4 | 91.8 | 97.8 |
| Ramchand | 2017 | Australia | Prospective | Patients with aortic stenosis | 138 | 61 | 74 | Echocardiography | 81 (58.7) | 49.4 | 25.9 | 13.6 | 84.2 | 82.5 | 93.0 |
Abbreviation: NR, not reported.
3.2. Meta‐analytic comparisons between criteria
Ten articles reporting on the accuracies of Peguero‐Lo Presti, Cornell, and Sokolow‐Lyon criteria for the diagnosis of LVH were included in our pooled analyses. For Peguero‐Lo Presti, there were a total of 1410 cases of LVH (928 TP and 402 FP) out of a total of 5984 (23.6%) patients. The sensitivity ranged from 14.5% to 73.3% (pooled sensitivity: 43.1%; 95% confidence interval [CI]: 30.2‐56.9) (Figure 1). The specificity ranged from 75.6% to 96.3% (pooled specificity: 90.5%; 95% CI: 86.3‐93.5) (Figure 2). Also, DOR ranged from 2.51 to 37.14 (pooled DOR: 6.63; 95% CI: 3.95‐11.13) (Figure 3). For Cornell, there was a total of 961 cases of LVH (16.1%, 726 were TP's, 235 were FP's). The sensitivity ranged from 5.8% to 56.0% (pooled sensitivity: 26.1%; 95% CI: 16.9‐37.9) (Figure 1). The specificity ranged from 82.5% to 99.0% (pooled specificity: 94.9%; 95% CI: 90.3‐97.3) (Figure 2). Also, the DOR ranged from 1.64 to 32.81 (pooled DOR: 5.50; 95% CI: 3.64‐8.30) (Figure 3), indicating that the discriminatory ability of Peguero‐Lo Presti was comparatively good. For Sokolow‐Lyon, there was a total of 961 cases of LVH (16.1%, 726 were TP's, 235 were FP's). The sensitivity ranged from 3.9% to 57.8% (pooled sensitivity: 22.0%; 95% CI: 14.1‐32.7) (Figure 1). The specificity ranged from 58.0% to 97.8% (pooled specificity: 92.1%; 95% CI: 86.2‐95.6) (Figure 2). Also, the DOR ranged from 1.62 to 11.90 (pooled DOR: 2.94; 95% CI: 2.2‐3.92) (Figure 3). As shown in Figure 4, the summary ROC curve for Peguero‐Lo Presti was the closest to the top left corner, indicating the best accuracy. This was well supported by the AUC's, which was 0.827 for Peguero‐Lo Presti, 0.715 for Cornell, and 0.623 for Sokolow‐Lyon (Figure 4).
FIGURE 1.
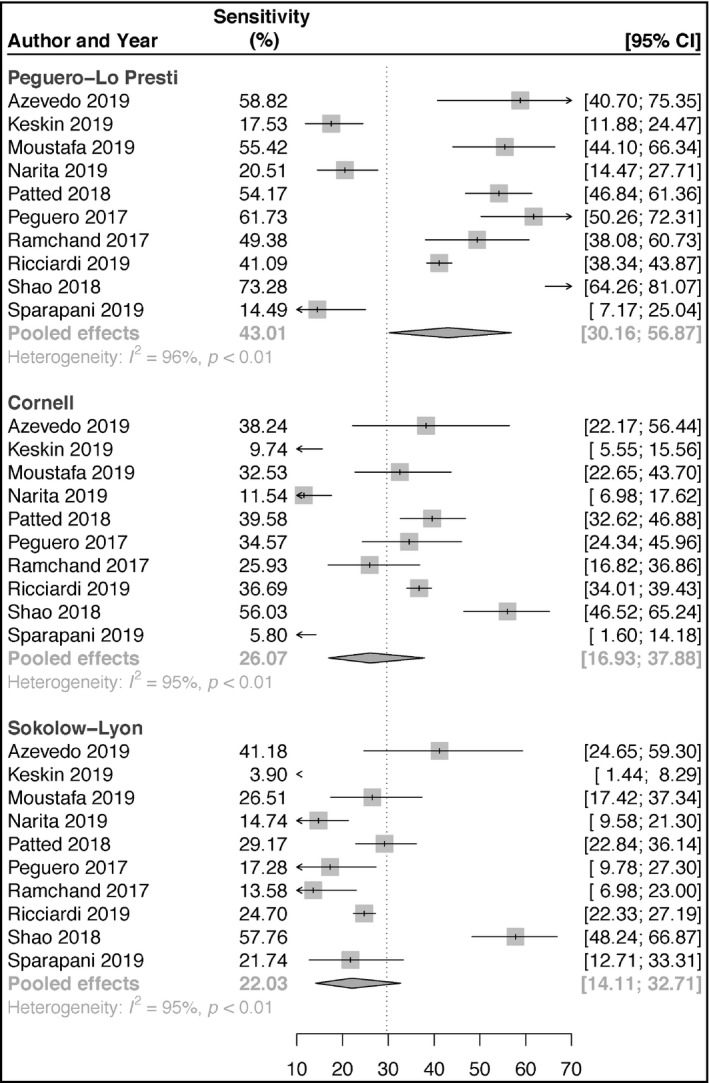
Forest plot of sensitivities of the Peguero‐Lo Presti, Cornell, and Sokolow‐Lyon criteria
FIGURE 2.
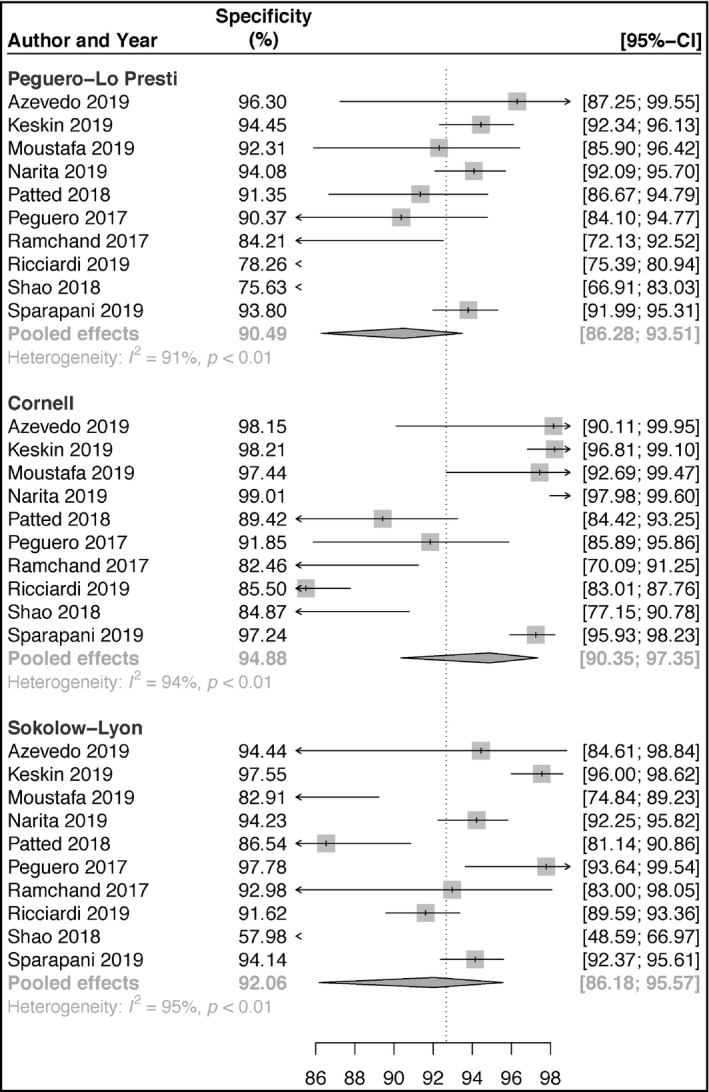
Forest plot of specificities of the Peguero‐Lo Presti, Cornell, and Sokolow‐Lyon criteria
FIGURE 3.
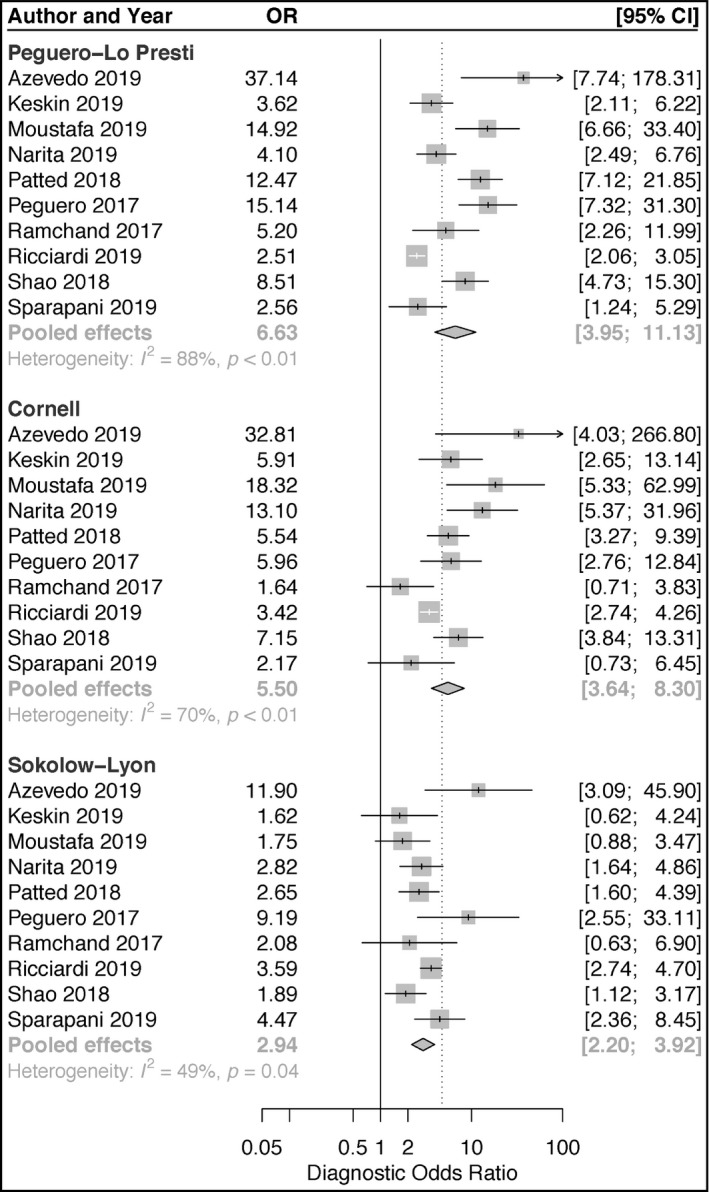
Forest plot of diagnostic odds rations of the Peguero‐Lo Presti, Cornell, and Sokolow‐Lyon criteria
FIGURE 4.
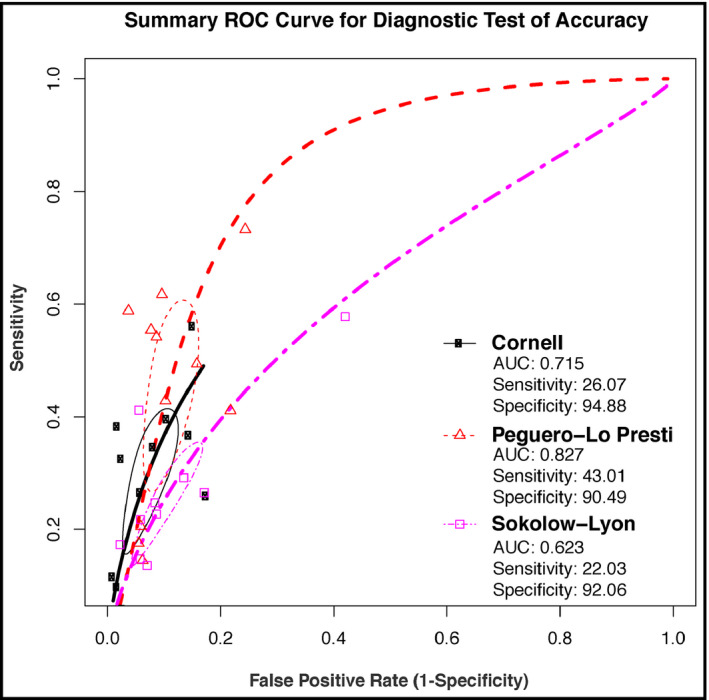
Summary receiver operating characteristic curves and areas under the curves for the Peguero‐Lo Presti, Cornell, and Sokolow‐Lyon criteria
3.3. Additional data
Among the four articles which could not be included in the meta‐analysis due to limited data, three of them from Australia, reported only precise sensitivity data for each criterion. A study done among 88 patients with type 2 diabetes showed that Peguero‐Lo Presti had a better sensitivity of 15.3% (95% CI: 6‐33), compared to Cornell criteria (7.7%; 95 CI: 2‐24) and Sokolow‐Lyon (3.8%; CI: 1‐18), with all P < .001. Specificities were reportedly all ≥93%. 25 Another study from the same group reported data from 33 patients with end‐stage kidney disease. With cardiac MRI as the reference standard, Peguero‐Lo Presti had a sensitivity of 55% (95% CI: 24‐82), Cornell 46% (95% CI: 18‐75), and Sokolow‐Lyon 18% (95% CI: 3‐52), with all P < .001. Considering cardiac echo as the reference standard, the sensitivity of Peguero‐Lo Presti to detect LVH was 50% (95% CI: 22‐78), Cornell 42% (95% CI: 16‐71), and Sokolow‐Lyon 8% (95% CI: 4‐40), with all P < .001. Specificities were ≥93% for all the criteria. 26 In another study in a general population of patients who had transthoracic cardiac echo (n = 144), Peguero‐Lo Presti had a better sensitivity of 16.0% compared to 7.6% for Cornell and 4.2% for Sokolow‐Lyon (P < .02). 27 In a large population from China (n = 10 614), Peguero‐Lo Presti criteria had the best sensitivity (57.0% in males and 41.9% in females) compared to Cornell (21.4% in males and 18.9% in females) and Sokolow‐Lyon (45.3% in males and 11.9% in females). However, the specificity of Peguero‐Lo Presti (66.6% in males and 83.1% in females) was markedly lower than those obtained with Cornell (96.1% in males and 95.7% in females) and Sokolow‐Lyon (79.7% in males and 96.9% in females). 8
4. DISCUSSION
Detection of LVH in routine clinical practice is pivotal for comprehensive cardiovascular risk assessment and for both primary and secondary cardiovascular prevention. Despite the wide availability of ECG and its ease to use, the performance of ECG to detect LVH has been shown to be poor. To improve its diagnostic accuracy, more than 30 criteria have been proposed, including the widely used Sokolow‐Lyon and Cornell criteria, 4 and the novel Peguero‐Lo Presti criterion. 7 Studies comparing these three criteria have shown inconsistent results. 8 , 9 , 10 Hence, we conducted a meta‐analytic comparison of the diagnostic performance of these criteria, from ten studies with low risk of bias and which provided data on 5984 individuals. It shows that Peguero‐Lo Presti has better sensitivity (43%) compared to Cornell (26%) and Sokolow‐Lyon (22%). Peguero‐Lo Presti has the lowest specificity (90%), but its overall performance is still the best based on ROC analysis, with an AUC of 0.83 compared to 0.72 and 0.62 for Cornell and Sokolow, respectively.
Several factors influence the performance of ECG in detecting LVH. In principle, ECG assesses the presence of LVH by estimating the electrical voltage changes detectable at the body surface and which are due to the increased left ventricular mass. Besides the thickness of the myocardium, the cardiac electrical voltage detected by the ECG electrodes is influenced by alterations in the myocardial interstitium such as fibrosis and deposition of other materials, left ventricular geometry, cardiac conduction abnormalities, the distance between the heart and the electrodes and their localization on the torsum, and pulmonary diseases. 5 , 28 , 29 Other factors shown to affect cardiac electrical voltage include age, sex, race, and body habitus. 5
Two fundamental assumptions underpinned the development of the Peguero‐Lo Presti criteria. One is that the detection of an increase in left ventricular mass would be improved by the measurement of the highest increase in voltage in any single lead, rather than in a fixed single lead. Flexible lead selection unlike fixed lead selection has the potential to alleviate the pitfalls related to the variations in the distance between the heart and the torsum as well as the position of the surface electrode and the body habitus. 7 The other premise is that the S wave, the second negative deflection of the QRS complex, might be a better representation of the activation of the myocardial and epicardial left ventricular free wall which occurs after 50 msec of the left ventricular depolarization. 7 , 30 Therefore, the overall hypothesis was that electrical cardiac changes shown by the S wave might be more sensitive to detect alterations in left ventricular mass. On the opposite, several previous criteria were based on the measurement of the highest amplitude of the R wave in various leads alone or in combination with other features. 4 In their landmark paper, Peguero et al found that the S waves of the precordial and limb leads had a better association with an increased left ventricular mass as compared to the R waves. 7 They suggested that this was the main reason why their criterion, which focuses on the S wave, had better performance than the Sokolow‐Lyon and Cornell which includes an amplitude of both R and S waves in different leads. 7
The much higher sensitivity of Peguero‐Lo Presti over Cornell and Sokolow‐Lyon is clinically meaningful. Indeed, the sensitivity is the most important parameter to consider when looking at a screening test, the goal of identifying the maximum of individuals with LVH (true positive) who need confirmation of the diagnosis with cardiac imaging, usually echocardiography. Although the specificity of the test is also important, it usually decreases as sensitivity increases, as the two moves in opposite directions. 31 Concomitant high sensitivity and specificity are only seen for tests measuring parameters that have a very central distribution and therefore a strong correlation. 31 For biological variables such as cardiac electrical voltage which are very variable and less reproducible, sensitivity, and specificity will tend to be wide apart. This might explain why the higher sensitivity of Peguero‐Lo Presti is mitigated by a lower specificity, although its overall performance in ROC analysis remains better.
As for the other ECG criteria, the Peguero‐Lo Presti diagnostic accuracy can be increased if some extracardiac determinants are taken into account. Indeed, we previously showed that the low performance of the ECG criteria in diagnosing LVH in a Black African population can be markedly improved after adjustment for factors such as age, sex, and body habitus. 5 We suggested such adjustments to Peguero et al 6 who, in response, declared that they were assessing further the accuracy of their proposed novel criterion after adjustments for the aforementioned factors. 32 For instance, a study showed that adjusting the diagnostic cutoff Sokolow‐Lyon index for BMI (overweight + 4 mm, obesity + 8 mm) improves the diagnostic accuracy for detecting LVH. 33
This study has some limitations. It has been shown that the performance of ECG criteria varies according to race. 5 Several racial/ethnic groups were not represented in this study including sub‐Saharan Africans. An important study done among a large Asian population was not included in the meta‐analysis because relevant data were not available from the manuscript and upon request from the authors. The study showed Peguero‐Lo Presti was not a better screening tool in that specific population. 8 Additionally, we did not consider, in our analyses, factors that might influence the accuracy of ECG criteria for LVH, such as sample size and comorbidities. An individual participant data meta‐analysis approach would have provided more accurate modeling of these moderating factors than the aggregate meta‐analysis presented herein. 34 Notably, an important finding in the study by Peguero et al is that the sensitivity of their proposed Peguero‐Lo Presti criteria in the derivation cohort (70%) fell markedly in the validation cohort (57%). 7 This discrepancy was attributed to the difference between the two cohorts, in terms of age and comorbidities. Furthermore, most of the studies included in this review used echocardiography as the reference standard, instead of cardiac MRI which is better for LV mass estimation. However, echocardiography is the most accessible cardiac imaging modality in routine practice and it would be difficult to use cardiac MRI in large diagnostic accuracy studies owing to its availability and cost.
5. CONCLUSION
This meta‐analysis suggests that Peguero‐Lo Presti has a better diagnostic performance than Cornell and Sokolow‐Lyon. Thus, it may prove to be more useful in routine clinical practice as a screening tool for LVH. However, more studies are needed for its validation especially across various ethnic groups. Furthermore, adjustment for some extracardiac factors should be considered to improve the diagnostic accuracy of the Peguero‐Lo Presti criterion.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
AUTHORS' CONTRIBUTIONS
Conception and Design: JJN. Search strategy: JJN. Studies selection: JJN and UFN. Data extraction: JJN and UFN. Data synthesis: JJN and TAA. Data interpretation: JJN and TAA. Manuscript drafting: JJN and TAA. Manuscript revision: JJN, TAA, UFN, CN, AMJ. Approval of the final manuscript: JJN, TAA, UFN, CN, AMJ. Guarantor of the review: JJN.
Supporting information
App S1
Noubiap JJ, Agbaedeng TA, Nyaga UF, Nkoke C, Jingi AM. A meta‐analytic evaluation of the diagnostic accuracy of the electrocardiographic Peguero‐Lo Presti criterion for left ventricular hypertrophy. J Clin Hypertens. 2020;22:1145–1153. 10.1111/jch.13923
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article and its supplementary information files.
REFERENCES
- 1. Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148‐2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bauml MA, Underwood DA. Left ventricular hypertrophy: an overlooked cardiovascular risk factor. Clevel Clin J Med. 2010;77:381‐387. [DOI] [PubMed] [Google Scholar]
- 3. Verdecchia P, Angeli F, Borgioni C, et al. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta‐analysis. Am J Hypertens. 2003;16:895‐899. [DOI] [PubMed] [Google Scholar]
- 4. Hancock EW, Deal BJ, Mirvis DM, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):992‐1002. [DOI] [PubMed] [Google Scholar]
- 5. Jingi AM, Noubiap JJ, Kamdem P, Kingue S. Determinants and improvement of electrocardiographic diagnosis of left ventricular hypertrophy in a black African population. PLoS ONE. 2014;9(5):e96783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noubiap JJ, Jingi AM. Adjustments of electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;70(5):686‐687. [DOI] [PubMed] [Google Scholar]
- 7. Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC, Tolentino A. Electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;69(13):1694‐1703. [DOI] [PubMed] [Google Scholar]
- 8. Sun GZ, Wang HY, Ye N, Sun YX. Assessment of novel peguero‐lo presti electrocardiographic left ventricular hypertrophy criteria in a large asian population: newer may not be better. Can J Cardiol. 2018;34(9):1153‐1157. [DOI] [PubMed] [Google Scholar]
- 9. Ricciardi D, Vetta G, Nenna A, et al. Current diagnostic ECG criteria for left ventricular hypertrophy: is it time to change paradigm in the analysis of data? J Cardiovasc Med (Hagerstown). 2020;21(2):128‐133. [DOI] [PubMed] [Google Scholar]
- 10. Shao Q, Meng L, Tse G, et al. Newly proposed electrocardiographic criteria for the diagnosis of left ventricular hypertrophy in a Chinese population. Ann Noninvasive Electrocardiol. 2019;24(2):e12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed). 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(4):412. [DOI] [PubMed] [Google Scholar]
- 13. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. [DOI] [PubMed] [Google Scholar]
- 14. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Res Synth Methods. 2010;1:97‐111. [DOI] [PubMed] [Google Scholar]
- 15. Stijnen T, Hamza TH, Ozdemir P. Random effects meta‐analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. 2010;29:3046‐3067. [DOI] [PubMed] [Google Scholar]
- 16. Arends L, Hamza T, Van Houwelingen J, Heijenbrok‐Kal M, Hunink M, Stijnen T. Bivariate random effects meta‐analysis of ROC curves. Med Decis Making. 2008;28(5):621‐638. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP. Commentary: heterogeneity in meta‐analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37(5):1158‐1160. [DOI] [PubMed] [Google Scholar]
- 18. Narita M, Yamada M, Tsushima M, et al. Novel electrocardiographic criteria for the diagnosis of left ventricular hypertrophy in the japanese general population. Int Heart J. 2019;60(3):679‐687. [DOI] [PubMed] [Google Scholar]
- 19. Keskin K, Ser OS, Dogan GM, et al. Assessment of a new electrocardiographic criterion for the diagnosis of left ventricle hypertrophy: a validation study. North Clin Istanb. 2019;7:231‐236. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patted SV, Porwal SC, Ambar SS, et al. Assessment of Peguero Lo‐Presti criteria for electrocardiographic diagnosis of LVH in Indian subjects. Cardiol Cardiovasc Med. 2018;2(3):65‐73. [Google Scholar]
- 21. Moustafa M, Shredah A, Marghany K, Zakaria A. Electrocardiography versus Echocardiography for assessment of left ventricular hypertrophy in ischemic heart disease patients with and without cardiovascular risk factors. Egypt J Hosp Med. 2019;74(5):1165‐1173. [Google Scholar]
- 22. Ramchand J, Sampaio Rodrigues T, Kearney LG, Patel SK, Srivastava PM, Burrell LM. The Peguero‐lo presti electrocardiographic criteria predict all‐cause mortality in patients with aortic stenosis. J Am Coll Cardiol. 2017;70(14):1831‐1832. [DOI] [PubMed] [Google Scholar]
- 23. Azevedo PM, Guerreiro C, Ladeiras‐Lopes R, et al. Diagnostic accuracy of a novel electrocardiographic criterion for the diagnosis of left ventricular hypertrophy in hypertrophic cardiomyopathy. Eur Heart J. 2019;40(S1):P1772. [Google Scholar]
- 24. Sparapani R, Dabbouseh NM, Gutterman D, et al. Detection of left ventricular hypertrophy using bayesian additive regression trees: the MESA. J Am Heart Assoc. 2019;8(5):e009959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodrigues TS, Ramchand J, Wai B, et al. Investigation of the Peguero‐Lo Presti criteria to improve the sensitivity of the electrocardiogram to diagnose left ventricular hypertrophy in patients with type 2 diabetes. J Hypertens. 2018;36:e252. [Google Scholar]
- 26. Rodrigues TS, Azraai M, Crosthwaite A, et al. The Peguero‐Lo Presti criteria improve the sensitivity of the electrocardiogram to diagnose left ventricular hypertrophy in patients with end‐stage kidney disease. Heart Lung Circ. 2019;28:S324. [Google Scholar]
- 27. Lim M, Fitzgerald M, Choi B, Tan C, Soward A. Validation of the Peguero‐Lo Presti criterion for the diagnosis of left ventricular hypertrophy on electrocardiogram. Heart Lung Circ. 2018;27:S358. [Google Scholar]
- 28. Bacharova L. Electrocardiography‐left ventricular mass discrepancies in left ventricular hypertrophy: electrocardiography imperfection or beyond perfection? J Electrocardiol. 2009;42:593‐596. [DOI] [PubMed] [Google Scholar]
- 29. Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81(3):815‐820. [DOI] [PubMed] [Google Scholar]
- 30. Durrer D, van Dam RT, Freud GE, Janse MJ, Meijler FL, Arzbaecher RC. Total excitation of the isolated human heart. Circulation. 1970;41:899‐912. [DOI] [PubMed] [Google Scholar]
- 31. Akobeng AK. Understanding diagnostic tests 1: sensitivity, specificity and predictive values. Acta Paediatr. 2007;96(3):338‐341. [DOI] [PubMed] [Google Scholar]
- 32. Peguero JG, Lo Presti S, Perez J, Issa O, Brenes JC, Tolentino A. Reply: adjustments of electrocardiographic criteria for the diagnosis of left ventricular hypertrophy. J Am Coll Cardiol. 2017;70(5):687. [DOI] [PubMed] [Google Scholar]
- 33. Rider OJ, Ntusi N, Bull SC, et al. Improvements in ECG accuracy for diagnosis of left ventricular hypertrophy in obesity. Heart. 2016;102(19):1566‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stewart GB, Altman DG, Askie LM, et al. Statistical analysis of individual participant data meta‐analysis: a comparison of methods and recommendation for practice. PLoS One. 2012;7(10):E46042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.


