Abstract
Dietary sodium intake and cardiovascular outcomes have a reported J‐shaped curve relationship. This study analyzes the relationship between dietary sodium and sugar intake as a potential mechanism to explain this association. The authors examined cross‐sectional data from the National Health and Nutrition Examination Survey (NHANES) 2001‐2016 where dietary sodium, carbohydrate, fat, cholesterol, and sugar intakes were assessed by 24‐hour dietary recall and were standardized to a total daily intake of 2000 calories. Sodium intake was categorized into sodium quintiles (SQ) as follows: SQ1(0.06‐2.6 g/d); SQ2(2.6‐3.0 g/d); SQ3(3.0‐3.4 g/d); SQ4(3.4‐4.0 g/d); and SQ5(4.0‐29.3 g/d). Simple and multivariate linear regression using SQ3 as reference were used to assess associations between daily sodium intake and the other nutrients. Our results showed that among 38 722 participants that met our study criteria, the mean age was 43.6 years (SD 16.8 years) and sex was equally distributed (48.8% male vs 51.2% female). Sugar intake went down across increasing SQs and was significantly higher in SQ1 (141.2 g/d) and SQ2 (118.6 g/d) and significantly lower in SQ4 (97.9 g/d) and SQ5 (85.6 g/d) compared to SQ3 (108.6 g/d; all P < .01). These same trends remained unchanged and significant in the fully adjusted multivariate model. In conclusion, NHANES study participants reporting low sodium intake on 24‐hour dietary recall have a higher consumption of sugar. The negative impact of low sodium diet on cardiovascular health may be explained at least partially by the associated high sugar intake.
Keywords: cardiovascular, fructose, outcome, sodium, sugar
1. INTRODUCTION
Awareness of sodium intake and its relationship with cardiovascular outcomes has been a patients of debate for decades. In 2012, the World Health Organization (WHO) strongly recommended <2 g sodium intake or <5 g salt intake daily because it was beneficial for blood pressure as well as risks of stroke, cardiovascular, and coronary heart diseases. 1 The 2019 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for prevention of cardiovascular disease have also recommended minimizing consumption of salt along with other dietary interventions as measures preventing cardiovascular morbidity. 2
Numerous studies have argued the merits of low sodium diet and suggested association with cardiovascular disease outcomes. 3 , 4 , 5 , 6 , 7 , 8 , 9 A meta‐analysis concluded the existence of a J‐shaped association between sodium intake and cardiovascular mortality. 10 Reverse causation where at‐risk participants lower their salt intake, the confounding effects of physical activity and frailty on total calorie intake along with questioned quality of the data and methods used to estimate sodium intake were consistent explanations that were employed in the discussion of limitations of these studies. 3 , 6 , 7 A scientific statement from the American Heart Association pointed to an average of 3 to 4 methodological issues that may account for the inconsistent findings in observational studies related to sodium and CVD. 11 Although such associations might exist, there was lack of evidence to establish causality. 12 The issue of low sodium diet and poor cardiovascular health remained unanswered and recommendations necessitated the need for quality research to tackle this major public health issue. 13
On the other hand, low‐quality carbohydrates such as sugar‐sweetened beverages (SSB), sweets, and refined grains have been shown to affect cardiometabolic risk by adversely affecting body weight and diabetes mellitus risk. 14 , 15 The 2015‐2020 Dietary Guidelines for Americans recognized the harmful effects of sugar and recommended consumption of less than ten percent of calories per day from added sugar. 16
The purpose of this study was to provide a plausible explanation of the association between low sodium intake and poor cardiovascular health by analyzing the relationship of dietary intakes of sodium and other nutrients including sugar.
2. METHODOLOGY
2.1. Data source and study design
The data for our study were obtained from the continuous National Health and Nutrition Examination Survey (NHANES), 2001‐2016. The NHANES is a multistage probability cross‐sectional survey that represents general health and nutritional status of the noninstitutionalized civilian population of the United States. The data retrieved included responses to the in‐person interviews and examinations on multiple demographic variables, medical history, vital and body measurements and nutritional intake history. The nutritional intake history was based on 24‐hour dietary recall, measured by two instances.
The NHANES was designed and executed by the National Center for Health Statistics (NCHS). All participants included in the data provided written consent. Data utilized for the current study are publicly available. 17
2.2. Study sample
A total of 82 097 participants from eight available continuous NHANES cycles (2001‐2016) were included in our study. We did not use the 1999‐2000 NHANES cycle because there was no information on sugar intake. We included participants from age 18 to 75 years with at least one 24‐hour dietary recall instance that was considered reliable with complete information on sodium and sugar intake. The NHANES dietary intake data were considered unreliable if there were no data on total nutrient intakes or the total number of foods reported. A total of 38 722 participants met our study criteria as shown in Figure 1.
Figure 1.
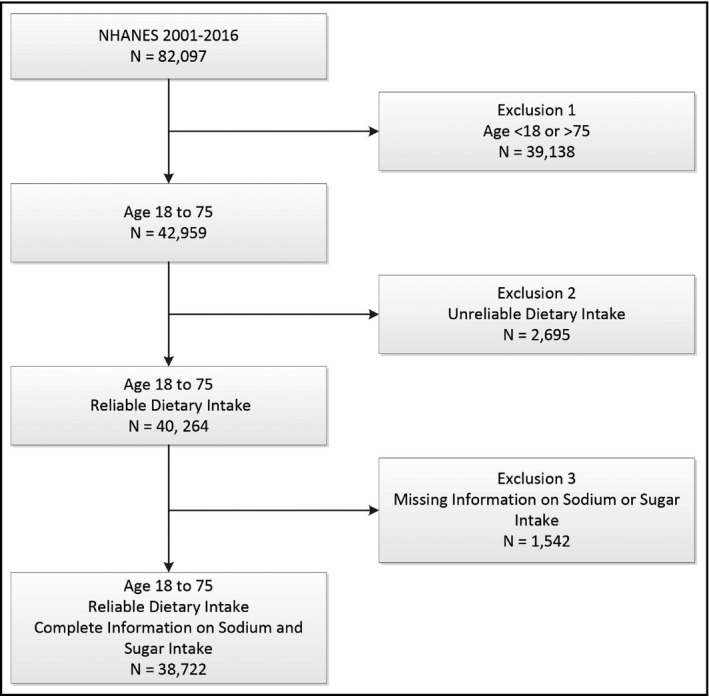
NHANES Sample Construction. All NHANES participants in cycle years 2001‐16 ages 18 to 75 with reliable dietary information and complete information on sodium and sugar intake were included in the final study sample
2.3. Twenty four‐hour dietary recall
Twenty four‐hour dietary recall interviews were administered by trained interviewers. Dietary data collected for the NHANES was based on the recall of the participants and was collected on both weekdays and weekends. Respondents were requested to recall their diet in the past 24 hours and to report all the food and beverages consumed (midnight to midnight). This information was collected with a computer‐assisted automated technique and utilized the U.S Department of Agriculture (USDA) automated multiple‐pass method to account for day to day variations. 18 There were two 24‐hour dietary recall instances for all NHANES cycles. The first instance was conducted by in‐person interview during a mobile physical examination session, and the second instance was conducted by phone interview executed 3‐10 days after the mobile examination session. Nutritional values of all dietary items and beverages were provided by the USDA's Food and Nutrient Database for Dietary Studies (FNDDS) which is regularly updated each cycle and supplies the nutrient profiles for every food and beverage reported in NHANES. 19
2.4. Demographics, body measurements, and other study variables
Age is reported as a continuous variable in years, and sex is reported dichotomously as either “male” or “female.” Race/ethnicity is reported categorically as “White,” “Black,” “Mexican Hispanics,” “Other Hispanics,” and “Others.” Season was divided into two values as either the first or second half of the year. Body mass index (BMI) in kg/m2 is reported as a continuous variable. Physical activity was recorded as either “moderate” or “vigorous” based on survey response to the level of activity over the past 30 days. Those not reporting “moderate” or “vigorous” activity were coded as “no moderate/vigorous.”
2.5. Medical history
Smoking was reported as a “yes” response to the question whether a participant smoked more than 100 cigarettes in a lifetime. We evaluated the following co‐morbid conditions as present if the respondent answered “yes” to the condition: hypertension, coronary artery disease, congestive heart failure, and angina. Diabetes was defined by self‐report of the condition or a glycosylated hemoglobin greater than or equal to 6.5%.
2.6. Dietary data
For all nutritional variables in NHANES, we used an average of the reported 24‐hour intake from the two 24‐hour dietary recall instances. We used the reported value when only one dietary instance occurred. We excluded participants where dietary information on sodium or sugar intake was absent. Observations were also excluded from analysis if the dietary history was unreliable by NHANES criteria (see Figure 1). Total sodium intake was recorded in milligrams and converted to grams. We classified sodium intake categorically into sodium quintiles (SQ) as follows: SQ1 (0.06‐2.6 g/d); SQ2 (2.6‐3.0 g/d); SQ3 (3.0‐3.4 g/d); SQ4 (3.4‐4.0 g/d); and SQ5 (4.0‐29.3 g/d). Carbohydrates, sugar, total protein, cholesterol, and fat were all recorded continuously in grams or milligrams as indicated. All nutritional variables were divided by reported total caloric intake and then multiplied by 2000 to standardize all nutritional variables to an arbitrarily defined 2000 calorie diet. Detailed information on NHANES data collection through 2016 is recorded by the Center for Disease Control and Prevention (CDC). 17 , 20
2.7. Statistical analysis
We performed all statistical analyses using the survey commands [svy] within Stata 16.0 to account for the complex sampling and survey design of the continuous NHANES. The data were analyzed according to the primary sampling units and strata and were weighted based on NHANES defined weights for the dietary intake data. Standard errors were estimated using the Taylor‐linearized variance estimation method. The NHANES weights from each cycle were combined into an eight‐cycle year weight as recommended by the NCHS.
We used simple descriptive statistics to report the overall characteristics of the defined NHANES participants. Categorical variables were compared across sodium groups using the design‐based probability. We performed categorical between group comparisons to the “average sodium intake group,” that is sodium quintile 3. Continuous variables were compared across sodium quintile groups using linear regression with sodium quintile 3 as the reference group. For these comparisons, a two‐tailed p value of less than 0.05 was accepted as statistically significant. We performed the same comparisons for the nutritional variables across sodium quintile groups, again using sodium quintile 3 as the reference group. We further explored the relationship between sugar and sodium intake using linear regression. Multiple linear regression was used to simultaneously adjust for age, sex, body mass index (BMI), physical activity, smoking, hypertension, diabetes, coronary artery disease, congestive heart failure, glycosylated hemoglobin, and season in an effort to attenuate reverse causation bias that can potentially affect both sodium and sugar intakes. We used boxplots with 95% confidence intervals to demonstrate the relationship between sugar and sodium intake.
Finally, we explored the relationship between sugar and sodium intake using liner regression while serially excluding participants with a history of cardiovascular disease (reported history of coronary artery disease, angina pectoris, myocardial infarction, congestive heart failure, and stroke), hypertension, and diabetes mellitus. In addition, after excluding all participants with a history of cardiovascular disease, we performed linear regression analysis on the relationship between dietary sugar and sodium intake in those with and without hypertension and in those with and without diabetes.
3. RESULTS
Among 38,722 NHANES participants that met our study criteria, the mean age was 43.6 years (SD 15.6 years) and sex was equally distributed (48.8 male vs 51.2% female). Overall BMI was an average of 28.7 kg/m2 (SD 6.8 kg/m2). Lifetime smoking greater than 100 cigarettes was reported in 46.4%, and reported prevalence of diabetes (13.0%) and hypertension (38.2%) was consistent with national prevalence rates. 21 , 22
Participant characteristics by level of sodium intake (quintiles) are presented in Table 1. Age was similar across sodium quintiles except sodium quintile 5 (SQ5) was significantly older compared to sodium quintile 3 (SQ3). Sex was similar across sodium intake groups. There were significant racial/ethnic differences by reported sodium intake when comparing reported race/ethnicity in sodium quintile groups 1 (SQ1) and 5 (SQ5) to SQ3. There was a notable decline in those reporting “black” and “Mexican Hispanic” race/ethnicity across sodium quintiles and a reciprocal increase in the proportion of those reporting “other” race/ethnicity. BMI (kg/m2) was lowest in SQ1 (27.9 kg/m2) increasing to the highest average BMI (29.3 kg/m2) in SQ5 (both P < .01 compared to SQ3). Physical activity was similar across sodium quintile groups. Reported smoking was higher in SQ1 compared to SQ3 (52.1 vs 45.1%; P < .01). The mean glycosylated hemoglobin increased across sodium quintiles. Hypertension was significantly lower in SQ2 (35.4%) and higher in SQ5 (41.5%) compared to SQ3 (38.7%; all P < .01). Diabetes trended up across sodium quintiles with 10.4% diabetes in SQ1 and 16.3% diabetes in SQ5, both significantly different from 13.0% diabetes in SQ3 (all P < .01). There were subtle differences across sodium groups for congestive heart failure, coronary artery disease, and angina with significant differences only for congestive heart failure.
Table 1.
Characteristics of NHANES participants by reported sodium intake
| Characteristic |
Sodium Quintile 1 (0.06‐2.6 g/d) No. (%) a (N = 7745) |
Sodium Quintile 2 (2.6‐3.0 g/d) No. (%) (N = 7744) |
Sodium Quintile 3 (3.0‐3.4 gm/day) No. (%) (N = 7745) |
Sodium Quintile 4 (3.4‐4.0 g/d) No. (%) (N = 7744) |
Sodium Quintile 5 (4.0‐29.3 gm/day) No. (%) (N = 7744) |
P value |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 42.9 (15.6) | 43.3 (15.6) | 43.6 (15.6) | 43.7 (15.5) | 44.5 (15.8) b | <.001 |
| Sex, male | 3858 (49.6) | 3748 (48.8) | 3697 (49.2) | 3739 (48.6) | 3723 (48.1) | .75 |
| Race | ||||||
| White | 3084 (65.0) b | 3186 (67.6) | 3251 (67.6) | 3387 (69.2) | 3312 (67.7) b | <.001 |
| Black | 1773 (13.0) b | 1850 (12.7) | 1790 (12.4) | 1721 (11.2) | 1523 (9.7) b | |
| Mexican Hispanic | 1126 (11.3) b | 1506 (9.2) | 1468 (8.8) | 1326 (8.3) | 1121 (7.0) b | |
| Other Hispanic | 615 (5.4) b | 720 (5.6) | 683 (5.3) | 668 (5.0) | 597 (4.5) b | |
| Other | 405 (5.2) b | 482 (4.9) | 553 (5.9) | 642 (6.3) | 1190 (11.2) b | |
| BMI (kg/m2), mean (SD) | 27.9 (6.3) b | 28.4 (6.7) b | 28.7 (6.7) | 29.0 (7.0) | 29.3 (7.2) b | <.001 |
| Physical Activity | ||||||
| Not moderate/vigorous | 3667 (41.1) | 3460 (39.1) | 3481 (40.2) | 3473 (38.6) | 3529 (39.4) | .38 |
| Moderate | 1915 (28.0) | 2065 (29.4) | 2098 (29.6) | 2090 (29.3) | 2040 (28.4) | |
| Vigorous | 2163 (30.9) | 2219 (31.5) | 2166 (30.2) | 2181 (32.1) | 2175 (32.2) | |
| Smoking >100 Cigs/Lifetime | 3457 (52.1) b | 3258 (46.0) | 3163 (45.1) | 3259 (45.5) | 3193 (43.9) | <.001 |
| Glycohemoglobin, mean (SD) | 5.45 (0.79) b | 5.49 (0.82) b | 5.53 (0.86) | 5.57 (0.93) b | 5.64 (1.04) b | <.001 |
| Hypertension | 3063 (37.2) | 2963 (35.4) b | 3106 (38.7) | 3166 (37.8) | 3379 (41.5) b | <.001 |
| Diabetes Mellitus | 1046 (10.4) b | 1049 (10.6) b | 1226 (13.0) | 13.9 (14.4) | 1680 (16.3) b | <.001 |
| Angina | 142 (1.9) | 130 (1.7) | 148 (1.8) | 167 (2.2) | 196 (2.4) | .35 |
| CHF | 166 (1.9) b | 144 (1.4) | 139 (1.6) | 184 (1.9) b | 228 (2.3) b | .005 |
| CAD | 197 (2.7) | 182 (2.3) | 201 (2.4) | 241 (3.0) | 267 (3.1) | .24 |
Abbreviations: BMI, body mass index; CAD, coronary artery disease.
Numbers represent N (percentage) unless otherwise indicated; CHF, congestive heart failure; SD, standard deviation.
Numbers may not sum to total N as they represent the actual observed N from the study while percentages were weighted taking into account the complex survey sampling.
P < .01 for individual between group comparisons with Sodium Quintile 3.
Dietary intake of major nutritional parameters by sodium intake is reported in Table 2. As can be seen in the table and in Figure 2, sugar intake went down across increasing sodium intake quintiles, and sugar intake was significantly higher in SQ1 (141.2 g/d) and SQ2 (118.6 g/d) and significantly lower in SQ4 (97.9 g/d) and SQ5 (85.6 g/d) compared to the average sodium intake of SQ3 (108.6 g/d; all P < .01). As with sugar intake, other nutritional parameters showed significant trends with sodium intake. Carbohydrate intake mirrored sugar intake across sodium quintiles, while protein and cholesterol intake increased across sodium intake quintiles. Fat intake increased across sodium intake up to SQ4 and leveled off with a similar intake in SQ5 (77.3 g/d) compared to SQ3 (76.5 g/d).
Table 2.
Nutritional parameters of NHANES participants by quintile of reported sodium intake
| Nutrients |
Sodium Quintile 1 (0.06‐2.6 g/d) (N = 7745) |
Sodium Quintile 2 (2.6‐3.0 g/d) (N = 7744) |
Sodium Quintile 3 (3.0‐3.4 g/d) (N = 7745) |
Sodium Quintile 4 (3.4‐4.0 g/d) (N = 7744) |
Sodium Quintile 5 (4.0‐29.3 g/d) (N = 7744) |
P value |
|---|---|---|---|---|---|---|
| Sugar (g/d) | 141.2 (60.9) a | 118.6 (43.4) a | 108.6 (39.8) | 97.9 (38.6) a | 85.6 (37.6) a | <.001 |
| Carbohydrate (g/d) | 262.6 (58.7) a | 250.0 (46.6) a | 242.7 (45.7) | 236.0 (46.0) a | 229.3 (49.8) a | <.001 |
| Protein (g/d) | 67.6 (21.9) a | 73.2 (18.0) a | 78.5 (19.3) | 83.2 (19.9) a | 93.3 (24.8) a | <.001 |
| Fat (g/d) | 68.9 (19.6) a | 74.8 (16.3) a | 76.5 (15.8) | 77.9 (16.9) a | 77.3 (18.4) | <.001 |
| Cholesterol (mg/d) | 219.7 (142.7) a | 248.6 (142.0) a | 276.6 (158.1) | 294.2 (165.6) a | 325.2 (192.2) a | <.001 |
Numbers in table represent the mean (standard deviation).
P < .01 for comparison to sodium quintile 3.
Figure 2.
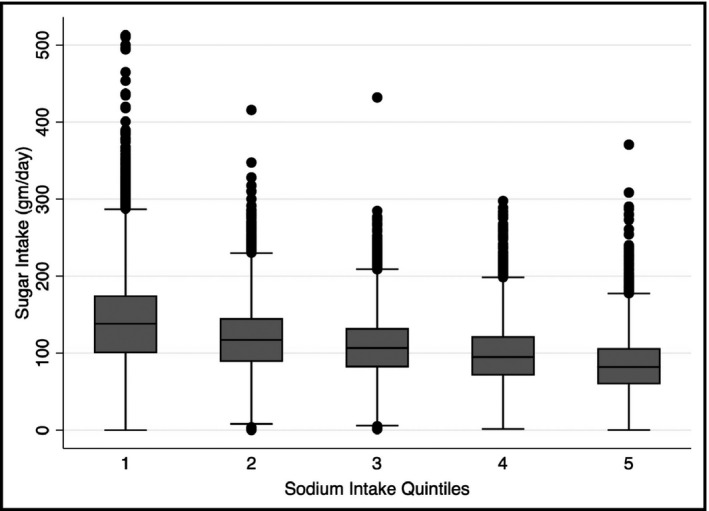
Boxplot showing the association between quantiles of sodium intake and reported 24‐h sugar intake. Sugar intake was significantly higher in SQ1 (141.2 g/d) and SQ2 (118.6 g/d) and significantly lower in SQ4 (97.9 g/d) and SQ5 (85.6 g/d) compared to the average sodium intake of SQ3 (108.6 g/d; all P < .01)
To further examine the relationship between reported dietary sugar intake and sodium intake,
we performed both simple and multivariate linear regression using the average sodium intake quintile, SQ3, as the reference group (Table 3). Unadjusted sugar intake was 32.6 gm/day (95% CI: 29.4 to 32.3 g/d) higher in SQ1 and 10.0 gm/day (95% CI: 9.7 to 12.5 g/d) higher in SQ2 compared to SQ3. Unadjusted sugar intake was 10.7 g/d (95% CI: 9.8 to 12.6 g/d) lower in SQ4 and 23.0 g/d (22.9 to 25.7) lower in SQ5 compared to SQ3 (all P < .001). The same trends continued in the multivariate model adjusted for age, sex, BMI, smoking, physical activity, co‐morbid disease, glycosylated hemoglobin, and season.
Table 3.
Unadjusted and adjusted mean sugar intake (g/d) by sodium intake quintile
| Sodium Group | Unadjusted Coefficient (95% CI) | P value | Adjusted Coefficient* (95% CI) | P value |
|---|---|---|---|---|
|
Quintile 1 (0.06‐2.6 g/d) (N = 7745) |
32.6 (30.1 to 35.0) | <.001 | 30.9 (28.4 to 33.4) | <.001 |
|
Quintile 2 (2.6‐3.00 g/d) (N = 7744) |
10.0 (8.1 to 11.9) | <.001 | 9.3 (7.3 to 11.3) | <.001 |
|
Quintile 3 (3.0‐3.4 g/d) (N = 7745) |
0 (Ref) | N/A | 0 (Ref) | N/A |
|
Quintile 4 (3.4‐4.0 g/d) (N = 7744) |
−10.7 (−9.0 to −12.4) | <.001 | −10.7 (−8.9 to −12.5) | <.001 |
|
Quintile 5 (4.0‐29.3 g/d) (N = 7744) |
−23.0 (−21.5 to −24.6) | <.001 | −22.1 (−20.4 to −23.8) | <.001 |
Adjusted for age, sex, body mass index, physical activity, smoking, diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, glycosylated hemoglobin, and season.
To further assess the robustness of the relationship between dietary sugar and sodium intake, we explored the relationship using simple linear regression while serially excluding participants with a history of cardiovascular disease, hypertension, and diabetes. Also, we performed additional subgroup analyses by first excluding those with cardiovascular disease and then assessing the relationship between dietary sugar and sodium intake in those with and without hypertension, and then repeated the analyses for those with and without diabetes. In all subsidiary analyses, the relationship between dietary sugar and sodium intake yielded similar results as our main analysis with a significant inverse relationship between the two nutrients (please see Tables A1, A2, A3 in Appendix A).
4. DISCUSSION
In this study of 38 722 adults from the United States, we aimed to characterize diet in reference to different levels of sodium intake in an effort to explore potential modifiers of interest to the reported relationship between low sodium intake and poor cardiovascular disease outcomes. Remarkably, we found a significant inverse association of the daily intake of sodium with estimated daily intake of sugar adjusted to a total daily intake of 2000 calories; this was most prominent at the lowest quintile of sodium intake. This association maintained strong significance after adjusting for cardiometabolic morbidities, BMI, glycosylated hemoglobin, season, and year of the survey. To the best of our knowledge, this is the first study to report the existence of an interplay between sodium intake and sugar consumption.
The controversy of dietary sodium and its association with cardiovascular disease outcomes started in late 1980s. A large epidemiology study, INTERSALT, reported that low salt diet decreases the risk for cardiovascular morbidity and mortality. 7 Since the introduction of this concept, numerous cohort studies and randomized clinical trials have attempted to delineate this association but rendered conflicting findings. 23 Proponents of the beneficial effects of low sodium diet proclaim that dietary sodium intake has a direct linear association with cardiovascular events. 5 , 24 , 25 , 26 On the contrary, there is a growing body of evidence suggesting that there is a J‐shaped association of cardiovascular events with dietary intake of sodium. 4 , 6 , 23 , 27 However, the methodology of these studies has been questioned and hence their provocative results were not sufficient to call for changes in dietary sodium intake as an isolated public health recommendation. 13
The hypothesis of the J‐shaped association between sodium intake and cardiovascular outcomes raises formidable issues and altogether with conflicting findings from large‐scale studies have resulted in important questions raised concerning the dietary recommendations given by physicians. Currently, general recommendations decree that sodium consumption should be restricted to less than 2.0 g/d for all individuals and to less than 1.5 g/d for males over 50 years of age, blacks, and hypertensive individuals. 2 , 28 Recommendations of low sodium diet for patients with hypertension are accepted 1 , 2 , 24 , 25 but application of low sodium diet to the general population as a public health measure is controversial. 13 Interestingly, Stolarz‐Skrzypek et al 8 reported increased morbidity and mortality with low sodium intake regardless of concomitant improvement in blood pressure control. Similarly, follow‐up of large‐scale observational studies which included a 70% proportion of hypertensive individuals have also yielded results which endorsed the J‐shaped association between sodium intake and adverse cardiac outcomes. 29
Cardiovascular health is affected by multiple factors associated with dietary habits including but not limited to sodium and sugar intake. The relationship between dietary factors and cardiovascular disease should not be underestimated at a time when the leading global cause of death in Western Countries is cardiovascular disease. 30 , 31 A large meta‐analysis revealed that a reduction in dietary sodium lowered blood pressure while adversely affected renin, angiotensin, catecholamine, cholesterol, and triglycerides serum levels. 3 It remains to be seen if the effects of these physiologic alterations can be offset by the benefits of blood pressure reduction on cardiovascular health. Of interest to this observation is that high sugar (fructose) diet is also associated with the exact same physiologic alterations. 3 , 32 High intake of dietary sugar in the setting of a pandemic of worldwide obesity and cardiovascular disease has heightened the concerns about the adverse effects of high sugar consumption. Sugar intake has been linked to obesity, insulin resistance, and adverse cardiovascular outcomes. 33 , 34 , 35 Excessive sugar consumption, particularly fructose, has been associated with the epidemic of coronary artery disease worldwide. 5 , 36 , 37 A scientific statement from the American Heart Association pointed out strong evidence supporting the association between added sugars and increased cardiovascular disease risk in children through increased energy intake, increased adiposity, and dyslipidemia. 38 In one study, high carbohydrate diet was associated with higher risk of total mortality while another study reported the presence of a J‐shaped curve between the intake of carbohydrates and total mortality. 39 , 40 In both reports, the species of carbohydrates and the relation between carbohydrates and sodium intake were not investigated. Based on known cardiometabolic effects of different species of carbohydrates, it is important to note that the species of ingested carbohydrates may be more important than their quantity. Furthermore, different species of fats (saturated vs unsaturated) and proteins (animal vs plant) can have opposing health and CV effects therefore a conclusion that these nutrients can explain the J‐shaped association could not be drawn from our results although a statistically significant trends were found between these nutrients and estimated sodium intake. 41 , 42
Our results have specific limitations. First and similar to all cross‐sectional observational studies, our findings do not allow us to establish causality. In our study, we aimed to characterize dietary habits; it was not an outcome study. The second limitation of our work was the assessment of dietary intake of nutrients. The 24‐hour recall method has inherent inaccuracies and may not reflect actual intakes thus can be subjected to recall bias with the potential for under and over‐reporting. Perhaps the major limitation in the present study is the failure to examine different types of dietary sugars. It is believed that the slowly released natural sugar from moderately sweet fruits is less harmful than the sugar from rapidly consumed sweetened beverages. 43 Moreover, physical activity during sugar consumption has a major impact on its metabolic fate. Low physical activity during a period of high fructose intake augments fructose‐induced postprandial lipidemia and inflammation while high physical activity minimizes these fructose‐induced metabolic disturbances. 44 Although we adjusted our results for levels of physical activity, controlling for physical activity during sugar consumption in a real‐time manner was not possible in our study.
From nationally representative US surveys conducted between 2001 and 2016, our results demonstrate that individuals consuming a low sodium diet are more likely to consume high sugar, a nutrient with an established link to poor cardiovascular outcomes. This finding offers a potential explanation to the J‐shaped association between sodium intake and cardiovascular outcomes and calls for carefully designed studies that analyze dietary characteristics and the interplay between all dietary factors including micronutrients of interest to reach a better understanding regarding the relationship between cardiovascular disease outcomes and these factors. This comes at a time when it is clear that dietary habits can be modified by populations strengthening the importance of such studies along with impact of public awareness and policy changes to help the fight against cardiovascular disease. 45
CONFLICT OF INTEREST
The authors declare they have no competing interest with the current work.
AUTHOR CONTRIBUTIONS
Designed research: (Project conception ZJK), (development of overall research plan TWG, JIS, ZJK); Data collection: KM; Performed statistical analysis: TWG; Analyzed data: YMR, RAK, JIS, ZJK; Wrote paper: KM, RFT, AHT, NGA, ZJK; Primarily responsible for final content of the manuscript: ZJK. All authors participated in drafting the work, approved the final version of the manuscript, and agreed to be accountable for aspects of the work.
Appendix A.
Table A1.
Regression coefficients for dietary sugar and sodium intake quintiles with serial exclusion of all cardiovascular disease (CVD), hypertension, and diabetes
| Sodium Group | Entire Cohort (N = 38 722) | Excluding all CVD (N = 35 731) | Excluding Hypertension (N = 22 417) | Excluding Diabetes (N = 20 378) |
|---|---|---|---|---|
| Quintile 1 (0.06‐2.6 g/d) | 32.6 (30.2 to 35.0) | 32.3 (29.8 to 34.8) | 33.3 (30.2 to 36.4) | 32.3 (29.1 to 35.6) |
| Quintile 2 (2.6‐3.0 g/d) | 10.0 (8.1 to 11.9) | 10.1 (8.1 to 12.2) | 9.1 (6.8 to 11.4) | 8.7 (6.2 to 11.1) |
| Quintile 3 (3.0‐3.4 g/d) | (Ref) | (Ref) | (Ref) | (Ref) |
| Quintile 4 (3.4‐4.0 g/d) | −10.7 (−12.4 to −9.0) | −10.5 (−12.3 to −8.7) | −10.8 (−13.0 to −8.6) | −10.6 (−12.9 to −8.4) |
| Quintile 5 (4.0‐29.3 g/d) | −23.0 (−24.6 to −21.4) | −23.2 (−25.0 to −21.5) | −22.4 (−24.4 to −20.4) | −22.2 (−24.4 to −20.0) |
All P values are <.001 for comparison of each sodium quintile to sodium quintile 3 (Reference Group).
Numbers represent the regression coefficient with the 95% confidence interval in parentheses.
Table A2.
Regression coefficients for dietary sugar intake and sodium intake quintiles in those with and without hypertension after excluding all with a history of cardiovascular disease
| Sodium Group | Hypertension/No CVD (N = 13 314) | No Hypertension/No CVD (N = 22 417) |
|---|---|---|
| Quintile 1 (0.06‐2.6 g/d) | 30.2 (26.0 to 34.4) | 33.3 (30.2 to 36.4) |
| Quintile 2 (2.6‐3.0 g/d) | 11.9 (8.4 to 15.5) | 9.1 (6.8 to 11.4) |
| Quintile 3 (3.0‐3.4 g/d) | (Ref) | (Ref) |
| Quintile 4 (3.4‐4.0 g/d) | −10.1 (−13.5 to −6.8) | −10.8 (−13.0 to −8.6) |
| Quintile 5 (4.0‐29.3 g/d) | −24.4 (−27.7 to −21.1) | −22.4 (−24.4 to −20.4) |
All P values are <.001 for comparison of each sodium quintile to sodium quintile 3 (Reference Group).
Numbers represent the regression coefficient with the 95% confidence interval in parentheses.
Table A3.
Regression coefficients for dietary sugar intake and sodium intake quintiles in those with and without diabetes after excluding all with a history of cardiovascular disease
| Sodium Group | Diabetes/No CVD (N = 5177) | No Diabetes/No CVD (N = 30 554) |
|---|---|---|
| Quintile 1 (0.06‐2.6 g/d) | 39.3 (31.6 to 46.9) | 31.4 (28.5 to 34.2) |
| Quintile 2 (2.6‐3.0 g/d) | 13.5 (8.7 to 18.3) | 9.6 (7.4 to 11.8) |
| Quintile 3 (3.0‐3.4 g/d) | (Ref) | (Ref) |
| Quintile 4 (3.4‐4.0 g/d) | −11.8 (−16.1 to −7.5) | −10.3 (−12.4 to −8.2) |
| Quintile 5 (4.0‐29.3 g/d) | −22.4 (−27.2 to −17.6) | −−23.2 (−25.1 to −21.2) |
All P values are <.001 for comparison of each sodium quintile to sodium quintile 3 (Reference Group).
Numbers represent the regression coefficient with the 95% confidence interval in parentheses.
Gress TW, Mansoor K, Rayyan YM, et al. Relationship between dietary sodium and sugar intake: A cross‐sectional study of the National Health and Nutrition Examination Survey 2001‐2016. J Clin Hypertens. 2020;22:1694–1702. 10.1111/jch.13985
REFERENCES
- 1. World Health Organization . Guideline: sodium intake for adults and children. World Health Organization; 2012. https://www.who.int/nutrition/publications/guidelines/sodium_intake/en/ (accessed 11 November 2019). [Google Scholar]
- 2. Arnett DK, Blumenthal RS, Albert MA et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563–e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graudal NA, Hubeck‐Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mente A, O'Donnell M, Rangarajan S et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388(10043):465–475. [DOI] [PubMed] [Google Scholar]
- 5. Micha R, Penalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. O'Donnell M, Mente A, Rangarajan S et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med. 2014;371(7):612–623. [DOI] [PubMed] [Google Scholar]
- 7. Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14(5):570–577. [DOI] [PubMed] [Google Scholar]
- 8. Stolarz‐Skrzypek K, Kuznetsova T, Thijs L et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305(17):1777–1785. [DOI] [PubMed] [Google Scholar]
- 9. Thomas MC, Moran J, Forsblom C et al. The association between dietary sodium intake, ESRD, and all‐cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34(4):861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low‐ and excessive‐sodium diets are associated with increased mortality: a meta‐analysis. Am J Hypertens. 2014;27(9):1129–1137. [DOI] [PubMed] [Google Scholar]
- 11. Cobb LK, Anderson CA, Elliott P et al. Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129(10):1173–1186. [DOI] [PubMed] [Google Scholar]
- 12. Webster J, Waqanivalu T, Arcand J et al. Understanding the science that supports population‐wide salt reduction programs. J Clin Hypertens (Greenwich). 2017;19(6):569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oparil S. Low sodium intake–cardiovascular health benefit or risk? N Engl J Med. 2014;371(7):677–679. [DOI] [PubMed] [Google Scholar]
- 14. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long‐term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith JD, Hou T, Ludwig DS et al. Changes in intake of protein foods, carbohydrate amount and quality, and long‐term weight change: results from 3 prospective cohorts. Am J Clin Nutr. 2015;101(6):1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. 2015–2020 Dietary Guidelines for Americans https://health.gov/dietaryguidelines/2015/ (accessed 13 November 2019).
- 17. NHANES survey methods and analytic guidelines. 2019. https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx. (accessed 14 November 2019).
- 18. Zipf GCM, Porter KS et al. National health and nutrition examination survey: plan and operations, 1999–2010. National Center for Health Statistics. Vital Health Stat. 2013;1(56):1‐37. [PubMed] [Google Scholar]
- 19. USDA . USDA Agricultural Research Service https://www.ars.usda.gov/northeast‐area/beltsville‐md‐bhnrc/beltsville‐human‐nutrition‐research‐center/food‐surveys‐research‐group/docs/wweianhanes‐overview/ (accessed 16 November 2019).
- 20. Ahluwalia N, Dwyer J, Terry A, Moshfegh A, Johnson C. Update on NHANES dietary data: focus on collection, release, analytical considerations, and uses to inform public policy12. Adv Nutr. 2016;7(1):121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention . National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention; Department of Health and Human Services; 2017. https://www.cdc.gov/diabetes/data/statistics/statistics‐report.html. Nucleic acids research, 35: D521‐526, 2007 (accessed 1 October 2019). [Google Scholar]
- 22. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon‐Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017;(289):1‐8. Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- 23. Alderman MH, Cohen HW. Dietary sodium intake and cardiovascular mortality: controversy resolved? Am J Hypertens. 2012;25(7):727–734. [DOI] [PubMed] [Google Scholar]
- 24. Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129(9):981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cook NR, Appel LJ, Whelton PK. Sodium intake and all‐cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol. 2016;68(15):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Donnell M, Mente A, Rangarajan S et al Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: prospective cohort study. BMJ. 2019;364:l772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow‐up Study. Arch Intern Med. 2002;162(14):1619–1624. [DOI] [PubMed] [Google Scholar]
- 29. O'Donnell MJ, Yusuf S, Mente A et al Urinary sodium and potassium excretion and risk of cardiovascular events. JAMA. 2011;306(20):2229–2238. [DOI] [PubMed] [Google Scholar]
- 30. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anand SS, Hawkes C, de Souza RJ et al Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol. 2015;66(14):1590–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DiNicolantonio JJ, Lucan SC, O'Keefe JH. The evidence for saturated fat and for sugar related to coronary heart disease. Prog Cardiovasc Dis. 2016;58(5):464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson RK, Appel LJ, Brands M et al Dietary sugars intake and cardiovascular health. Circulation. 2009;120(11):1011–1020. [DOI] [PubMed] [Google Scholar]
- 34. Malik VS, Popkin BM, Bray GA, Despres JP, Hu FB. Sugar‐sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mirtschink P, Jang C, Arany Z, Krek W. Fructose metabolism, cardiometabolic risk, and the epidemic of coronary artery disease. Eur Heart J. 2018;39(26):2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D. Estimated global, regional, and national disease burdens related to sugar‐sweetened beverage consumption in 2010. Circulation. 2015;132(8):639–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vos MB, Kaar JL, Welsh JA et al Added sugars and cardiovascular disease risk in children: a scientific statement from the american heart association. Circulation. 2017;135(19):e1017–e1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dehghan M, Mente A, Zhang X et al Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050–2062. [DOI] [PubMed] [Google Scholar]
- 40. Seidelmann SB, Claggett B, Cheng S et al Dietary carbohydrate intake and mortality: a prospective cohort study and meta‐analysis. Lancet Public Health. 2018;3(9):e419–e428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sacks FM, Lichtenstein AH, Wu JHY et al Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1–e23. [DOI] [PubMed] [Google Scholar]
- 42. Song M, Fung TT, Hu FB et al Association of animal and plant protein intake with all‐cause and cause‐specific mortality. JAMA Intern Med. 2016;176(10):1453–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choo VL, Viguiliouk E, Blanco Mejia S et al Food sources of fructose‐containing sugars and glycaemic control: systematic review and meta‐analysis of controlled intervention studies. BMJ. 2018;363:k4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bidwell AJ, Fairchild TJ, Redmond J, Wang L, Keslacy S, Kanaley JA. Physical activity offsets the negative effects of a high‐fructose diet. Med Sci Sports Exerc. 2014;46(11):2091–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999–2012. JAMA. 2016;315(23):2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]


