Abstract
Masked hypertension (MH) is defined as normal office blood pressure (BP) and elevated ambulatory BP (ABP) or home BP or both. This study assessed the association of MH (ie, isolated home, isolated ABP and dual MH) with echocardiographic left ventricular hypertrophy (LVH). The present analysis of the PAMELA study included 1087 untreated and treated participants with normal office BP and a measurable LV mass (LVM). A total of 193 individuals (17.7%) had any MH (ie, normal office BP, elevated ABP or home BP or both), 48 had dual MH (25%), 62 isolated ambulatory MH (32%), and 83 isolated home MH (43%). Average LVM indexed to body surface area was superimposable in the three MH phenotypes (being the largest difference between groups <3 g/m2) and significantly higher than in true normotensives. This was also for the LVH prevalence that varied across the MH subgroups in a narrow range (from 8.3% to 10.8%). In conclusion, individuals from the general population with isolated MH, in which either home or ABP was elevated, exhibited an increased risk of LVH similar to that entailed by dual MH. Our findings add the notion both home and ABP measurements are useful to more accurately assess the risk of LVH associated with MH in the community.
Keywords: general population, left ventricular hypertrophy, masked hypertension
1. INTRODUCTION
A large amount of evidence supports the view that blood pressure (BP) measured outside the office is a strong predictor of left ventricular hypertrophy (LVH), a cardinal marker of hypertension‐mediated organ damage (HMOD), compared to conventional measurements in the office setting.1
The increasing use of combined office and out‐of‐office (home or ambulatory) BP measurements has allowed to provide a comprehensive information on the risk of LVH entailed by different BP phenotypes that is white coat hypertension, sustained hypertension, and masked hypertension (MH, normal office, and elevated out‐of‐office BP).2
In particular, several individual cross‐sectional and prospective studies and their meta‐analysis have shown that MH individuals have a greater likelihood of LVH compared to true normotensive individuals.3, 4
In such studies, the MH phenotype was indifferently defined by out‐of‐office BP assessed by home or ambulatory BP (ABP) monitoring as outcome findings have shown these two methods convey similar information on the cardiovascular prognosis of MH.5, 6 So far, to the best of our knowledge, no study provided data on the association of LVH and MH assessed by each of these two methods.
We have addressed this topic in the Pressioni Monitorate E Loro Associazioni (PAMELA) population, taking advantage of the fact that an echocardiographic examination, office, home BP measurements, and ABP measurements were collected in all participants.
2. METHODS
The PAMELA study was performed in 2051 subjects representative of the population of Monza (Italy) for sex, age, and other characteristics. As described in detail elsewhere,7 data collection included medical history, office, home and ambulatory BP, standard blood examinations, and LVM as assessed by echocardiography.
2.1. BP measurements
Office BP was measured three times with the subject in the sitting position, using a mercury sphygmomanometer. To measure ABP, subjects were fitted with an ABP monitoring (ABPM) device (Spacelabs 90207) set to obtain automated BP and heart rate oscillometric readings every 20 minutes over 24 hours. During the ABPM period, subjects were asked to self‐measure BP at home twice, namely around 8.00 am and 8.00 pm, using a semiautomatic oscillometric device (Philips, model HP 5331) on the arm contralateral to the one used for ambulatory BP monitoring. (ie, usually the dominant).
2.2. Echocardiography
M‐mode and two‐dimensional echo examinations were carried out with a commercially available instrument (Acuson 128 CF, Computer Sonography). End‐diastolic (d) and end‐systolic (s) LV internal diameters (LVID), interventricular septum (IVS), and posterior wall (PW) thickness were measured off‐line from two‐dimensionally guided M‐mode tracings, during at least three consecutive cycles. LVM was estimated by using the corrected ASE method and normalized to body surface area (BSA). LVH was defined as LVM index (LVMI) higher than: 114 g/m2 in men and 99 g/m2 in women.8 These cutoffs have been derived from sex‐specific upper limits of normality (mean plus 1.96 standard deviation) for LV mass indexed to BSA in 675 healthy individuals with sustained normotension belonging to PAMELA population.
2.3. Clinical setting and definitions
Participants with normal office BP values at entry (<140 mm Hg systolic and/or <90 mm Hg diastolic) were divided into four groups: true normotensives, isolated ambulatory MH (ie, normal office and home BP, and elevated ABP), isolated home MH (ie, normal office and ABP, and elevated home BP), and dual MH (ie, normal office, elevated ABP, and home BP) based on ambulatory and home values in the normal or elevated range according to the hypertension guidelines that is 24‐hour mean BP <or ≥130 mm Hg systolic and/or 80 mm Hg diastolic, home BP <or ≥135 mm Hg systolic and/or 85 mm Hg diastolic, respectively.9
2.4. Data analysis
In each subject, the three office and four home BP measurements were averaged. ABP readings were also averaged and analyzed to obtain 24‐hour mean (±standard deviation) systolic/diastolic BP. Values are expressed as means ± SD or percentages. Comparisons between groups were performed by t test or ANOVA with Bonferroni correction (mean values) and by chi‐square test or Fisher's exact test (prevalence). Data regarding the main outcome of the study (ie, LVM and prevalence of LVH) were adjusted for major confounders. A P value <.05 was considered statistically significant. Statistical analysis was performed by SAS System (version 9.4; SAS Institute Inc).
3. RESULTS
This cross‐sectional analysis included 1087 untreated and treated participants with normal office BP and a measurable LVMI at the initial evaluation. In this population sample, a total of 193 individuals (17.7%) had any MH (ie, normal office BP, elevated ABP or home BP, or both). The prevalence of isolated home, isolated ambulatory, and dual MH were 43%, 32%, and 25%, respectively.
Table 1 reports demographic clinical data of PAMELA participants with normal office BP classified according to their home and ABP values. Overall, individuals with MH, regardless of their pattern, showed a greater male prevalence, age, body mass index, office, home, and 24‐hour mean systolic/diastolic BP and LVMI compared to subjects with true normotension. This was also the case for fasting blood glucose, total serum cholesterol, and prevalence of the metabolic syndrome and antihypertensive treatment. Some of these differences such as fasting blood glucose, total cholesterol, serum creatinine disappeared after adjustment for age, sex, and BMI. Among participants with MH, individuals with isolated home and dual MH were older than their counterparts with isolated ambulatory MH. A worse metabolic risk profile was evident in the subgroup with dual MH as compared to isolated ABP and home MH subgroups. Of note, participants with isolated home MH were more frequently taking BP lowering drugs than those with isolated ABP and dual MH.
Table 1.
Clinical characteristics of PAMELA participants classified according to their blood pressure (BP) status in normotensive (ie, normal office, ambulatory, and home BP), dual masked hypertension (ie, MH normal office, elevated home, and ambulatory BP), isolated ambulatory MH (ie, normal office and home, and elevated ambulatory BP) and isolated home MH (ie, normal office, elevated home, and normal ambulatory BP)
| Variables |
Normotensive N = 894 |
Dual MH N = 48 |
Isolated Ambulatory MH N = 62 |
Isolated Home MH N = 83 |
|---|---|---|---|---|
| Male, % | 42.51a, b, c | 70.83 | 70.97 | 62.65 |
| Age, years | 44 ± 12.1a, c | 52.8 ± 11.5b | 46.2 ± 10.8c | 53.7 ± 14.5 |
| BMI, kg/m2 | 24 ± 3.6a, c | 27.1 ± 3.8 | 25.1 ± 3.4 | 25.8 ± 3.5 |
| Office SBP, mm Hg | 117.7 ± 10.3a, b, c | 127.7 ± 7.8 | 124.8 ± 7.5 | 125.6 ± 8.4 |
| Office DBP, mm Hg | 76.8 ± 7a, b, c | 82.6 ± 4.5c | 82.6 ± 5.3c | 79.4 ± 5.2 |
| 24 h SBP, mm Hg | 112.9 ± 7a, b, c | 130.5 ± 6.9a, c | 126.8 ± 5.3c | 118.2 ± 6.2 |
| 24 h DBP, mm Hg | 70.3 ± 5a, b, c | 82.2 ± 4.7c | 81.6 ± 3.3c | 72.4 ± 4.5 |
| Home SBP, mm Hg | 111.6 ± 10.8a, b, c | 138.4 ± 12.2b | 121.2 ± 8.2c | 136.9 ± 13.3 |
| Home DBP, mm Hg | 69.9 ± 7.1a, b, c | 86.5 ± 5.5b | 77.3 ± 4.8c | 85 ± 12.2 |
| Serum glucose mg/dL | 85.8 ± 12.4a, b | 105.3 ± 52.9b, c | 92.3 ± 26.3 | 87.9 ± 14.4 |
| Serum total cholesterol, mg/dL | 214.1 ± 41.1a, c | 231.4 ± 45.3 | 221.1 ± 41.7 | 230.4 ± 46.2 |
| Serum HDL cholesterol, mg/dL | 57.5 ± 15.7 | 54.6 ± 18 | 52.4 ± 13.7 | 53.2 ± 15.5 |
| Creatinine, mg/dL | 0.85 ± 0.15c | 0.86 ± 0.14 | 0.91 ± 0.15 | 0.94 ± 0.31 |
| LVMI, g/m2 | 77.7 ± 16.4a, b, c | 87.8 ± 17.4 | 85.6 ± 19.0 | 88 ± 17.1 |
| Prevalence of MS, % | 4.9a | 18.7 | 9.8 | 12.3 |
| LVMI >114/99 g/m2, % | 4.6a, b, c | 8.7 | 8.6 | 10.6 |
| Antihypertensive treatment, % | 4.82c | 6.25c | 8.06c | 25.30 |
| Type 2 Diabetes (%) | 3.1 | 3.9 | 3.5 | 4.0 |
| Current Smoking (%) | 22.1c | 18.0 | 19.7 | 16.1 |
As for differences in LVMI and prevalence of LVH data are adjusted for age, sex and BMI, glycemia, total cholesterol, serum creatinine, smoking, and antihypertensive treatment.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; LVMI, left ventricular mass index, MS, metabolic syndrome; SBP, systolic blood pressure.
P < .05 vs dual MH.
P < .05 vs isolated ambulatory MH.
P < .05 vs isolated home MH.
Average LVM indexed to BSA was superimposable in the three MH phenotypes and significantly higher than in true normotensives. This was also the case when LVMI was treated as a categorical variable. Indeed, compared to normotensive participants, a higher percentage of subjects with MH, regardless their pattern, fulfilled the diagnostic criteria for LVH. Statistical difference between MH subgroups and true normotensive individuals persisted after adjusting for several confounders including age, sex, BMI, glycemia, total cholesterol, creatinine, smoking, and antihypertensive treatment.
3.1. Additional analysis
In a sensitivity analysis, the assessment of subclinical LV damage by LVM indexed to height to allometric power of 2.7 provided similar results (ie, 35 ± 8 g/h2.7 normotensive, 40 ± 8 g/h2.7 dual MH, 39 ± 9 g/h2.7, isolated ambulatory MH, and 41 ± 9 g/h2.7 isolated home MH).
Furthermore, we excluded from the analysis all participants treated with BP lowering drugs, due to the potential impact of the treatment on LVM. Differences in LVM as well as in prevalence of LVH between true normotensive individuals (n = 849), dual MH (n = 45), isolated ambulatory MH (n = 57), and isolated home MH turned out to be significant (Figure 1).
Figure 1.
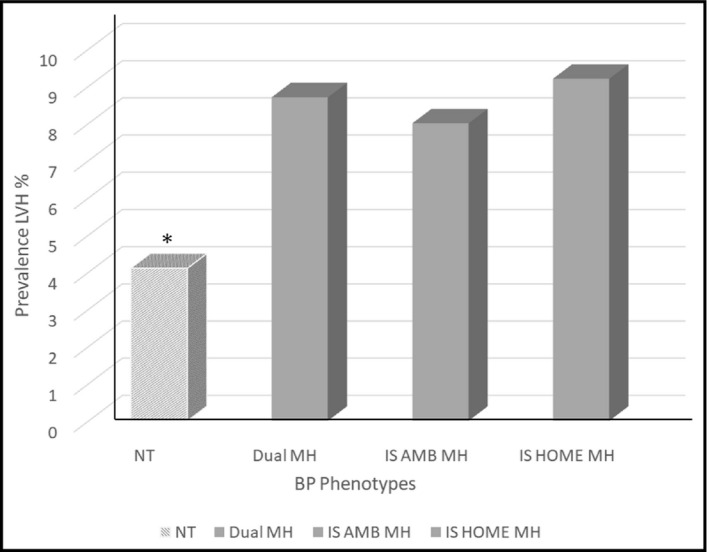
Prevalence rates of left ventricular hypertrophy (LVH) assessed according to LV mass normalized to body surface area in true in untreated normotensive (NT), dual masked hypertension (MH), isolated ambulatory (IS AMB) MH, and isolated home (I home) MH. *< 0.05 normotensive vs dual, IS AMB, and IS home MH
4. DISCUSSION
The novel findings of the present analysis of the PAMELA population are that participants with MH had a higher risk of subclinical LV damage than that of the reference group with BP normality defined by all three BP measurement methods, regardless of the circumstances in which out‐of‐office BP elevation has been documented (ie, home, isolated ABP, or both), after adjusting for major confounders. A further relevant aspect is that the extent of LV involvement, as assessed by a prognostically validated marker such as LVMI, was not different among the MH subtypes, even when the analysis was restricted to untreated individuals. This means that even a partial elevation BP can play an important role in the pathogenesis of organ damage, and, consequently, the complementary use of the two methods allows to identify a larger number of subjects at risk10. In our series, dual (or complete) MH (which included only a quarter of the entire MH population) did not have a greater degree of cardiac HMOD as compared to isolated (ie, partial) MH and therefore this phenotype would not significantly refine the risk stratification.
Additional aspects of our results merit to be briefly discussed. First, the prognostic value of the different subtypes of MH is largely unknown and the information on this topic is limited to the general population. Participants of the Ohasama study with complete MH (both home and 24‐h ambulatory hypertension and office normotension) and partial MH (either home or 24‐h ambulatory hypertension and office normotension) had a significantly increased risk for stroke (OR: 2.08 and 2.46, respectively) compared to sustained normotensive groups during a median follow‐up period of 17 years.[10, 11] Of note, when partial MH groups were further divided into participants only with home hypertension and those only with 24‐h hypertension, both subgroups exhibited a similar and significantly greater stroke risk. As for white coat hypertension (WCH), the opposite condition of MH, a pioneering report in this research field, showed that the members of the general population with partial WCH (ie, subjects with only one out‐of‐office BP normal) had a greater adjusted risk of cardiovascular and all‐cause mortality.[11, 12]
Second, in the present analysis dual or complete MH was found in only a quarter of individuals with any MH, having the majority of them either isolated home (43%) or ambulatory hypertension (32%). Disagreement between home and ABPM criteria in diagnosing MH has also been reported in the few previous studies carried out in population‐based samples or in patients attending hypertension clinics. In such studies, the diagnostic agreement of the two BP measurement methods in identifying out‐of‐office hypertension ranged from 32% to 48%11, 13, 14, 15 suggesting that dual or complete MH can be diagnosed in less than half of the subjects with office normotension. Third, as compared participants with partial MH to those with dual MH had a worse metabolic profile, greater prevalence of obesity, and higher average office and out‐of‐office BP values, all important pathogenetic factors for development and progression of cardiac HMOD. Therefore, the failure to find differences, despite adjustments for possible confounders, in LVMI and LVH prevalence between MH subtypes, could be related to a first type error due to small population sample.
Fourth, in virtually all studies information on the clinical and prognostic value of MH has been collected by measuring out‐of‐office BP by a single ABPM or home BP monitoring performed for short periods of time. The stability of this pattern over time has been investigated by few studies with somewhat variable results but, on the whole, indicative of the poor reproducibility of this phenotype. Particularly relevant are the findings recently provided by the ELSA study which have shown that only about 40% of treated hypertensive patients with MH at the first set of office and ambulatory BP measurement remained in the same condition at the second set of measurements.16 Thus, the evidence that MH is not a stable trait should be taken into account in outcome studies, like the present, based on a single set of measurements.
4.1. Limitations
The present study needs to be interpreted within the context of its potential limitations. The cross‐sectional analysis does not allow direct assessment of causal relationship between MH and LVH. Our findings refer to a middle‐aged population with low prevalence of comorbidities and might not to be extended to populations with different clinical characteristics. Home BP was based on the mean of four readings recorded only a single day, which may have led to an inaccurate classification of participants according to home BP criteria. Furthermore, per protocol in the PAMELA study the home BP measurement was carried out on the dominant arm as the nondominant was used for the ABPM. This may actually represent a limit that may have influenced the results to some extent. Participants on antihypertensive treatment were not excluded to reflect real‐world conditions.
5. CONCLUSIONS
In conclusion, individuals from the general population with isolated MH, in which either home or ABP was really elevated, exhibited an increased risk of LVH similar to those with dual or complete MH. To current evidence, our findings add the notion both home and ABP measurements are useful to more accurately assess the risk of LVH associated with MH, and this is because the two out‐of‐office BP measurement methods cannot be regarded as interchangeable and their combined use can improve cardiovascular risk stratification. However, in view of the limitations of our analysis, further prospective studies on larger populations are needed in this research area.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
Cuspidi C, Facchetti R, Quarti‐Trevano F, et al. Left ventricular hypertrophy in isolated and dual masked hypertension. J Clin Hypertens. 2020;22:673–677. 10.1111/jch.13808
REFERENCES
- 1. Mancia G, Zanchetti A, Agabiti‐Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment‐induced regression of left ventricular hypertrophy. SAMPLE study group. Study on ambulatory monitoring of blood pressure and lisinopril evaluation. Circulation. 1997;95:1464‐1470. [DOI] [PubMed] [Google Scholar]
- 2. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (pressione arteriose monitorate E loro associazioni [PAMELA] study). Circulation. 2001;104:1385‐1392. [DOI] [PubMed] [Google Scholar]
- 3. Cuspidi C, Sala C, Tadic M, et al. Untreated masked hypertension and subclinical cardiac damage: a systematic review and meta‐analysis. Am J Hypertens. 2015;28:806‐813. [DOI] [PubMed] [Google Scholar]
- 4. Cuspidi C, Facchetti R, Quarti‐Trevano F, et al. Incident left ventricular hypertrophy in masked hypertension. Hypertension. 2019;74:56‐62. [DOI] [PubMed] [Google Scholar]
- 5. Stergiou GS, Asayama K, Thijs L, et al. Prognosis of white‐coat and masked hypertension: international database of HOme blood pressure in relation to cardiovascular outcome. Hypertension. 2014;63:675‐682. [DOI] [PubMed] [Google Scholar]
- 6. Zhang DY, Guo QH, An DW, et al. A comparative meta‐analysis of prospective observational studies on masked hypertension and masked uncontrolled hypertension defined by ambulatory and home blood pressure. J Hypertens. 2019;37:1775‐1785. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Sega R, Bravi C, et al. Ambulatory blood pressure normality: results from the PAMELA study. J Hypertens. 1995;13:1377‐1390. [PubMed] [Google Scholar]
- 8. Cuspidi C, Facchetti R, Sala C, et al. Normal values of left‐ventricular mass: echocardiographic findings from the PAMELA study. J Hypertens. 2012;30:997‐1003. [DOI] [PubMed] [Google Scholar]
- 9. Williams B, Mancia G, Spiering W, et al. 2018 practice guidelines for the management of arterial hypertension of the european society of hypertension and the european society of cardiology: ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2018;36:2284‐2309. [DOI] [PubMed] [Google Scholar]
- 10. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self‐measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26:1919‐1927. [DOI] [PubMed] [Google Scholar]
- 11. Satoh M, Asayama K, Kikuya M, et al. Long‐term stroke risk due to partial white‐coat or masked hypertension based on home and ambulatory blood pressure measurements: the ohasama study. Hypertension. 2016;67:48‐55. [DOI] [PubMed] [Google Scholar]
- 12. Mancia G, Bombelli M, Brambilla G, et al. Long‐term prognostic value of white coat hypertension: an insight from diagnostic use of both ambulatory and home blood pressure measurements. Hypertension. 2013;62:168‐174. [DOI] [PubMed] [Google Scholar]
- 13. Stergiou GS, Salgami EV, Tzamouranis DG, et al. Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18:772‐778. [DOI] [PubMed] [Google Scholar]
- 14. Stergiou GS, Kyriakoulis KG, McManus RJ, et al. Phenotypes of masked hypertension: isolated ambulatory, isolated home and dual masked hypertension. J Hypertens. 2019;38:218‐223. [DOI] [PubMed] [Google Scholar]
- 15. Anstey DE, Muntner P, Bello NA, et al. Diagnosing masked hypertension using ambulatory blood pressure monitoring, home blood pressure monitoring, or both? Hypertension. 2018;72:1200‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mancia G, Facchetti R, Cuspidi C, et al. Limited reproducibility of MUCH and WUCH: evidence from the ELSA study. Eur Heart J. 2019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


