Abstract
Previous studies describing renal denervation (RDN) from the intima of the renal artery for the treatment of resistant hypertension have reported variable efficacies, and RDN triggers renal intimal injury and atherosclerosis. This study aimed to evaluate the efficacy and safety of RDN from the adventitia of renal artery plus unilateral laparoscopic adrenalectomy to treat patients with resistant hypertension caused by unilateral aldosterone‐producing adenoma (APA). A total of 60 consecutive patients with resistant hypertension caused by unilateral APA were enrolled in this study. Patients were randomly assigned to undergo RDN from the adventitia of the renal artery plus adrenalectomy (RDN group, n = 30) or adrenalectomy alone (control group, n = 30) and were followed up for 12 months. The primary efficacy end point was the change in 24‐hours mean ambulatory systolic blood pressure (SBP) from baseline to 12 months. At the 12‐month follow‐up, the mean reduction of 24‐hours average SBP and office SBP in the RDN group was 20.7 ± 15.2 and 37.1 ± 26.0 mm Hg, respectively, which was significantly higher than the mean reduction of 24‐hours average SBP (11.9 ± 11.1 mm Hg, P = .017) and the office SBP (25.9 ± 16.8 mm Hg, P = .035) in the control group. Serum potassium levels returned to normal 12 months post‐procedure. Patients in the RDN group had higher proportion of cured clinical and biochemical outcomes than those in the control group (35.7% vs 17.9% in clinical outcome; 96.4% vs 89.3% in biochemical outcome, respectively). There were no procedural‐, device‐, or treatment‐related safety events during the 12‐month follow‐up period between the groups. In conclusion, RDN from the adventitia of the renal artery plus unilateral laparoscopic adrenalectomy is more effective than adrenalectomy alone for treating resistant hypertension caused by unilateral APA.
Keywords: aldosterone‐producing adenoma, renal denervation, resistant hypertension
1. INTRODUCTION
Hypertension has a growing impact and currently affects over 1 billion individuals worldwide. 1 It is estimated that 12%‐18% of hypertensive patients have resistant hypertension, 2 defined as blood pressure (BP) that does not remain within the normal range despite the administration of three antihypertensive medications at maximally tolerated doses, including a diuretic. 3 Among patients with resistant hypertension, primary aldosteronism (PA) is considered the most common identifiable cause, which accounts for approximately 20% of this population. 4 Aldosterone‐producing adenoma (APA) is a benign functional adrenal tumor that oversecretes aldosterone resulting in PA. Laparoscopic adrenalectomy is currently recommended as the standard treatment of APA 5 , 6 ; however, the clinical outcome of adrenalectomy for APA is not satisfactory. While most adrenalectomy‐treated patients exhibit some extent of decrease in BP and hypokalemia, remission of high BP has been reported in 20%‐72% of patients. 7 , 8 In this context, renal denervation (RDN) has emerged as a treatment option for resistant hypertension. 9 By denervating the renal arteries, general sympathetic tone is reduced by decreased norepinephrine spillover and muscle sympathetic nerve activity. 10 , 11 Clinical studies have documented that catheter‐based RDN leads to clinically meaningful reduction in SBP and diastolic BP (DBP) in patients with resistant hypertension. However, when catheter‐based RDN was used, it could damage the intima of renal arteries and induce progression of underlying atherosclerotic lesions. 12 , 13
A case study reported by our team showed that laparoscopic‐based RDN from the adventitia of renal artery could effectively treat resistant hypertension. 14 In that report, our team for the first time used laparoscopic RDN from the adventitia of renal artery for resistant hypertension. The tissue surrounding the renal artery was stripped, and radiofrequency (RF) ablation was applied to the renal artery to destroy residuary renal nerve bundles distributed deeper in the arterial wall in order to increase the effects of ablation. Based on this first clinical report, we conducted animal experiments relative to RDN on the adventitia of renal artery in pigs and dogs. Our results showed that the adventitia‐RDN and the intima‐RDN were equally safe and the adventitia‐RDN could inhibit the activity of the sympathetic nervous system, with a significant reduction in the levels of norepinephrine and tyrosine hydroxylase activity in renal tissue. 15 Therefore, we chose to apply RDN from the adventitia of the renal artery during unilateral laparoscopic adrenalectomy to treat resistant hypertension complicated by unilateral APA. The aim of the present study was to compare the clinical outcomes of laparoscopic‐based RDN plus adrenalectomy with adrenalectomy alone for the treatment of patients with resistant hypertension caused by unilateral APA.
2. METHODS
2.1. Study design and participants
This was a single‐center, single‐blinded, randomized controlled trial. Patients with resistant hypertension were screened, and the diagnosis of APA was confirmed at the Henan Provincial People's Hospital (Zhengzhou, China) between December 2016 and March 2018. All patients were willing and able to comply with the protocol and provided written informed consent. The study was approved by the local medical ethical committee of the Henan Provincial People's Hospital (Zhengzhou, China) and was registered at clinicaltrial.gov (NCT02642445).
Patients with a history of renal artery stenosis of >50%, renal artery aneurysm, prior renal artery intervention, multiple renal arteries, a renal artery of <4 mm in diameter, or a treatable segment of <20 mm in length were excluded from the trial. All patients underwent renal computed tomography (CT) angiography or magnetic resonance (MR) angiography before randomization to evaluate the feasibility of the renal artery. A total of 60 consecutive patients diagnosed with resistant hypertension caused by APA according to the above criteria were randomized 1:1 to receive RDN plus adrenalectomy (RDN group, n = 30) or adrenalectomy only (control group, n = 30).
2.2. Diagnosis of PA and unilateral APA
Diagnosis of PA was based on “The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline”. 16 Firstly, ARR screening was conducted in all patients, and those with positive ARR were selected to be confirmed by saline load test or captopril inhibition test. Then, adrenal computed tomography (CT) was selected, if patients with contraindications to CT were selected by MR imaging (MRI). The diagnosis of APA was established when a unilateral adrenal adenoma and a normal contra lateral adrenal gland could be detected unequivocally on CT or MRI in patients with confirmed PA, and adrenal venous sampling (AVS) was performed. 17 , 18
CSI was defined as the ratio of aldosterone/cortisol (ALD/C) of non‐dominant adrenal vein to ALD/C in peripheral vein. In our study, the ratio of dominant and non‐dominant side blood ALD/C was ≥3, suggesting high unilateral aldosterone secretion, combined with imaging examination of unilateral APA; meanwhile, CSI < 1 also confirms the diagnosis of unilateral APA.
2.3. Surgical procedures
All procedures were performed by professional members of our team, composed of cardiologists, urologists, hypertensive physicians, and professional nurses. Patients were placed on the affected side, in the lateral position after anesthesia. A 10‐mm trocar was inserted approximately 2‐3 cm beneath the costal spinal angle, and two 5‐mm trocars were placed below the 11th rib, approximately 3 cm laterally, followed by CO2 insufflation. The laparoscope was introduced through the 10‐mm trocar, and 2 graspers were introduced through the 5‐mm trocars. After excision of the adenoma, the homolateral renal artery on the same side was carefully separated and exposed. RDN was performed using an electrode (Johnson & Johnson) and a temperature‐controlled cardiac radiofrequency catheter (NS7TCDL174HS, Biosense Webster). RF ablations of 8 W were applied for 120 seconds to obtain up to 4 to 6 discreated ablations 8 separated both longitudinally and rotationally along the renal artery from the adventitia (temperature ≤ 45°C).
2.4. Primary end points and postoperative follow‐up
Patients were evaluated during clinical visits at baseline, and 6 and 12 months after the surgical procedures. The 24‐hours ambulatory BP, office BP, antihypertensive medications, plasma aldosterone concentration (PAC), plasma renin activity (PRA), and other laboratory assessments were performed at each visit. The primary efficacy end point was the change in 24‐hours average ambulatory SBP from baseline to 12 months after the procedure. The secondary efficacy end points were changes in 24‐hours average ambulatory DBP, office SBP, and DBP; antihypertensive medications; and PAC, PRA, and serum potassium concentrations. Additional specified secondary efficacy end points included the proportion of patients with clinically and biochemically successful outcomes. The primary safety end points included changes in kidney function (estimated glomerular filtration rate, eGFR), and a composite of major adverse events, defined as death from all causes, end‐stage renal disease, renal artery or other vascular complications, or new renal artery stenosis of >70% within 12 months.
2.5. BP measurement
BP was determined by trained nurses using a validated semiautomatic manometer (Omron 705CP, Omron Healthcare). Three measurements of BP were obtained in the sitting position, with a 5‐minutes rest period between measurements. Ambulatory blood pressure monitoring (ABPM) was measured using an automatic dynamic BP monitor (SunTech Oscar Type 2), adapted to the patient's arm circumference. BP recordings were registered every 15 minutes during daytime (7:00 am‐10:00 pm) and every 30 minutes during nighttime (10:00 pm‐7:00 am). 19
2.6. Clinical and biochemical outcomes
Cured clinical outcome was defined as normal blood pressure without any antihypertensive medication. Improved clinical outcome was defined as same blood pressure as before surgery with less antihypertensive medication or a reduction in blood pressure with either the same amount or less antihypertensive medication. Failed clinical outcome was defined as unchanged or increased blood pressure with either the same amount or an increase in antihypertensive medication. Cured and improved clinical outcomes effective were considered to be effective clinical outcomes.
Cured biochemical outcome was defined as correction of hypokalemia and normalization of the raised aldosterone‐to‐renin ratio (ARR). Improved biochemical outcome was defined as correction of hypokalemia and an ARR with one or both of the following ≥50% decrease in baseline plasma aldosterone concentration. Failed biochemical outcome was defined as persistent hypokalemia or persistent raised ARR. Cured and improved biochemical outcomes were considered to be effective biochemical outcomes.
2.7. Statistical analysis
Statistical analysis was performed using IBM SPSS statistics version 22.0 (IBM Inc, Armonk NY, USA). All variables were tested for normality of distribution. For the comparison of normally distributed variables, a paired t test was used and continuous variables were expressed as mean ± standard deviation (SD). For non‐normally distributed variables, the Mann‐Whitney U test was used and continuous variables were expressed as median (interquartile range, IQR). Some results were analyzed by repeated measures of variance. All categorical variables were reported as percentages, and categorical data were analyzed using Pearson chi‐square test. P‐values < .05 were considered statistically significant.
3. RESULTS
3.1. Baseline characteristics
Between December 2016 and March 2018, 306 patients with a history of hypertension were enrolled into the trial. Overall, 60 patients met both resistant hypertension and unilateral APA criteria and were assigned 1:1 to two groups to undergo RDN from the adventitia of renal artery plus unilateral laparoscopic adrenalectomy (RDN group, n = 30) or unilateral laparoscopic adrenalectomy alone (control group, n = 30). Two patients missed visit in the RDN group and 1 patient missed visit, 1 patient withdrew consent in the control group at the 6‐month post‐procedure follow‐up, and no patient was lost at the 12‐month post‐procedure follow‐up.
Baseline characteristics for the study population are shown in Table 1. Patients were similar in mean age, body mass index (BMI), PAC, PRA, and other laboratory biomarkers between groups. The mean baseline office and 24‐hours BP were similar between groups (Table 1), and the distribution of baseline antihypertensive medications used in the two groups was similar (for all, P > .05; Table 2).
Table 1.
Baseline characteristics of the patients
| Characteristics | RDN group (n = 30) | Control group (n = 30) | P |
|---|---|---|---|
| Age (years) | 50.0 ± 10.9 | 50.3 ± 9.7 | .928 |
| Sex (male) | 11 (36.7%) | 13 (43.3%) | .792 |
| Body mass index (kg/m2) | 26.0 ± 3.3 | 26.3 ± 3.3 | .710 |
| Current smokers (%) | 9 (30.0%) | 6 (20.0%) | .371 |
| Type 2 diabetes mellitus (%) | 6 (20.0%) | 4 (13.3%) | .488 |
| Family history of hypertension (%) | 14 (46.7%) | 8 (26.7%) | .108 |
| Number of years with hypertension (years) | 9.4 (6.6‐12.2) | 8.8 (5.9‐11.7) | .592 |
| PAC (pg/mL) | 434.5 (227.9‐680.3) | 310.0 (176.0‐538.0) | .241 |
| PRA (ng/mL)/h | 0.20 (0.20‐0.55) | 0.20 (0.20‐0.30) | .150 |
| ARR | 185.8 (62.3‐329.4) | 103.0 (54.2‐257.5) | .372 |
| Serum potassium (mmol/L) | 3.44 ± 0.71 | 3.43 ± 0.70 | .939 |
| eGFR (mL/min/1.73 m2) | 88.2 ± 11.4 | 84.3 ± 14.9 | .939 |
| 24‐h SBP (mmHg) | 147.9 ± 15.1 | 146.0 ± 5.5 | .552 |
| 24‐h DBP (mmHg) | 91.9 ± 10.1 | 90.8 ± 7.3 | .643 |
| Office SBP (mmHg) | 170.0 ± 25.4 | 168.0 ± 13.0 | .717 |
| Office DBP (mmHg) | 99.6 ± 14.2 | 98.4 ± 5.7 | .676 |
Data are expressed as N (%) or mean ± SD, or median (IQR). Statistically significant differences between the RDN and control group are defined as those with P‐value < .05.
Abbreviations: ARR, aldosterone‐to‐renin ratio; eGFR, estimated glomerular filtration rate, calculated using the Cockcroft‐Gault formula.
Table 2.
Antihypertensive medication at baseline of the two groups
| Antihypertensive medications | RDN group (n = 30) | Control group (n = 30) | P |
|---|---|---|---|
| ACE inhibitor | 10 (33.3) | 11 (36.7) | .783 |
| Angiotensin receptor blocker | 12 (40.0) | 11 (36.7) | .786 |
| β‐Blockers | 10 (33.3) | 11 (36.7) | .783 |
| Calcium channel blocker | 27 (90.0) | 23 (76.7) | .195 |
| Other diuretics | 6 (20.0) | 9 (30.0) | .371 |
| Aldosterone antagonists | 24 (80.0) | 21 (70.0) | .313 |
| α‐Adrenergic blocker | 11 (36.7) | 11 (36.7) | 1.000 |
| Direct‐acting vasodilators | 1 (3.3) | 0 | 1.000 |
| Other antihypertensive agents | 1 (3.3) | 2 (6.7) | 1.000 |
Data are expressed as N (%).
Abbreviation: ACE, angiotensin‐converting enzyme.
3.2. Tumor and operation characteristics
Tumor and operation characteristics for the study population are shown in Table 3. Patients were similar in lesion location, AVS performed, tumor size, and operation time between groups (Figure 1).
Table 3.
Tumor and operation characteristics
| RDN group (n = 30) | Control group (n = 30) | P | |
|---|---|---|---|
| Lesion location, right (%) | 13 (43.3) | 11 (36.7) | .792 |
| AVS performed, n (%) | 23 (76.7) | 25 (83.3) | .519 |
| Tumor size, cm | 1.50 ± 0.19 | 1.27 ± 0.11 | .498 |
| Operation time, min | 45.7 ± 4.3 | 32.1 ± 2.9 | .054 |
Data are expressed as mean ± SD. Statistically significant differences between the RDN and control groups are defined as those with P‐value < .05.
Abbreviation: AVS, adrenal vein sampling.
FIGURE 1.
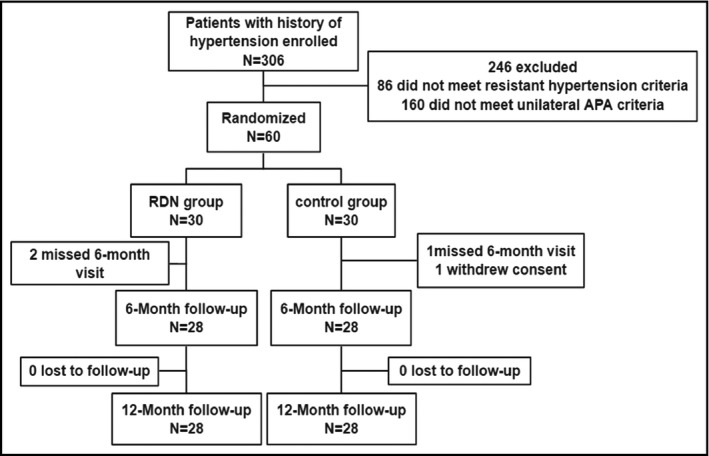
Study flow
3.3. Efficacy analysis
At the 12‐month follow‐up visit, there were 28 patients in the RDN group and 28 patients in the control group. Changes from baseline 24‐hours ambulatory BP to the 12‐month BP are shown in Figure 2 for both groups. The change in 24‐hours ambulatory BP and office BP was greater at 12 months for the RDN group than for the control group. At 12 months post‐procedure, the 24‐hours ambulatory BP in the RDN group had decreased by 20.7 ± 15.2/11.8 ± 10.7 mm Hg and that in the control group had decreased by 11.9 ± 11.1/6.7 ± 6.4 mm Hg. The difference in the degree of reduction at 12 months between the two groups was statistically significant (P = .017 for 24‐hours SBP, P = .036 for 24‐hours DBP; Figure 2). All 24‐hours mean SBP and DBP values for the two groups were significantly lower than the baseline values (for all, P < .001; Figure 2).
FIGURE 2.
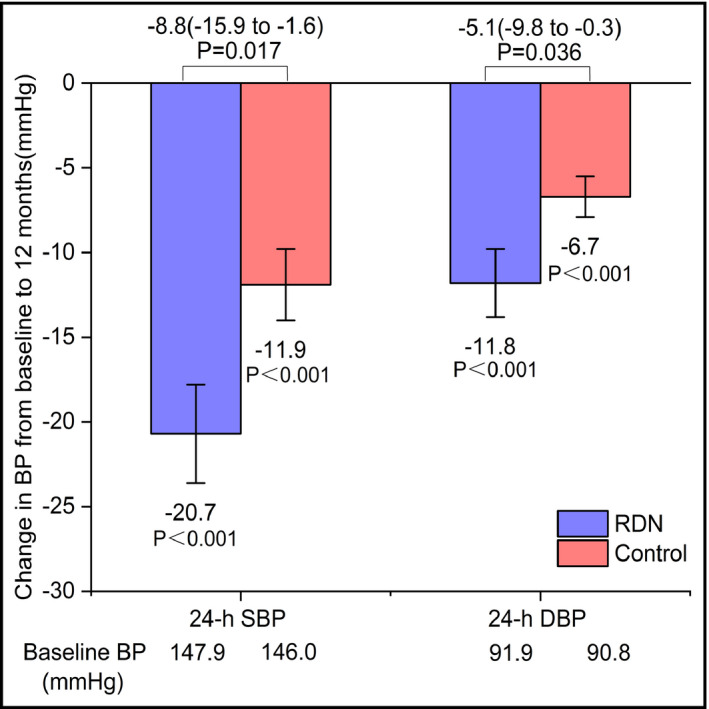
Change in 24‐h SBP and DBP for RDN and control groups. Both the RDN group and the control group at 12 mo after the procedure experienced significant reductions in 24‐h SBP and DBP. BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure
Changes from baseline office BP and 12‐month BP are displayed in Figure 3 for the two groups. The change in BP was greater at 12 months for the RDN group than for the control group for office SBP and DBP. At 12 months post‐procedure, the office BP in the RDN group was reduced by 37.1 ± 26.0/16.5 ± 12.8 mm Hg. The 12‐month changes in office BP were significantly greater than those observed in the control group (25.9 ± 16.8/9.9 ± 5.1 mm Hg). The difference in office BP reduction at 12 months between the two groups was statistically significant (P = .035 for SBP, P = .013 for DBP; Figure 3). All office SBP and DBP values for the two groups were significantly lower than baseline (all P < .001; Figure 3).
FIGURE 3.
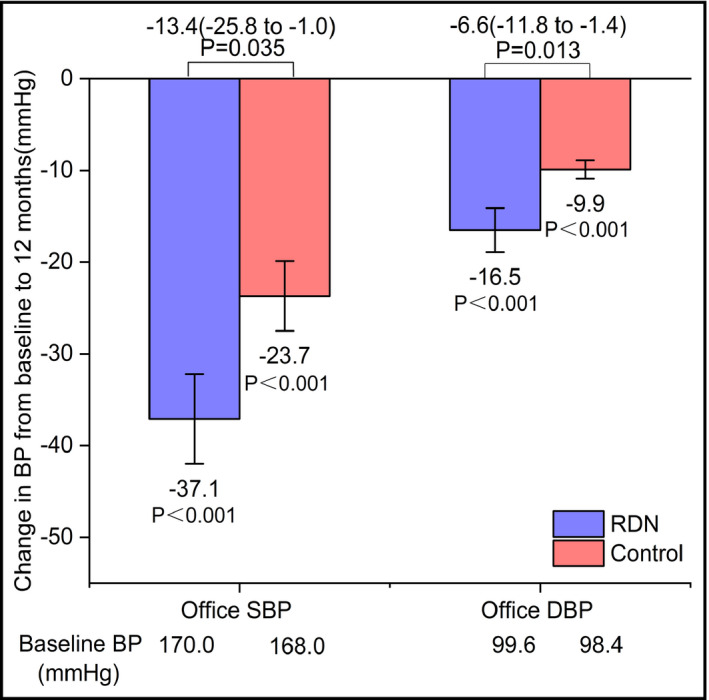
Changes in office SBP and DBP for RDN and control groups. Both the RDN group and the control group at 12 mo after the procedure experienced significant drops in office SBP and DBP. BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure
Individual patient changes in 24‐hours BP from baseline to 12 months are shown in Figure 4. BP values at 6 and 12 months after the procedure for office and ABPM are shown in Table 3. There was a significant difference in 24‐hours and office BP between the RDN group and control group at 12 months after the procedure (all P < .05; Table 4).
FIGURE 4.
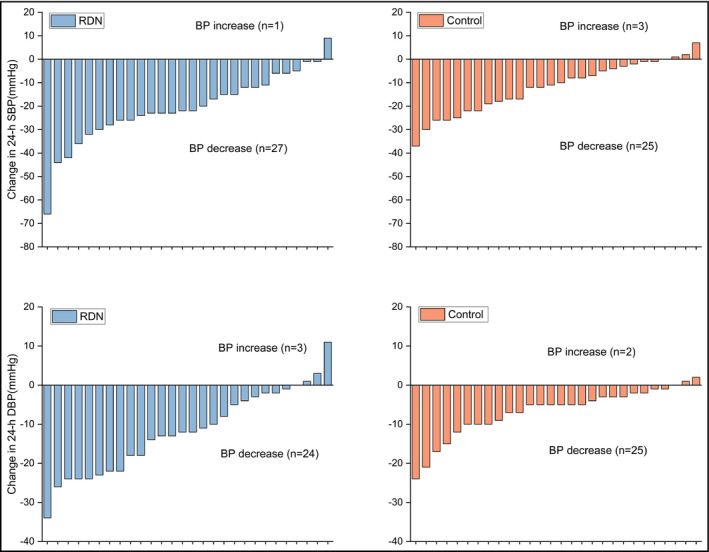
Individual patient changes in 24‐h BP from baseline to the 12‐mo post‐procedure follow‐up
Table 4.
Twenty‐four‐hours average BP and office BP at the 6‐ and 12‐mo post‐procedure follow‐up
| BP | RDN group (n = 28) | Control group (n = 28) | P |
|---|---|---|---|
| 6 mo after procedure | |||
| 24‐h SBP (mmHg) | 132.0 ± 9.8 | 137.6 ± 10.0 | .034 |
| 24‐h DBP (mmHg) | 79.3 ± 7.4 | 84.5 ± 8.8 | .012 |
| SBP (mmHg) | 135.7 ± 15.0 | 143.7 ± 12.8 | .076 |
| DBP (mmHg) | 86.3 ± 9.5 | 91.1 ± 6.0 | .014 |
| 12 mo after procedure | |||
| 24‐h SBP (mmHg) | 127.2 ± 10.7 | 134.1 ± 11.2 | .021 |
| 24‐h DBP (mmHg) | 75.7 ± 7.0 | 81.2 ± 7.6 | .006 |
| SBP (mmHg) | 133.3 ± 11.7 | 141.8 ± 12.1 | .004 |
| DBP (mmHg) | 83.8 ± 7.2 | 88.5 ± 5.6 | .034 |
Data are expressed as mean ± SD. Statistically significant differences between the RDN and control groups are defined as those with P‐value < .05.
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
The number of antihypertensive medications used changed from pre‐procedure treatment to 6 and 12 months post‐procedure. Fewer antihypertensive medications were used in the RDN group than in the control group at 6 and 12 months post‐procedure (Table 5).
Table 5.
The change in the number of antihypertensive medications for the two groups at baseline and at the 12‐mo post‐procedure follow‐up
| Number of antihypertension medications | Use (%) at Baseline | Use (%) at 6 mo | Use (%) at 12 mo | |||
|---|---|---|---|---|---|---|
| RDN group (n = 30) | Control group (n = 30) | RDN group (n = 28) | Control group (n = 28) | RDN group (n = 28) | Control group (n = 28) | |
| 0 | 0 | 0 | 12 (42.9) | 6 (21.4) | 12 (42.9) | 7 (25.0) |
| 1 | 0 | 0 | 5 (17.5) | 14 (50.0) | 16 (57.1) | 16 (57.1) |
| 2 | 0 | 0 | 11 (39.3) | 6 (21.4) | 0 | 3 (10.7) |
| 3 | 15 (53.6) | 17 (60.7) | 0 | 1 (3.6) | 0 | 2 (7.1) |
| 4 | 10 (35.7) | 11 (39.3) | 0 | 1 (3.6) | 0 | 0 |
| 5 | 3 (10.7) | 0 | 0 | 0 | 0 | 0 |
Data are expressed as N (%).
At 6 and 12 months post‐procedure, PAC and ARR in either group were lower than the baseline levels. PRA in either group was higher than the baseline levels. For all patients, serum potassium levels returned to normal at 6 and 12 months post‐procedure. There were no significant changes in eGFR in either group from baseline to 6 and 12 months post‐procedure (Table 6).
Table 6.
Biochemistry test results at 6‐ and 12‐mo post‐procedure follow‐up
| Characteristics | RDN group (n = 28) | Control group (n = 28) | P |
|---|---|---|---|
| PRA (ng/mL)/h | |||
| Baseline | 0.20 (0.20 ~ 0.55) | 0.20 (0.20 ~ 0.30) | .150 |
| 6 mo | 1.40 (1.20 ~ 2.20) | 1.40 (1.20 ~ 1.89) | .625 |
| 12 mo | 1.64 (1.14 ~ 2.40) | 1.40 (1.15 ~ 1.67) | .097 |
| PAC (pg/mL) | |||
| Baseline | 434.5 (227.9 ~ 680.3) | 310.0 (176.0 ~ 538.0) | .150 |
| 6 mo | 93.3 (74.0 ~ 140.4) | 143.0 (103.0 ~ 188.0) | .010 |
| 12 mo | 95.0 (70.0 ~ 128.2) | 141.0 (94.3 ~ 187.0) | .053 |
| ARR | |||
| Baseline | 185.8 (62.3 ~ 329.4) | 103.00 (54.2 ~ 257.5) | .372 |
| 6 mo | 6.8 (4.7 ~ 10.7) | 9.9 (5.8 ~ 15.5) | .074 |
| 12 mo | 8.4 (6.5 ~ 10.0) | 7.9 (5.7 ~ 13.5) | .501 |
| Serum potassium (mmol/L) | |||
| Baseline | 3.44 ± 0.71 | 3.43 ± 0.70 | .939 |
| 6 mo | 4.19 ± 0.20 | 4.14 ± 0.29 | .453 |
| 12 mo | 4.22 ± 0.27 | 4.11 ± 0.44 | .310 |
| eGFR (mL/(min/1.73 m2)) | |||
| Baseline | 88.2 ± 11.4 | 84.3 ± 14.9 | .939 |
| 6 mo | 89.8 ± 9.0+ | 86.7 ± 10.6+ | .771 |
| 12 mo | 82.3 ± 10.2+ | 84.8 ± 9.5+ | .692 |
Data are presented as mean ± SD, or median (IQR). +: compared with baseline level in the same group, P > .05.
Abbreviations: ARR, aldosterone‐to‐renin ratio; eGFR, estimated glomerular filtration rate, calculated using the Cockcroft‐Gault formula; PAC, plasma aldosterone concentration; PRA, plasma renin activity.
3.4. Clinical and biochemical outcomes
No significant difference was found between groups in clinical and biochemical outcomes, but patients in the RDN group had a higher proportion of cured clinical and biochemical outcomes than those in the control group (Tables 7 and 8). The proportion of patients without clinically response in the control group was higher than in the RDN group (7.1% vs 0; Table 7).
Table 7.
Clinical outcomes in the RDN and control groups at 12‐mo post‐procedure follow‐up
| Clinical outcomes | RDN group (n = 28) | Control group (n = 28) | P |
|---|---|---|---|
| Cured (%) | 10 (35.7) | 5 (17.9) | .131 |
| Improved (%) | 18 (64.3) | 21 (75.0) | .383 |
| Failed (%) | 0 | 2 (7.1) | .471 |
Data are expressed as N (%).
Table 8.
Biochemical outcomes in the RDN and control groups at the 12‐mo post‐procedure follow‐up
| Biochemical outcomes | RDN group (n = 28) | Control group (n = 28) | P |
|---|---|---|---|
| Cured (%) | 27 (96.4) | 25 (89.3) | .604 |
| Improved (%) | 1 (3.6) | 3 (10.7) | .604 |
| Failed (%) | 0 | 0 | 1.000 |
Data are expressed as N (%).
3.5. Safety end points
No major procedural or clinical safety events were observed in either the RDN or control groups throughout the 12‐month follow‐up. Specifically, there were no significant changes in eGFR (Table 6), no deaths from all causes, end‐stage renal disease, renal artery or other vascular complications, or new renal artery stenosis of >70%.
4. DISCUSSION
Adrenalectomy is the only treatment that can achieve biochemical cure by removing the lesion responsible for hyperaldosteronism. Although APA is considered correctable with removal of an adrenal adenoma, the proportions of patients reported that achieve lower hypertension vary widely (16%‐72%). 7 , 8
Surgical sympathectomy was first performed for the treatment of severe hypertension in the 1920s. 20 However, because of the severe side effects, such as orthostatic hypotension, palpitations, anhydrosis, intestinal disturbances, loss of ejaculation, thoracic duct injuries, and atelectasis, surgery did not become a popular procedure. 20 Catheter‐based RDN has been used to treat resistant hypertension by ablating sympathetic nerves from the intima of renal arteries. 21 RDN was considered to be effective to control resistant hypertension before the SYMPLICITY HTN‐3 clinical trial. 21 Despite the failure of the SYMPLICITY HTN‐3 trial to meet its primary efficacy end point, recent positive results from the SPYRAL HTN‐ON MED 22 and RADIANCE‐HTN SOLO 23 trials sparked our interest in studying the denervation methods. However, we believed that direct stimulation using RF energy and the use of catheter and wire on the arterial intima could result in intimal injury and/or thrombosis, 13 , 24 which may trigger the progression of atherosclerosis. Moreover, an inappropriate ablation device may lead to inadequate ablation, which in turn results in uncertainty in the effects of RDN. 14
Previous studies have shown that as many as 50% of the sympathetic nerve fibers may reside at depths of >3 mm from the intimal surface of the renal artery 25 ; in other words, closer to the adventitia renal artery. Therefore, to minimize the above side effects, we tried to perform RDN from the adventitia of renal artery with the help of laparoscopy. Previously, we already had sufficient basis for animal experiments on RDN through the adventitia of renal artery, and the results showed that this innovative technology was safe and effective. 15 Considering that patients with unilateral aldosterone‐producing adenoma have poor blood pressure control after laparoscopic unilateral adrenalectomy and RDN from the intima of the renal artery may cause intimal injury and/or thrombosis, also the surgical method happened to require laparoscopic technology, we tried to implement laparoscopy‐based RDN from the adventitia of renal artery. We selected patients with resistant hypertension caused by unilateral APA, and these patients were also willing to enter the study. This clinical trial was an exploratory trial in order to apply this innovative technology and explore the validity and feasibility of this approach in clinical trial for the first time. Fortunately, the results were satisfactory with this unilateral procedure and there were no periprocedural complications; furthermore, eGFR data indicated no substantial deterioration of renal function.
Patients in both the RDN plus adrenalectomy group (RDN group) and the adrenalectomy group (control group) achieved sustained reductions in office BP and 24‐hours BP from baseline to 12 months after undergoing the procedures. The SBP reductions in the RDN group are higher than the reductions reported in the treatment‐resistant hypertension (TR‐HTN) studies (35.7 vs 28.1 mm Hg). 26 This may be due to the collective effect of RDN and adrenalectomy on lowering hypertension. Furthermore, the reductions in 24‐hours BP and office BP in the RDN group were also higher than those in the adrenalectomy‐only group. This may be due to the additional effect of RDN. The RF used during laparoscopic‐based RDN is transmitted from the adventitia to the lumen, and we also stripped the tissue surrounding the renal arteries to separate and expose the renal artery, so that most of the renal nerves distributed in the adventitia may be destroyed, which may cause damage to more nerve fibers than traditional RDN surgery.
Several studies have shown a higher prevalence of cardiovascular and cerebrovascular morbidity and mortality in patients with PA than in patients with primary hypertension matched for age, sex, and BP, 19 and some experimental studies have suggested that long‐term exposure to increased aldosterone levels might result in cardiovascular 27 and renal 28 , 29 structural damage, independent of the BP level. In our study, the PAC was lower in the RDN group than in the control group at 12 months post‐procedure, but there was no significant difference between groups. We considered that RDN may have no significant effect on the concentration of RAS in the blood.
Many researches found that after unilateral laparoscopic adrenalectomy in patients with APA, serum potassium concentrations improved in nearly 100% of patients postoperatively and plasma aldosterone levels and renin levels gradually returned to normal after unilateral laparoscopic adrenalectomy. 30 , 31 , 32 , 33 , 34 , 35 In our study, cured and improved rate of clinical outcomes in the RDN group was achieved in 35.7% and in 64.3%, respectively, of all the patients, which were higher than those in the control group (17.9% and 75%, respectively). Therefore, all patients achieved a clinical benefit (cured and improved clinical outcomes) in the RDN group, which was also higher than patients in the control group (100% vs 92.9%). And all patients in the two groups achieved the effective biochemical outcomes (cured and improved biochemical outcomes).
Primary aldosterone is caused by excessive secretion of aldosterone, but the result of high blood pressure is not only caused by excess aldosterone. In many cases, hypertension may persist after treatment. 36 We consider that patients with older age, longer duration of hypertension course, elevated serum creatinine, taking more than two antihypertensive drugs, and family history of hypertension before surgery are predictors of persistent hypertension after surgery. 35 For the patients selected in our study, excess aldosterone may be the origin of hypertension, but it is not the only factor for such special patients, so that not all patients' blood pressure returned to normal after surgery in the both groups. The positive results of our clinical trials indicated that the selected patients may have primary hypertension on the basis of secondary hypertension. Therefore, with the help of the additional effect of RDN, the blood pressure of RDN group decreased more than the control group.
Regrettably, we were unable to accurately distinguish between the weight of primary hypertension in the maintenance of patients' hypertension and the proportion of patients with primary hypertension between the two groups for such special patients in this study. Although there are still deficiencies in this study, we have completed the first exploration about RDN from the adventitia of renal artery in the field of hypertension. For our team, this innovative technology has become more mature; we will set strict selection criteria and explore more suitable ablation catheters from the adventitia of renal artery, which is expected to yield more convincing results in the follow‐up clinical trials.
4.1. Limitations
The data presented here may be have been subjected to potential biases and limitations. The number of included cases was not sufficiently large, and it was also difficult to exclude the influence of other internal and external factors on BP. Furthermore, after surgery, inadvertent changes in lifestyle habits may also have affected the BP. Therefore, clarifying the effects of these factors would require larger sample sizes and controls.
5. CONCLUSIONS
Despite the relatively small number of patients enrolled, the study results showed sustained reductions in office BP and 24‐hours mean BP at the 12‐month follow‐up. RDN from the adventitia of renal artery through laparoscopy has proven to be safe. Further, RDN from the adventitia of renal artery plus unilateral laparoscopic adrenalectomy is more effective than unilateral laparoscopic adrenalectomy alone for treating resistant hypertension caused by unilateral APA.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
AUTHOR CONTRIBUTIONS
Yahui Liu conceived and designed the study, analyzed and interpreted the data, and drafted the manuscript. Lijie Zhu conceived and designed the data. Linwei Zhao analyzed and interpreted the data. Degang Ding, Zhonghua Liu, Zhiqiang Fan, and Qiuping Zhao revised the manuscript. You Zhang conceived and designed the study, and analyzed and interpreted the data. Jiguang Wang revised the manuscript critically for important intellectual content. Chuanyu Gao gave final approval of the manuscript submitted.
Liu Y, Zhu B, Zhu L, et al. Clinical outcomes of laparoscopic‐based renal denervation plus adrenalectomy vs adrenalectomy alone for treating resistant hypertension caused by unilateral aldosterone‐producing adenoma. J Clin Hypertens. 2020;22:1606–1615. 10.1111/jch.13963
Funding information
This study was supported by the National Natural Science Foundation of China (Number: U1604184).
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 2. Chernova I, Krishnan N. Resistant hypertension updated guidelines. Curr Cardiol Rep. 2019;21(10):117. [DOI] [PubMed] [Google Scholar]
- 3. Tsioufis C, Kordalis A, Flessas D, et al. Pathophysiology of resistant hypertension: the role of sympathetic nervous system. Int J Hypertens. 2011;11:642416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Byrd JB, Turcu AF, Auchus RJ. Primary aldosteronism. Circulation. 2018;138(8):823‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Assalia A, Gagner M. Laparoscopic adrenalectomy. Br J Surg. 2004;91(10):1259‐1274. [DOI] [PubMed] [Google Scholar]
- 6. Meria P, Kempf BF, Hermieu JF, Plouin PF, Duclos JM. Laparoscopic management of primary hyperaldosteronism: clinical experience with 212 cases. J Urol. 2003;169(1):32‐35. [DOI] [PubMed] [Google Scholar]
- 7. Steichen O, Zinzindohoue F, Plouin PF, Amar L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm Metab Res. 2012;44(3):221‐227. [DOI] [PubMed] [Google Scholar]
- 8. Muth A, Ragnarsson O, Johannsson G, Wängberg B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br J Surg. 2015;102(4):307‐317. [DOI] [PubMed] [Google Scholar]
- 9. Huan Y, Cohen DL. Renal denervation: a potential new treatment for severe hypertension. Clin Cardiol. 2013;36(1):10‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Symplicity HTN‐1 Investigators . Catheter‐based renal sympathetic denervation for resistant hypertension: durability of BP reduction out to 24 months. Hypertension. 2011;57(5):911‐917. [DOI] [PubMed] [Google Scholar]
- 11. Parrish DC, Gritman K, van Winkle DM, Woodward WR, Bader M, Habecker BA. Postinfarct sympathetic hyperactivity differentially stimulates expression of tyrosine hydroxylase and norepinephrine transporter. Am J Physiol Heart Circ Physiol. 2008;294(1):99‐106. [DOI] [PubMed] [Google Scholar]
- 12. Jadczyk T, Partyka L, Smolka G, et al. Long‐term follow‐up of renal arteries after radio‐frequency catheter‐based denervation using optical coherence tomography and angiography. Int J Cardiovasc Imaging. 2016;32(6):855‐862. [DOI] [PubMed] [Google Scholar]
- 13. Delgado‐Silva J, Fernandes R, Pita IR, et al. Intravascular imaging, histopathological analysis, and catecholamine quantification following catheter‐based renal denervation in a swine model: the impact of prebifurcation energy delivery. Hypertens Res. 2018;41(9):708‐717. [DOI] [PubMed] [Google Scholar]
- 14. Gao C, Zhao L, Zhu L, et al. Laparoscopic‐based perivascular unilateral renal sympathetic nerve denervation for treating resistant hypertension: a case report. Hypertens Res. 2019;42(8):1162‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bai M, Yang C, Gao C, et al. Effects of renal denervation from the intima and the adventitia of renal arteries on renal sympathetic nerve activity in dogs: a comparative Study. Cardiology. 2015;131(3):189‐196. [DOI] [PubMed] [Google Scholar]
- 16. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889‐1916. [DOI] [PubMed] [Google Scholar]
- 17. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63(1):151‐160. [DOI] [PubMed] [Google Scholar]
- 18. Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017;5(9):689‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. O'Brien E, Parati G, Stergiou G, et al. European society of hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731‐1768. [DOI] [PubMed] [Google Scholar]
- 20. Page IH, Heuer GJ. A surgical treatment of essential hypertension. J Clin Invest. 1935;14(1):22‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393‐1401. [DOI] [PubMed] [Google Scholar]
- 22. Kandzari DE, Böhm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6‐month efficacy and safety results from the SPYRAL HTN‐ON MED proof‐of‐concept randomised trial. Lancet. 2018;391(10137):2346‐2355. [DOI] [PubMed] [Google Scholar]
- 23. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE‐HTN SOLO): a multicentre, international, single‐blind, randomised, sham‐controlled trial. Lancet. 2018;391(10137):2335‐2345. [DOI] [PubMed] [Google Scholar]
- 24. Roleder T, Skowerski M, Wiecek A, et al. Long‐term follow‐up of renal arteries after radio‐frequency catheter‐based denervation using optical coherence tomography and angiography. Int J Cardiovasc Imaging. 2016;32(6):855‐862. [DOI] [PubMed] [Google Scholar]
- 25. Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri‐arterial renal nerves in man. J Am Coll Cardiol. 2014;64(7):635‐643. [DOI] [PubMed] [Google Scholar]
- 26. McBride M, Krum H, Schlaich M, et al. Effectiveness of catheter‐based renal denervation for treatment resistant hypertension‐results of a systematic review and meta‐analysis. Value Health. 2014;17(7):A757‐758. [DOI] [PubMed] [Google Scholar]
- 27. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin‐angiotensin‐aldosterone system alterations. Circ Res. 2015;116(6):960‐975. [DOI] [PubMed] [Google Scholar]
- 28. Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest. 1996;98(4):1063‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int. 2004;66(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 30. Blumenfeld JD, Sealey JE, Schlussel Y, et al. Diagnosis and treatment of primary hyperaldosteronism. Ann Intern Med. 1994;121(11):877‐885. [DOI] [PubMed] [Google Scholar]
- 31. Harris DA, Au‐Yong I, Basnyat PS, Sadler GP, Wheeler MH. Review of surgical management of aldosterone secreting tumours of the adrenal cortex. Eur J Surg Oncol. 2003;29(5):467‐474. [DOI] [PubMed] [Google Scholar]
- 32. Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002;15(10 Pt 1):896‐902. [DOI] [PubMed] [Google Scholar]
- 33. Stowasser M, Klemm SA, Tunny TJ, Storie WJ, Rutherford JC, Gordon RD. Response to unilateral adrenalectomy for aldosterone‐producing adenoma: effect of potassium levels and angiotensin responsiveness. Clin Exp Pharmacol Physiol. 1994;21(4):319‐322. [DOI] [PubMed] [Google Scholar]
- 34. Young WF Jr. Minireview: primary aldosteronism– changing concepts in diagnosis and treatment. Endocrinology. 2003;144(6):2208‐2213. [DOI] [PubMed] [Google Scholar]
- 35. Meyer A, Brabant G, Behrend M. Long‐term follow‐up after adrenalectomy for primary aldosteronism. World J Surg. 2005;29(2):155‐159. [DOI] [PubMed] [Google Scholar]
- 36. Sechi LA, Novello M, Lapenna R, et al. Long‐term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638‐2645. [DOI] [PubMed] [Google Scholar]


