Abstract
Recently, neutrophil‐to‐lymphocyte ratio (NLR) and monocyte‐to‐lymphocyte ratio (MLR) are frequently used to evaluate disease progression and outcome. Here, we aim to analyze the associations between NLR or MLR and kidney function in undiagnosed hypertensive individuals from general population during routine health checkup. Liver function was analyzed for comparison. From 2011 to 2016, 53 939 examiners have registered for health checkup in Yanbian University Hospital, Yanbian, China. Among 15 219 participants who have complete datasets, 4997 individuals were hypertensive (HTN, SBP/DBP: ≥ 140/90 mm Hg). NLR, glucose, lipids (Chol, TG, LDL), kidney (CREA, BUN), and liver (AST, ALT, GGT, ALB, TBIL) functional parameters were significantly higher in HTN. Pearman correlation analysis showed that NLR was positively correlated with SBP and CREA only in HTN. MLR was associated with CREA in both HTN and non‐HTN. NLR or MLR was associated with liver functions similarly in HTN and non‐HTN. The authors then divided NLR or MLR into tertiles (NLR: 0‐1.7276, 1.7276‐3, >3; MLR: 0‐0.1845, 0.1845‐0.3, >0.3). NLR was positively associated with BUN at NLR >1.7276 and with CREA at all tertiles in HTN. MLR was correlated with CREA and BUN at high MLR in non‐HTN. Further analysis showed that age or gender did not affect the associations of NLR and MLR with kidney function in HTN, but strong association was observed in male or aged (>65 years old) non‐HTN group. These results showed that NLR could be used as a cost‐effective predictor of kidney abnormality in HTN patients even in a general population.
Keywords: general population, hypertension, kidney function, monocyte‐to‐lymphocyte ratio, neutrophil‐to‐lymphocyte ratio
1. INTRODUCTION
Hypertension is an important precursor of cardiovascular diseases, if left untreated, hypertension can be life‐threatening due to the comorbidities including cardiac arrhythmogenesis (sudden death), atherosclerosis, heart failure, brain hemorrhagic stroke, and kidney failure. Hypertensive population is in an upsurge in both developed and developing countries.1 The numbers of hypertensive patients are expected to increase significantly when new guidelines are applied (ie, systolic and diastolic blood pressure exceeds 130 mm Hg and 80 mm Hg, respectively, instead of 140 mm Hg and 90 mm Hg1). Therefore, it will be fundamentally important to detect whether one is hypertensive and examine hypertension‐induced organ disorders to prevent adverse outcome.
Pressure overload and neurohormonal disturbances are the direct causes of endothelial disruption, oxidative stress, and pathological remodeling in vasculature and target organs.2 In addition, it has been well established that inflammation plays critical roles in the pathogenesis of hypertension and its comorbidities.3, 4 For example, transferring lymphocytes of diseased animals to recipient animals induced high blood pressure,5 whereas immunosuppression lowers blood pressure,6, 7 indicating that inflammation is the upstream of blood pressure elevation. Mice lacking the recombination‐activating gene 1 (RAG1‐/‐, which lack both T and B cells) blunted angiotensin II or deoxycorticosterone acetate salt‐induced hypertension and preserved vascular endothelial function.8 Therefore, inflammation is indispensable for the initiation of hypertension and hypertension‐associated organ damages.
It has been documented that various types of immune cells are accumulated in peripheral tissues in hypertensive rodent models and in humans, representing that inflammation is associated with hypertension or hypertensive phenomenon.4, 9, 10, 11 In particular, infiltration of monocytes/macrophages and adaptive immune cells (T lymphocytes) into perivascular adipose tissues, kidneys, and myocardium could increase the expression of adhesion molecules and chemokines, the production and release of various pro‐inflammatory cytokines, and subsequently the generation of reactive oxygen species (ROS) function as downstream mediators, which are implicated in hypertension and cardiovascular injury.3, 4
Recently, convincing evidences revealed that neutrophil‐to‐lymphocyte ratio (NLR) or monocyte‐to‐lymphocyte ratio (MLR) in peripheral blood could be regarded as reliable indicators of system inflammation, which is used for early prediction of the prognosis and outcome of cardiovascular diseases, including mortality of myocardial infarction and heart failure patients.12, 13 Studies have shown that NLR and neutrophil counts are increased in resistant hypertensive patients compared with those of normotension or hypertensive patients whose blood pressure is controlled.3 Similarly, epidemiological studies indicated that the elevation of monocyte counts is independently related to all‐cause cardiovascular mortality in hemodialysis patients.14 Until now, whether NLR or MLR in hypertensive individuals among general population is related to kidney or liver dysfunction has not been studied yet.
Therefore, the aim of the present study was to analyze NLR and MLR and their correlation with parameters of kidney and liver functions among general population who underwent routine health checkup. Our results indicate that high NLR is associated with kidney dysfunction in HTN subjects in general population.
2. METHODS
2.1. Participants
The study was conducted at Yanbian University Affiliated Hospital. We have collected annual health checkup data in the Department of Medical Examination. Information about medical history was collected through questionnaires by trained interviewers. A total of 53 939 physical examinations were conducted from 2011 to 2016. 16 298 cases undergone blood pressure measurements and took blood for clinical examination. We have excluded individuals with medical history of cardiovascular diseases, diabetes, chronic and acute infection, hepatitis, or any infectious disease because those conditions may affect leukocyte concentration; we also excluded participants with no complete clinical examination data. The number of examiners was 15 219 individuals. Among them, 7158 were men (accounted for 47.0% of the total number). All participants received written informed consent for the program.
With SBP/DBP 140/90 mm Hg as the standard, there were 4997 hypertensive individuals (accounted for 32.8% of the total number). Among the hypertensive subjects, 2720 were men (accounted for 54.43%); in non‐hypertensive subjects, 4438 were men (accounted for 43.42%). The protocol used in this study was approved by the Yanbian University Affiliated Hospital.
2.2. Measurement of parameters in the blood sample and calculation of NLR and MLR
Peripheral blood samples from all participants were collected. The blood cell counts were measured systematically using Sysmex XN‐1000Q automated blood analyzer (Yanbian University Hospital, Clinical Laboratory). NLR was calculated as neutrophil count ÷ lymphocyte count; MLR count was calculated as monocyte count ÷ lymphocyte count. NLR and MLR were divided into three groups, respectively, according to the average and upper limits (the upper limit formula was as follows: 75% quantile + [75% quantile −25% quantile] × 1.5). The average of NLR was 1.7276, and the upper limit of NLR was 3; the average of MLR was 0.18, and the upper limit of MLR was 0.3. Therefore, NLR: 0‐1.7276,1.7276‐3, >3 or MLR: 0‐0.1845, 0.1845‐0.3, >0.3 was taken for the analysis. Roche Cobas automatic biochemical analyzer was used to detect "blood sugar," "lipid," "kidney function," and "liver function."
2.3. Statistical analysis
Data analysis was performed using SPSS 19.0 statistical software. The test data consistent with the normal distribution were expressed as mean ± standard deviation (mean ± SD) or nonparametric distribution as median (P25, P75); single‐factor ANOVA was used between hypertension and non‐hypertension group analysis; Pearman analysis was used for NLR, MLR and test parameter correlations, and P < .05 was considered statistically significant.
3. RESULT
A total of 15 219 individuals with no diagnosed disease and with complete dataset were included in the analysis. As shown in Table 1, baseline characteristics and laboratory results showed that mean systolic blood pressure and diastolic blood pressure (SBP and DBP) in all the subjects were 125 mm Hg and 80 mm Hg. 4997 subjects were hypertensive, and mean SBP and DBP were 147 mm Hg/94 mm Hg and 118 mm Hg/75 mm Hg in HTN and non‐HTN, respectively (P < .0001). There were more males in HTN (54.4%, P < .0001), and this group tends to be older: 52.6 ± 12 in HTN vs 43.6 ± 13 in non‐HTN (P < .0001). BMI and WBC were significantly higher in HTN group (P < .0001; P < .0001). Median HGB, NEU, MON, EOS, and BAS counts, which were within the normal ranges, were significantly higher in HTN than in non‐HTN (P < .0001). PLT was significantly lower (P < .001). Glucose (Glu), triacylglycerol (TG), cholesterol (Chol), and low‐density lipoprotein (LDL) levels were all significantly higher in HTN group (P < .0001).
Table 1.
Descriptive statistical analysis of healthy checkup population
| Variable | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | P |
|---|---|---|---|---|
| HTN | 4997(32.8%) | |||
| SBP (90‐140 mm Hg) | 125(113, 139) | 147(140, 157) | 118(109, 126) | .000 |
| DBP(60‐90 mm Hg) | 80(72, 90) | 94(90, 101) | 75(69, 81) | .000 |
| Male | 7158(47.0%) | 2720(54.43%) | 4438(43.42%) | .000 |
| Age | 46.6 ± 13.4 | 52.6 ± 12.0 | 43.61 ± 13.04 | .000 |
| BMI | 24.41(21.91, 26.87) | 25.80(23.44, 28.22) | 23.66(21.36, 26.08) | .000 |
| WBC (4‐10) × 109/L | 6.16(5.17, 7.35) | 6.35(5.37, 7.56) | 6.05(5.07, 7.25) | .000 |
| HGB (110‐160) g/L | 144(133, 157) | 148(136, 160) | 141(131, 155) | .000 |
| PLT (100‐300) × 109/L | 222(190, 258) | 221(187, 257) | 222(191, 258) | .001 |
| NEU# (2‐7.7) × 109/L | 3.39(2.67, 4.26) | 3.55 (2.80, 4.40) | 3.31 (2.61, 4.18) | .000 |
| LYM# (0.8‐4) × 109/L | 2.16(1.80, 2.60) | 2.21 (1.83, 2.66) | 2.14 (1.78, 2.56) | .000 |
| MON# (0.12‐0.8) × 109/L | 0.37(0.29, 0.47) | 0.38(0.30, 0.48) | 0.36(0.29, 0.46) | .000 |
| EOS# (0‐0.5) × 109/L | 0.1(0.06, 0.17) | 0.11(0.06, 0.18) | 0.10(0.06, 0.17) | .018 |
| BAS# (0‐0.1) × 109/L | 0.02(0.01, 0.03) | 0.02(0.01, 0.03) | 0.02(0.01, 0.03) | .000 |
| GLU (3.6‐6.1) mmol/L | 5.2 (4.9, 5.6) | 5.4 (5.0, 5.9) | 5.1 (4.8, 5.5) | .000 |
| CHOL (3.35‐6.45) mmol/L | 4.8 (4.2, 5.47) | 5.02 (4.4, 5.7) | 4.7 (4.1, 5.33) | .000 |
| TG (0.48‐1.88) mmol/L | 1.3 (0.89, 1.97) | 1.57 (1.08, 2.35) | 1.18 (0.83, 1.77) | .000 |
| HDL >0.9 mmol/L | 1.39 (1.18, 1.65) | 1.34 (1.14, 1.59) | 1.42 (1.19, 1.67) | .227 |
| LDL (0.00‐3.12) mmol/L | 2.76(2.29, 3.27) | 2.93 (2.44, 3.43) | 2.67 (2.22, 3.17) | .000 |
| BUN (2.5‐7.0) mmol/L | 4.8(4.0, 5.8) | 5.0(4.1, 6.0) | 4.7(3.9, 5.7) | .000 |
| CREA (44‐80) umol/L | 66(56.6, 78) | 69(59, 80) | 65(55.9, 77) | .000 |
| AST (0‐40) U/L | 20(17, 25) | 22(18, 27) | 20(17, 24) | .000 |
| ALT (0‐40) U/L | 19(14, 29) | 21(15.7, 32) | 18(13, 27) | .000 |
| GGT (8‐58) U/L | 25 (16, 47) | 33 (20, 60) | 22 (15, 39) | .000 |
| ALB (37‐53) g/L | 47 (45, 49) | 47 (45, 49) | 47 (45, 49) | .017 |
| TBIL (5.1‐25.6) umol/L | 13.2 (10.6, 16.8) | 13.4 (10.7, 17) | 13.1 (10.5, 16.6) | .009 |
An estimate of kidney function (BUN and CREA) was significantly higher in HTN compared with those in non‐HTN (P < .0001). Similarly, ALT, AST, GGT, ALB, and TBIL were significantly higher in HTN group (P < .0001, P = .017 for ALB and P = .009 for TBIL). It should be noted that all the parameters were within the normal ranges.
Mean NLR for all the subjects was 1.7276, and mean NLR in HTN and non‐HTN group was 1.7665 and 1.7085, respectively, and there was a significant difference between HTN and non‐HTN (P = .001, Figure 1A). Median NLR for all the subjects was 1.55 (minimum: 0.19, maximum: 70.88, lower quartile: 1.21, and higher quartile: 2.01). Median NLR for HTN was 1.59 (minimum: 0.37, maximum: 11.18, lower quartile: 1.24, and higher quartile: 2.08) and non‐HTN was 1.53 (minimum: 0.19, maximum: 70.88, lower quartile: 1.20, and higher quartile: 1.97; Figure 1A).
Figure 1.
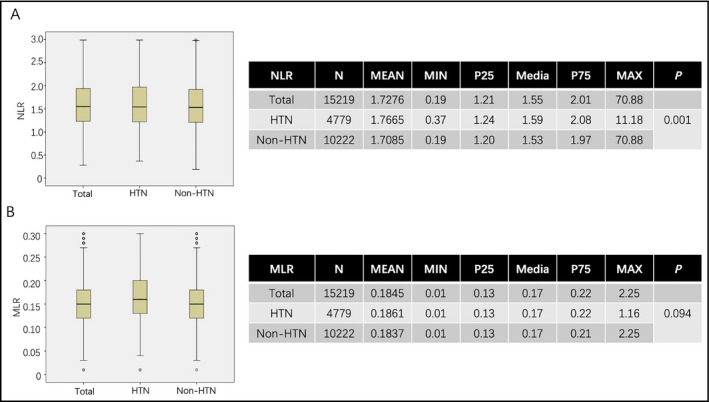
A and B are box plot analysis of NLR and MLR, which are divided into total, HTN, and non‐HTN groups. The tables on the right are descriptive analysis of NLR and MLR in all three groups and the difference between HTN and non‐HTN groups
Mean MLR for all the subjects was 0.1845, and MLR in HTN and non‐HTN group was 0.1861 and 0.1837, respectively, and there was no significant difference between two groups (P = .09, Figure 1B). Median MLR for all the subjects was 0.17 (minimum: 0.01, maximum: 2.25, lower quartile: 0.13, and higher quartile: 0.22). Median MLR for HTN was 0.17 (minimum: 0.01, maximum: 1.16, lower quartile: 0.13, and higher quartile: 0.22) and non‐HTN was 0.17 (minimum: 0.01, maximum: 2.25, lower quartile: 0.13, and higher quartile: 0.21; Figure 1B).
Next, we analyzed the correlation between NLR or MLR and kidney functional parameters. NLR was positively correlated with BUN and CREA in HTN (r = .09, P < .0001; r = .142, P < .0001, respectively). This is similar to that in all the individuals included in the study (Table 2, Figure 2). In non‐HTN, NLR was not associated with CREA (r = .009, P = .355), but correlation was observed between NLR and BUN in non‐HTN (r = .028, P = .005; Table 2, Figure 2). In contrast, NLR showed similar correlation with liver function parameters (AST, ALT, ALB) in HTN, non‐HTN, and all the individuals (Table 3). These results suggest that NLR can be a sensitive predictor of CREA in HTN in particular and NLR is associated with liver function regardless of high blood pressure.
Table 2.
Correlation between NLR and kidney function parameters
| NLR | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| BUN (2.5‐7.0) mmol/L | .048 | .000 | .090 | .000 | .028 | .005 |
| CREA (75‐115) mmol/L | .058 | .000 | .142 | .000 | .009 | .355 |
Figure 2.
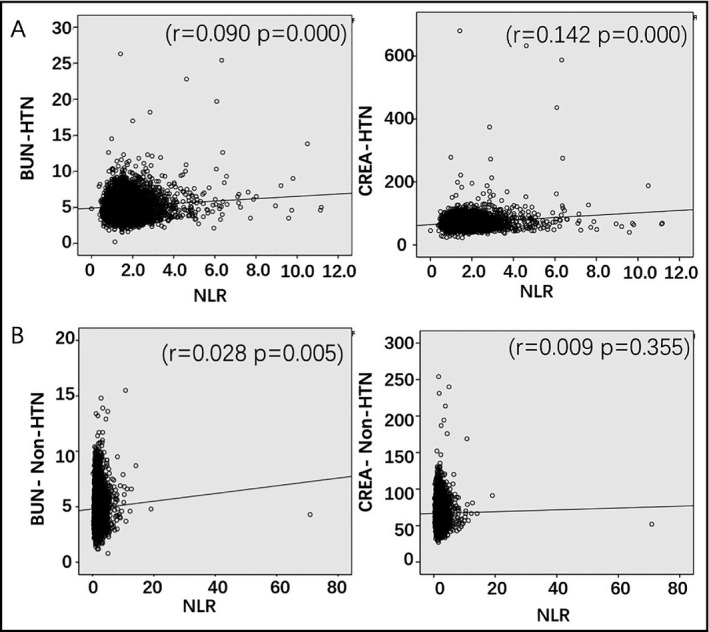
Analysis of the correlation between NLR and BUN, and CREA parameters in HTN (A) and non‐HTN (B) groups
Table 3.
Correlation between NLR and liver function parameters
| NLR | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| AST (0‐40) U/L | −.033 | .000 | −.039 | .005 | −.033 | .001 |
| ALT (0‐40) U/L | −.029 | .000 | −.042 | .003 | −.027 | .007 |
| GGT (8‐58) U/L | .004 | .631 | −.002 | .825 | .002 | .820 |
| ALB (37‐53) g/L | −.045 | .000 | −.045 | .002 | −.044 | .000 |
| TBIL (5.1‐25.6) umol/L | .009 | .292 | .003 | .842 | .010 | .313 |
Monocyte‐to‐lymphocyte ratio showed positive correlation with CREA but not with BUN in all the individuals, including HTN and non‐HTN (Table 4, Figure 3). However, MLR was associated with AST, ALT, GGT, ALB, and TBIL in HTN and in non‐HTN (Table 5).
Table 4.
Correlation between MLR and kidney function parameters
| MLR | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| BUN (2.5‐7.0) mmol/L | −.006 | .428 | .000 | .983 | −.012 | .228 |
| CREA (75‐115) mmol/L | .120 | .000 | .142 | .000 | .107 | .000 |
Figure 3.
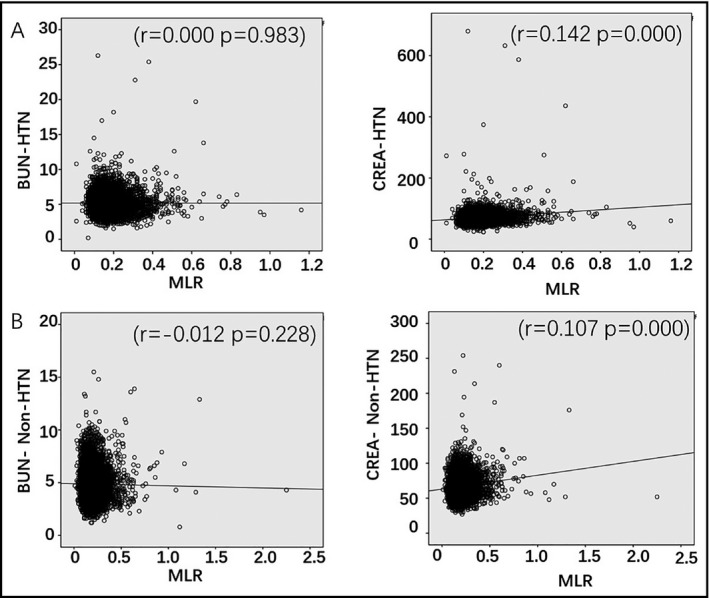
Analysis of the correlation between MLR and BUN, and CREA parameters in HTN (A) and non‐HTN (B) groups
Table 5.
Correlation between MLR and liver function parameters
| MLR | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | |||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| AST (0‐40) U/L | .074 | .000 | .071 | .000 | .075 | .000 |
| ALT (0‐40) U/L | .044 | .000 | .035 | .012 | .047 | .000 |
| GGT (8‐58) U/L | .090 | .000 | .114 | .000 | .076 | .000 |
| ALB (37‐53) g/L | −.088 | .000 | −.081 | .000 | −.092 | .000 |
| TBIL (5.1‐25.6) umol/L | .065 | .000 | .067 | .000 | .063 | .000 |
As shown in Tables 6 and 7, we also analyzed the correlation of NLR or MLR with other clinical examination parameters in HTN and non‐HTN groups. Majority of parameters showed similar correlation or noncorrelation between two groups. Intriguingly, NLR showed positive correlation with PLT and HDL only in HTN (r = .03, P = .01 for PLT and r = .03, P = .03 for HDL); NLR was correlated with Glu only in non‐HTN (r = .02, P = .03).
Table 6.
Correlation between NLR and blood sample laboratory parameters
| Variables | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | |||
|---|---|---|---|---|---|---|
| NLR correlation | r | P | r | P | r | P |
| Age | .026 | .001 | .018 | .200 | .020 | .048 |
| SBP (90‐140 mm Hg) | .032 | .000 | .044 | .002 | .055 | .613 |
| DBP(60‐90 mm Hg) | .021 | .009 | .024 | .094 | −.007 | .466 |
| BMI | −.016 | .043 | −.033 | .019 | −.021 | .035 |
| WBC (4‐10) × 109/L | .347 | .000 | .417 | .000 | .321 | .000 |
| HGB (110‐160) g/L | .007 | .414 | −.001 | .924 | .005 | .644 |
| PLT (100‐300) × 109/L | .021 | .011 | .034 | .016 | .017 | .094 |
| NEU# (2‐7.7) × 109/L | .594 | .000 | .709 | .000 | .555 | .000 |
| LYM# (0.8‐4) × 109/L | −.360 | .000 | −.437 | .000 | −.336 | .000 |
| MON# (0.12‐0.8) × 109/L | .178 | .000 | .221 | .000 | .162 | .000 |
| EOS# (0‐0.5) × 109/L | −.055 | .000 | −.071 | .000 | −.050 | .000 |
| BAS# (0‐0.1) × 109/L | −.056 | .000 | −.072 | .000 | −.052 | .000 |
| GLU (3.6‐6.1) mmol/L | .028 | .001 | .027 | .052 | .021 | .035 |
| CHOL (3.35‐6.45) mmol/L | −.056 | .000 | −.059 | .000 | −.063 | .000 |
| TG (0.48‐1.88) mmol/L | −.036 | .000 | −.061 | .000 | −.033 | .001 |
| HDL >0.9 mmol/L | −.005 | .521 | .031 | .031 | −.006 | .531 |
| LDL (0.00‐3.12) mmol/L | −.049 | .000 | −.050 | .000 | −.056 | .000 |
Table 7.
Correlation between MLR and blood sample laboratory parameters
| Variables | ALL n = 15 219 | HTN n = 4997 | Non‐HTN n = 10 222 | |||
|---|---|---|---|---|---|---|
| MLR correlation | r | P | r | P | r | P |
| Age | .045 | .000 | .044 | .002 | .042 | .000 |
| SBP (90‐140 mm Hg) | .017 | .036 | .009 | .507 | .011 | .246 |
| DBP(60‐90 mm Hg) | .013 | .112 | ‐.006 | .692 | .011 | .260 |
| BMI | ‐.036 | .000 | ‐.045 | .002 | ‐.038 | .000 |
| WBC (4‐10) ×10^9/L | .164 | .000 | .171 | .000 | .159 | .000 |
| HGB (110‐160) g/L | .001 | .900 | .031 | .027 | ‐.016 | .106 |
| PLT (100‐300)×10^9/L | ‐.031 | .000 | ‐.019 | .177 | ‐.036 | .000 |
| NEU# (2‐7.7)×10^9/L | .316 | .000 | .327 | .000 | .311 | .000 |
| LYM# (0.8‐4)×10^9/L | ‐.382 | .000 | ‐.397 | .000 | ‐.377 | .000 |
| MON# (0.12‐0.8)×10^9/L | .643 | .000 | .659 | .000 | .635 | .000 |
| EOS# (0‐0.5)×10^9/L | ‐.006 | .496 | ‐.009 | .533 | ‐.004 | .652 |
| BAS# (0‐0.1)×10^9/L | .013 | .115 | .008 | .575 | .014 | .151 |
| GLU (3.6‐6.1) mmol/L | .023 | .005 | .018 | .200 | .023 | .023 |
| CHOL (3.35‐6.45) mmol/L | ‐.091 | .000 | ‐.092 | .000 | ‐.095 | .000 |
| TG (0.48‐1.88) mmol/L | ‐.062 | .000 | ‐.079 | .000 | ‐.058 | .000 |
| HDL > 0.9 mmol/L | .000 | .995 | .034 | .016 | ‐.001 | .957 |
| LDL (0.00‐3.12) mmol/L | ‐.081 | .000 | ‐.082 | .000 | ‐.085 | .000 |
Similarly, MLR was correlated with HDL only in HTN (r = .03, P = .01) and with Glu in non‐HTN (r = .02, P = .02). In addition, MLR showed positive correlation with HGB only in HTN (r = .03, P = .02). MLR was negatively correlated with PLT only in non‐HTN (r = −.03, P < .0001).
Furthermore, we divided NLR and MLR into tertiles (NLR: 0‐1.7276, 1.7276‐3, >3 and MLR: 0‐0.1845, 0.1845‐0.3, >0.3) and analyzed their associations with kidney or liver function parameters. As shown in Table 8 and Table 9, NLR showed gradual but significant increase in its associations with BUN and CREA in HTN (NLR and BUN: r = .042 at 1.7276‐3 and r = .199 at >3, P = .077 and P = .001, respectively; NLR and CREA: r = .052 at 0‐0.17276, r = .066 at 1.7276‐3, and r = .14 at >3, P = .005, .005, and .015, respectively.) MLR showed correlation with CREA and BUN more in non‐HTN (Table 9). In addition, MLR correlated with TBIL and GGT at high MLR (>0.3) only in non‐HTN.
Table 8.
Correlation between NLR and kidney and liver functions in HTN and non‐HTN
| Variables | NLR | Number | HTN | Non‐HTN | ||
|---|---|---|---|---|---|---|
| r | P | r | P | |||
| BUN (2.5‐7.0) mmol/L | 0‐1.7276 | 9313 | .023 | .214 | .011 | .363 |
| 1.7276‐3 | 5065 | .042 | .077 | −.019 | .287 | |
| >3 | 841 | .199 | .001 | .049 | .259 | |
| CREA (75‐115) mmol/L | 0‐1.7276 | 9313 | .052 | .005 | .028 | .023 |
| 1.7276‐3 | 5065 | .066 | .005 | −.021 | .233 | |
| >3 | 841 | .140 | .015 | .001 | .982 | |
| AST (0‐40) U/L | 0‐1.7276 | 9313 | −.029 | .122 | −.071 | .000 |
| 1.7276‐3 | 5065 | −.004 | .850 | −.019 | .271 | |
| >3 | 841 | −.047 | .423 | −.030 | .486 | |
| ALT (0‐40) U/L | 0‐1.7276 | 9313 | −.003 | .891 | −.026 | .039 |
| 1.7276‐3 | 5065 | −.035 | .144 | −.018 | .301 | |
| >3 | 841 | −.068 | .241 | −.006 | .898 | |
| TBIL (5.1‐25.6) umol/L | 0‐1.7276 | 9313 | −.003 | .854 | .005 | .672 |
| 1.7276−3 | 5065 | −.028 | .234 | −.022 | .204 | |
| >3 | 841 | −.076 | .190 | .013 | .759 | |
| ALB (37−53) g/L | 0‐1.7276 | 9313 | .018 | .337 | .027 | .031 |
| 1.7276‐3 | 5065 | −.014 | .547 | −.047 | .008 | |
| >3 | 841 | −.143 | .013 | −.022 | .615 | |
| GGT (8‐58) U/L | 0‐1.7276 | 9313 | .013 | .500 | −.011 | .399 |
| 1.7276‐3 | 5065 | −.017 | .471 | −.012 | .510 | |
| >3 | 841 | −.049 | .399 | −.023 | .600 | |
Table 9.
Correlation between MLR and kidney and liver functions in HTN and non‐HTN
| Variables | MLR | Number | HTN | Non‐HTN | ||
|---|---|---|---|---|---|---|
| r | P | r | P | |||
| BUN (2.5‐7.0) mmol/L | 0‐0.1845 | 9114 | −.042 | .022 | −.055 | .000 |
| 0.1845‐0.3 | 5089 | −.026 | .294 | .029 | .096 | |
| >0.3 | 1016 | .070 | .182 | .096 | .015 | |
| CREA (75‐115) mmol/L | 0‐0.1845 | 9114 | .042 | .022 | .077 | .000 |
| 0.1845‐0.3 | 5089 | .046 | .058 | .045 | .009 | |
| >0.3 | 1016 | .077 | .139 | .113 | .004 | |
| AST (0‐40) U/L | 0‐0.1845 | 9114 | .003 | .873 | .007 | .594 |
| 0. 0.1845‐0.3 | 5089 | .031 | .201 | .012 | .496 | |
| >0.3 | 1016 | .117 | .025 | .039 | .328 | |
| ALT (0‐40) U/L | 0‐0.1845 | 9114 | .015 | .414 | .013 | .302 |
| 0.1845‐0.3 | 5089 | .016 | .522 | .009 | .591 | |
| >0.3 | 1016 | .087 | .094 | .020 | .610 | |
| TBIL (5.1‐25.6) umol/L | 0‐0.1845 | 9114 | .032 | .081 | .045 | .000 |
| 0.1845‐0.3 | 5089 | .022 | .366 | .019 | .277 | |
| >0.3 | 1016 | −.017 | .744 | .085 | .031 | |
| ALB (37‐53) g/L | 0‐0.1845 | 9114 | −.010 | .575 | .002 | .850 |
| 0.1845‐0.3 | 5089 | −.049 | .044 | −.019 | .277 | |
| >0.3 | 1016 | −.198 | .000 | −.140 | .000 | |
| GGT (8‐58) U/L | 0‐0.1845 | 9114 | .072 | .000 | .030 | .020 |
| 0.1845‐0.3 | 5089 | .080 | .001 | .023 | .183 | |
| >0.3 | 1016 | −.019 | .714 | .021 | .602 | |
Therefore, NLR at high level was significantly associated with kidney dysfunction in HTN. MLR was linked with kidney dysfunction more in non‐HTN.
Finally, we have analyzed the effects of gender and age on the correlations of NLR or MLR with kidney or liver functional parameters in HTN and non‐HTN groups. Statistical analysis showed that NLR was significantly increased in male or age >65 groups both in HTN (mean NLR values for male and female: 1.8151 and 1.7093, P < .0001; age <65 and age >65:1.7472 and 1.8805, P < .0001) and in non‐HTN groups (mean NLR values for male and female: 1.7394 and 1.6848; age < 65 and age >65:1.6977 and 1.9290, P = .013, P < .0001). Similarly, MLR was significantly increased in male or age >65 groups in HTN (mean values for male and female: 0.1995 and 0.1700, P < .0001; age <65 and age >65:0.1834 and 0.2015, P < .0001) and in non‐HTN groups (mean values for male and female: 0.1954 and 0.1747, P < .0001; for age <65 and age >65, 0.1821 and 0.2163, P < .0001).
Correlation analysis results showed that in HTN, gender and age did not affect the positive correlation between NLR and CREA or BUN (Table 10). However, among non‐HTN, age >65 group showed correlation between NLR and CREA (P = .001, Table 10), and male group showed correlation between NLR and BUN (P < .0001, Table 10).
Table 10.
Effects of gender and age on the correlations between NLR, MLR, and kidney function parameters
| NLR | HTN n = 4997 | Non‐HTN n = 10 222 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male n = 2720 | Female n = 2277 | Age <65 n = 4259 | Age ≥ 65 n = 738 |
Male n = 4438 |
Female n = 5784 | Age <65 n = 9744 | Age ≥65 n = 478 | |||||||||
| r | P | r | P | R | P | r | P | r | P | r | P | r | P | r | P | |
| BUN (2.5‐7.0) mmol/L | .078 | .000 | .091 | .000 | .053 | .001 | .179 | .000 | .076 | .000 | −.010 | .466 | .017 | .088 | .078 | .089 |
| CREA (75‐115) mmol/L | .113 | .000 | .143 | .000 | .095 | .000 | .242 | .000 | .000 | .975 | −.019 | .158 | −.007 | .461 | .151 | .001 |
| MLR | HTN n = 4997 | Non‐HTN n = 10 222 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Male n = 2720 |
Female n = 2277 | Age <65 n = 4259 | Age ≥ 65 n = 738 |
Male n = 4438 |
Female n = 5784 | Age <65 n = 9744 | Age ≥65 n = 478 | |||||||||
| r | P | r | P | R | P | r | P | r | P | r | P | r | P | r | P | |
| BUN (2.5‐7.0) mmol/L | ‐.018 | .348 | ‐.020 | .336 | ‐.039 | .011 | .067 | .067 | .004 | .771 | ‐.084 | .000 | ‐.044 | .000 | .136 | .003 |
| CREA (75‐115) mmol/L | .077 | .000 | .081 | .000 | .131 | .000 | .174 | .000 | .049 | .001 | .009 | .492 | .079 | .000 | .248 | .000 |
Similarly, age and gender did not affect the correlation between MLR and CREA in HTN (Table 10). Among non‐HTN, the correlation between MLR and CREA was lost in female population (P = .492). And MLR was positively correlated with BUN only in aged group (P = .003).
4. DISCUSSION
Using retrospective data analysis, this study demonstrated the first report of NLR and MLR and their associations with kidney function from over 15 000 individuals among general population who underwent health checkup. More importantly, we have analyzed the differences of association between HTN and non‐HTN groups. Our data have shown that NLR was elevated in HTN group even when the history of diseases (including previous diagnosis of high blood pressure) was excluded. MLR was not elevated in HTN. Notably, parameters in the blood were within normal ranges. However, majority of parameters, including WBC, Glu, lipids, or kidney functional parameters (CREA and BUN) and liver functional parameters (AST, ALT, ALB, TBIL), were higher in HTN comparing to non‐HTN group. PLT was lower in HTN. Pearman correlation analysis has indicated that BUN and CREA showed positive correlation with high NLR only in HTN. MLR showed similar correlation with kidney or liver function parameters between HTN and non‐HTN. When the groups were divided according to gender and age, without changing the correlation in HTN, NLR and kidney functions were correlated in male and aged groups among non‐HTN population. Similarly, stronger correlation was observed between MLR and kidney function in males and in aged groups, especially among non‐HTN population. These results suggest that NLR can be a sensitive predictor of kidney dysfunction among general population with high blood pressure.
Recently, a number of reports analyzed NLR, MLR, and their associations with organ function or hypertension from general population.15, 16 Interestingly, NLR and MLR values in our study (mean NLR 1.7265 and MLR 0.1845) are in agreement with those by Lee et al, who analyzed the values in 12 160 samples from healthy Korean population, showing that mean NLR was 1.65 and mean MLR was 0.188.15 Similar mean NLR numbers were found in 132 participants without HTN was 1.6917 and in 30 healthy subjects was 1.75.18 In another study by Tonyali et al, mean NLR from 46 healthy controls was 2.1419 and the level was increased significantly in chronic kidney disease (to 3.5219). Similar increment in NLR was observed in disease conditions.18, 20, 21 Previously, we have shown that mean NLR in 715 acute myocardial infarction patients on admission without failure or infection was 2.7622; mean NLR was 2.65 when GRACE risk score was 100 and NLR was >6.5 when GRACE risk score was over 140.23 Arbel et al explored that NLR (>6.5) was associated with lower EF and increased 30 days and 5‐year mortality.24 These results strongly support that as a marker of inflammation, the level of NLR increases with the severity of diseases and high NLR is correlated with high mortality and poor prognosis.
Among 15 219 general population who underwent health checkup, about 32% were hypertensive. This is after diabetes, cancer, cardiovascular or kidney diseases, history of hypertension were excluded. Furthermore, most of WBCs were significantly higher and NLR was significantly increased in HTN. This is in line with Belen and colleagues who demonstrated that NLR and neutrophil count increased in patients with resistant hypertension compared with normotension or patients whose hypertension was controlled.25 Similar results were found by Wang H et al,17 who showed that WBC counts in hypertensive patients were increased compared with those in normotension. Demir M et al26 have shown that NLR is positively correlated with blood pressure and NLR is significantly elevated in non‐dippers compared with dippers. It is possible that mechanical stress in the arteries of hypertensive patients can cause endothelial disruption, arterial damage, and inflammation, a mechanism that is indispensable for hypertension and its comorbidities, lead to fatal stroke and myocardial infarction, etc.. In line with these results, here, our results indicate that inflammatory parameters should be studied more carefully, especially parameters such as NLR in hypertensive individuals should be compared to those from healthy population. It can be informative for early prevention and intervention before disease development.
Inflammation, as a predictor of organ damage, has been linked with the kidney dysfunction in hypertension.21, 27 This is also shown to be the case in our study from general population: That is, NLR showed significant positive correlation with CREA in HTN, a kidney function parameter, especially at high NLR values. The association was also observed among non‐HTN groups, in male, and with aged groups (>65). Recently, it was reported that elevated blood neutrophil activity (which leads to superoxide anion generation and myeloperoxidase activity) was significantly associated with high blood pressure in spontaneously hypertensive rat.28 Neutrophils are shown to stick to vascular endothelium, which initiates endothelial, vascular inflammation, and dysfunction; consequently, increased pro‐inflammatory cytokines and smooth muscle proliferation affect arterial functions, such as stiffness, which deteriorates organ damage.17 Interestingly, NLR correlation with liver function parameters was not different between HTN and non‐HTN, indicating that inflammation in hypertension shows organ specificity.
Until recently, MLR and its association with organ damage in HTN have not been reported. As one of the active inflammatory cells, monocytes are essential in the generation of atherosclerosis in vascular smooth muscle and in endothelium, as such, monocytes play key roles in the pathogenesis of cardiovascular diseases. Although monocyte counts were significantly increased in HTN, MLR did not show significant difference between HTN and non‐HTN (P = .09). Nevertheless, MLR showed significant correlation with CREA and liver parameters in all individuals. This is similar when the groups were divided into male, female, or aged (>65) or not aged (<65) groups, although stronger correlation was observed among aged and male groups. In addition, high MLR was associated with kidney functions more in non‐HTN. These results indicate that MLR and NLR are different in linking the changes in organ function in HTN.
Taken together, our results confirm that NLR can be a useful indicator of organ damage (eg, kidney dysfunction) in hypertensive group from general population of normal health checkup. Overall, the higher the MLR, the worse the performance of liver or kidney, probably more in healthy population. Larger prospective studies and measurement of inflammatory markers, both pro‐inflammatory and anti‐inflammatory cytokines in the peripheral blood samples and in the tissue, are warranted to define the significance of the inflammatory changes in hypertension.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Yin Hua Zhang was involved in the conception of the work. Chen Chen and Hai Yan Zhao were involved in data collection, analysis, and preparation of the manuscript. Chen Chen, Hai Yan Zhao, and Yin Hua Zhang were involved in revision, reviewing, and final approval of the revised work.
Chen C, Zhao HY, Zhang YH. Correlation between neutrophil‐to‐lymphocyte ratio and kidney dysfunction in undiagnosed hypertensive population from general health checkup. J Clin Hypertens. 2020;22:47–56. 10.1111/jch.13749
Chen and Zhao both authors contributed equally to the study
Funding information
This work is supported by National Natural Science Foundation of China (NSFC 31660284).
REFERENCES
- 1. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2018;138(17):e426‐e483. [DOI] [PubMed] [Google Scholar]
- 2. Schiffrin EL. Hypertension in 2017: novel mechanisms of hypertension and vascular dysfunction. Nat Rev Nephrol. 2018;14(2):73‐74. [DOI] [PubMed] [Google Scholar]
- 3. Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215(1):21‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Higaki A, Caillon A, Paradis P, Schiffrin EL. Innate and innate‐like immune system in hypertension and vascular injury. Curr Hypertens Rep. 2019;21(1):4. [DOI] [PubMed] [Google Scholar]
- 5. White FN, Sambhi MP, Grollman A. Renal function in hypertensive cardiovascular disease of rat. Am J Physiol. 1960;198:221‐222. [DOI] [PubMed] [Google Scholar]
- 6. White FN, Grollman A. Autoimmune factors associated with infarction of the kidney. Nephron. 1964;1:93‐102. [DOI] [PubMed] [Google Scholar]
- 7. Okuda T, Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25(2):257‐264. [PubMed] [Google Scholar]
- 8. Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449‐2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Olsen F. Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A. 1972;80(2):253‐256. [PubMed] [Google Scholar]
- 10. Rodríguez‐Iturbe B, Quiroz Y, Nava M, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am J Physiol Renal Physiol. 2002;282(2):F191‐F201. [DOI] [PubMed] [Google Scholar]
- 11. Vinh A, Chen W, Blinder Y, et al. Inhibition and genetic ablation of the B7/CD28 T‐cell costimulation axis prevents experimental hypertension. Circulation. 2010;122(24):2529‐2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short‐ and long‐term mortality after non‐ST‐elevation myocardial infarction. Am J Cardiol. 2010;106:470‐476. [DOI] [PubMed] [Google Scholar]
- 13. Uthamalingam S, Patvardhan EA, Subramanian S, et al. Utility of the neutrophil to lymphocyte ratio in predicting long‐term outcomes in acute decompensated heart failure. Am J Cardiol. 2011;107:433‐438. [DOI] [PubMed] [Google Scholar]
- 14. Kato A, Takita T, Furuhashi M, Maruyama Y, Kumagai H, Hishida A. Blood monocyte count is a predictor of total and cardiovascular mortality in hemodialysis patients. Nephron Clin Pract. 2008;110(4):c235‐c243. [DOI] [PubMed] [Google Scholar]
- 15. Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil‐lymphocyte ratio, lymphocyte‐monocyte ratio, platelet‐lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine. 2018;97:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jhuang YH, Kao TW, Peng TC, et al. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9‐year cohort study in Taiwan. Hypertens Res. 2019. 42(8):1209‐1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Hu Y, Geng Y, et al. The relationship between neutrophil to lymphocyte ratio and artery stiffness in subtypes of hypertension. J Clin Hypertens (Greenwich). 2017;19(8):780‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okyay GU, Inal S, Oneç K, et al. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 19. Tonyali S, Ceylan C, Yahsi S, Karakan MS. Does neutrophil to lymphocyte ratio demonstrate deterioration in renal function? Ren Fail. 2018;40(1):209‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kocyigit I, Eroglu E, Unal A, et al. Role of neutrophil/lymphocyte ratio in prediction of disease progression in patients with stage‐4 chronic kidney disease. J Nephrol. 2013;26(2):358‐365. [DOI] [PubMed] [Google Scholar]
- 21. Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil‐to‐lymphocyte ratio and inflammation in end‐stage renal disease patients. Ren Fail. 2012;34:155‐159. [DOI] [PubMed] [Google Scholar]
- 22. Chen C, Cong BL, Wang M, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr Med Res. 2018;7(2):192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oncel RC, Ucar M, Karakas MS, et al. Relation of neutrophil‐to‐lymphocyte ratio with GRACE risk score to in‐hospital cardiac events in patients with ST‐segment elevated myocardial infarction. Clin Appl Thromb Hemost. 2015;21:383‐388. [DOI] [PubMed] [Google Scholar]
- 24. Arbel Y, Shacham Y, Ziv‐Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long‐term all‐cause mortality in ST‐elevation myocardial infarction patients. Can J Cardiol. 2014;30:1177‐1182. [DOI] [PubMed] [Google Scholar]
- 25. Belen E, Sungur A, Sungur MA, Erdoğan G. Increased neutrophil to lymphocyte ratio in patients with resistant hypertension. J Clin Hypertens. 2015;17(7):532‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demir M. The relationship between neutrophil lymphocyte ratio and non‐dipper hypertension. Clin Exp Hypertens. 2013;35(8):570‐573. [DOI] [PubMed] [Google Scholar]
- 27. Magen E, Mishal J, Paskin J, et al. Resistant arterial hypertension is associated with higher blood levels of complement C3 and C‐ reactive protein. J Clin Hypertens (Greenwich). 2008;10:677‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang R, Inagawa H, Kazumura K, et al. Evaluation of a hypertensive rat model using peripheral blood neutrophil activity, phagocytic activity and oxidized LDL evaluation. Anticancer Res. 2018;38(7):4289‐4294. [DOI] [PubMed] [Google Scholar]


