Abstract
Reversing left ventricular hypertrophy (LVH) can reduce the incidence of adverse cardiovascular events. However, there is no clear superiority–inferiority differentiation between angiotensin‐converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta‐blockers (BB), calcium channel blockers (CCB), and diuretics in reversing LVH in hypertensive patients. To provide further evidence for choosing the optimal antihypertensive drug for improving LVH, we performed a network meta‐analysis of randomized controlled trials (RCTs) based on the Cochrane library database, Embase, and Pubmed, and identified 49 studies involving 5402 patients that were eligible for inclusion. It was found that ARB could improve LVH in hypertensive patients more effectively than CCB (MD −4.07, 95%CI −8.03 to −0.24) and BB (MD −4.57, 95%CI −8.07 to −1.12). Matched comparison of renin‐angiotensin system inhibitors (RASi) showed that the effect of ACEI in reducing left ventricular mass index (LVMi) was not effective as that of ARB (MD −3.72, 95%CI −7.52 to −0.11). The surface under the cumulative ranking for each intervention indicated that the use of ARB was more effective among the different types of antihypertensive drugs (97%). This network meta‐analysis revealed that the use of ARB in antihypertensive therapy could achieve better efficacy in reversing LVH in hypertensive patients.
Keywords: antihypertensive drug, Bayesian network analysis, hypertension, left ventricular hypertrophy
1. INTRODUCTION
Hypertension is a major risk factor for cardiovascular disease (CVD) and is significantly associated with increased morbidity and mortality from CVD. 1 , 2 Left ventricular hypertrophy (LVH) is a common target organ damage of hypertension, which can cause abnormal changes in the ultrastructure and energy metabolism of cardiomyocytes, resulting in adverse cardiovascular events such as abnormal cardiac contraction and diastolic function, and arrhythmia. 3 , 4 , 5 The left ventricular mass index (LVMi), which reflects LVH, plays an important role in predicting the risk of adverse cardiovascular events in the future. 6 , 7
The European Society of Cardiology (ESC)/ European Society of Hypertension (ESH) 2018 Guidelines for Hypertension Diagnosis and Treatment indicate that antihypertensive therapy reverses LVH as represented by a reduction of CV events and mortality. 8 , 9 On the basis of preliminary clinical studies, the American expert consensus on hypertension points out that angiotensin receptor blockers (ARB) or angiotensin‐converting enzyme inhibitors (ACEI) are generally used in hypertensive patients with LVH. 10
Many current clinical studies have shown that there has been controversy over whether patients with hypertension can reverse LVH and the pros and cons of reversing LVH after treatment with antihypertensive drugs. 11 , 12 This also brings great confusion to clinical decision makers in the treatment of hypertensive LVH which antihypertensive drugs can obtain the maximum benefit. In addition, single randomized controlled trials or traditional meta‐analysis cannot provide strong evidence support. At the same time, the lack of direct comparison between different antihypertensive drugs cannot evaluate the superiority–inferiority differentiation of different antihypertensive drugs in reversing LVH. The purpose of this network meta‐analysis was to compare the efficacy of different types of antihypertensive drugs in reversing LVH in hypertensive patients.
2. MATERIALS AND METHODS
2.1. Search strategy for identifying eligible studies
We searched PubMed, Cochrane Library, and EMBASE databases up to May 2020 for evaluating the effects of different types of antihypertensive drugs on LVH in hypertensive patients by using the following search terms: (a) hypertension; (b) LVH; and (c) each class of antihypertensive drugs. We identify the grey literature by retrieving relevant institutions and clinical trial registries. All analyses were based on previously published studies and therefore did not require ethical approval and patient consent. The detailed search strategies are displayed in Figure 1.
Figure 1.
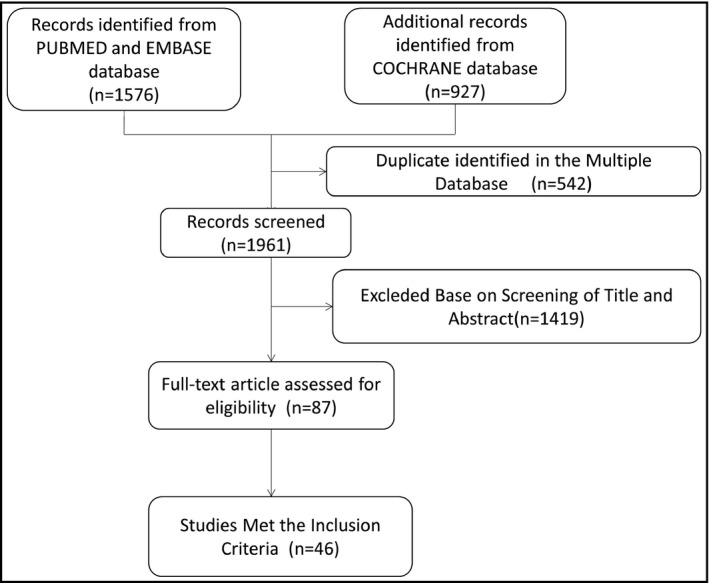
Flow diagram showing the study selection process
2.2. Eligibility criteria
Studies meeting the following criteria were considered for inclusion: (a) comparisons of six classes of antihypertensive drugs were performed and did not include any other non‐drug treatment modality; (b) the shortest follow‐up time was 3 months; (c) randomized controlled studies; and (d) LVMI was evaluated by echocardiography. Studies that did not meet these requirements were excluded.
2.3. Data extraction
Two researchers independently screened the literature, extracted and cross‐checked the data. Any disagreement was resolved through discussion or judgment by a third party. Data extraction follows objective principles and faithful original data. We reported details of study design, participants, intervention, follow‐up time, age, baseline systolic and diastolic blood pressure.
2.4. Statistical analysis
Stata SE‐64 and GeMTC‐GUI‐0.14.3 were used for statistical analysis. Continuous variables were analyzed using the mean difference (MD) with 95% CI. The significance level was set to 0.05. A chi‐square test was used to judge the heterogeneity between the results of each study (the test level was α = 0.10). The specific steps are as follows:(a) if there was no statistical heterogeneity between the studies or the heterogeneity was small (I2<50%, P > .1), the fixed effect model was used for analysis; (b) if the heterogeneity was large(I2>50%, P < .1), the heterogeneity source would be further determined by sensitivity analysis. Bayesian statistical method was used for network meta‐analysis. We used the Markov Chains Monte Carlo methods to perform 20 000 tuning iterations and 5000 simulation iterations with 3 Markov chains. The convergence degree of the model was ensured according to the results of orbit diagrams and density diagram. We performed the node‐splitting model to check whether the analysis of the trials in the network was indeed consistent. In addition, when the 95% CI of the median of the inconsistency factors included zero and if the inconsistency standard deviation was less than or equal to the random‐effects standard deviation, the inconsistency was considered insignificant. According to the surface under the cumulative ranking, we evaluated the superiority–inferiority of multiple antihypertensive drugs in reversing LVH.
3. RESULTS
3.1. Study characteristics
Overall, the systematic review and network meta‐analysis included 46 clinical studies involving 5074 hypertensive patients. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 The follow‐up time ranged from 3 months to 24 months with a mean of 7 months. The mean age of the participants was 55 (46‐ 67) years, and 61% of the patients were male. The mean baseline systolic blood pressure was 161 (143‐180) mmHg. In these RCTs, 1332 patients (26.25%) were assigned to ACEI; 1040 (20.50%) to ARB; 967 (19.06%) to BB; 970 (19.12%) to CCB; and 765(15.08%) were randomized to DIU. The characteristics of the included studies and the associated patient characteristics are summarized in Table 1. The network comparison between different processing strategies is constructed as shown in Figure 2. The PRISMA checklist and PRISMA Protocol are presented in S1 Appendix and S2 Appendix respectively. A quality assessment of the included studies can be found in S3 Appendix.
Table 1.
Characteristics of the studies included in this Meta‐analysis
| Study (author, year) | Treatment class | Sample size | Mean age | LVMI (baseline) | SBP (mmHg) | DBP (mmHg) | Durations(months) |
|---|---|---|---|---|---|---|---|
| Futoshi 2005 13 | ARB/ACEI | 10/11 | 59/59 | 151 ± 16/149 ± 16 | 157/156 | 97/97 | 10 |
| Azizi 2014 14 | DIU/ACEI | 46/40 | 56/55 | 97 ± 17/98 ± 27 | 150/150 | 90/90 | 3 |
| Bilge 2005 15 | CCB/ACEI | 14/13 | 46/49 | 122 ± 26/118 ± 23 | 151/161 | 101/103 | 6 |
| Fogari 2005 16 | CCB/ACEI | 60/61 | 61/60 | 116 ± 16/115 ± 15 | 148/148 | 89/90 | 24 |
| Grandi 2008 17 | ARB/CCB | 12/12 | 49/51 | 115 ± 19/146 ± 18 | 146/144 | 95/93 | 6 |
| Neutel 2004 18 | CCB/ACEI | 35/34 | 51/51 | NA | 156/160 | 93/91 | 6 |
| Ogunyankin 2009 19 | CCB/DIU | 18/20 | 54/55 | NA | 144/143 | 91/91 | 6 |
| Okura 2013 20 | DIU/CCB | 28/25 | 61/63 | 137 ± 34/146 ± 44 | 156/160 | 90/91 | 12 |
| Scaglione 2007 21 | ARB/ACEI | 19/19 | 56/56 | 47 ± 14/49 ± 10 | 162/159 | 94/98 | 6 |
| Dahlof 2005 51 | DIU/ACEI | 284/272 | 55/56 | 144 ± 30/143 ± 28 | 164/165 | 99/99 | 6 |
| Fountoulaki 2005 47 | BB/ARB | 20/20 | 54/56 | 98 ± 16/97 ± 13 | 156/153 | 99/98 | 3 |
| Galzerano 2005 46 | ARB/BB | 36/34 | 59/60 | 140 ± 13/135 ± 16 | 160/158 | 98/96 | 11 |
| Agabiti 2005 38 | BB/CCB | 78/96 | 53/53 | 106 ± 23/104 ± 28 | 160/161 | 100/101 | 6 |
| Schneider 2004 48 | ARB/BB | 119/121 | 54/55 | 117 ± 27/119 ± 26 | 160/161 | 94/93 | 18 |
| Richard 2004 40 | ARB/BB | 457/459 | NA | NA | NA | NA | 12 |
| Koldas 2003 45 | ACEI/CCB | 20/20 | 60/59 | 202 ± 62/203 ± 56 | 173/180 | 99/95 | 3 |
| Sakata 2003 39 | CCB/ACEI | 30/30 | NA | 121 ± 32/127 ± 20 | NA | NA | 12 |
| Dahlof 2002 49 | ARB/BB | 115/110 | 57/57 | 149 ± 30/146 ± 31 | 165/169 | 98/99 | 9 |
| Gaudio 2003 44 | ARB/CCB | 30/30 | 50/53 | 141 ± 14/136 ± 17 | 168/168 | 107/108 | 6 |
| Cuspidi 2002 42 | ARB/ACEI | 91/105 | 53/53 | 141 ± 24/143 ± 28 | 163/162 | 102/101 | 12 |
| Yoshida 2011 50 | CCB/ARB | 22/22 | 57/57 | 102 ± 15/102 ± 17 | 162/159 | NA | 12 |
| Richard 2001 36 | ACEI/CCB | 148/155 | 64/63 | 131 ± 25/133 ± 25 | 172/171 | 98/98 | 12 |
| Malmqvist 2001 57 | ACEI/BB | 25/26 | 50/51 | 113 ± 23/116 ± 19 | 159/158 | 103/101 | 12 |
| Kuperstein 2000 56 | ACEI/BB | 10/11 | NA | 98 ± 9/101 ± 11 | 148/149 | 97/98 | 6 |
| Nalbantgil 2000 43 | ARB/ACEI | 20/20 | 54/53 | 162 ± 22/165 ± 24 | 166/165 | 101/100 | 6 |
| Philippe 2000 41 | DIU/ACEI | 206/206 | 55/54 | 144 ± 40/138 ± 36 | 172/172 | 101/102 | 12 |
| Willem 2001 37 | CCB/ACEI | 81/81 | 67/67 | 109 ± 20/114 ± 23 | 175/175 | 92/93 | 24 |
| Sihm 2000 32 | CCB/ACEI/DIU | 12/11/14 | 47/50/48 | 182 ± 52/152 ± 26 | 168/153/153 | 108/101/103 | 12 |
| Martina 1999 25 | ARB/CCB | 11/11 | 47/51 | NA | 154/145 | 102/100 | 4 |
| Agabiti 1998 23 | CCB/DIU | 15/17 | 50/56 | 142 ± 25/142 ± 26 | 156/159 | 102/102 | 6 |
| Thurmann 1998 24 | ARB/BB | 34/35 | 55/57 | 127 ± 23/127 ± 25 | NA | NA | 8 |
| Hoglund 1998 27 | CCB/BB | 33/33 | 52/53 | 117 ± 12/123 ± 18 | 163/163 | 104/103 | 6 |
| Topouchian 1999 28 | CCB/ACEI | 23/23 | NA | 52 ± 11/52 ± 11 | 156/160 | 96/101 | 3 |
| Radevski 1999 29 | CCB/ACEI | 47/48 | 53/43 | 146 ± 40/139 ± 36 | 179/181 | 118/117 | 4 |
| Tedesco 1998 31 | ARB/DIU | 44/33 | 54/56 | 139 ± 19/140 ± 23 | 157/158 | 96/97 | 6 |
| Athanasios 1998 35 | CCB/ACEI | 15/15 | NA | 140 ± 15/139 ± 15 | NA | NA | 6 |
| Roman 1998 33 | ACEI/DIU | 22/28 | 52/51 | 134 ± 20/93 ± 19 | 153/146 | 96/93 | 6 |
| Scognamiglio 1997 22 | ACEI/CCB | 36/37 | 58/57 | 87 ± 2/89 ± 2 | 165/167 | 100/101 | 9 |
| Papademetriou 1997 34 | CCB/DIU | 89/45 | 56/58 | 170 ± 36/165 ± 36 | 158/161 | 101/101 | 6 |
| Yang 1995 58 | ACEI/CCB | 26/27 | 48/49 | 162 ± 10/165 ± 12 | 162/160 | 105/102 | 12 |
| Kirpizidis 1995 26 | ACEI/CCB | 16/15 | 59/61 | 146 ± 17/146 ± 14 | NA | 102/103 | 6 |
| Ernesto 1994 55 | BB/ACEI | 8/9 | NA | 110 ± 6/125 ± 12 | 148/147 | 99/99 | 12 |
| Trenkwalder 1994 30 | CCB/DIU | 21/21 | NA | 138 ± 25/134 ± 21 | 194/195 | 102/101 | 3 |
| Senior 1993 52 | DIU/CCB/ACEI/BB | 23/22/11/20 | 56/60/49/59 | 151 ± 6/170 ± 7/142 ± 7/157 ± 8 | 167/168/172/166 | 102/103/106/102 | 6 |
| Ranieri 1993 53 | BB/DIU | 20/20 | NA | 113 ± 12/114 ± 15 | 165/162 | 106/106 | 6 |
| Schulte 1992 54 | CCB/ACEI | 20/20 | NA | 141 ± 6/148 ± 5 | 157/149 | 106/104 | 6 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blockers; CCB, calcium channel blocker; DIU, diuretic; LVMI, left ventricular mass index; NA, not available.
Figure 2.
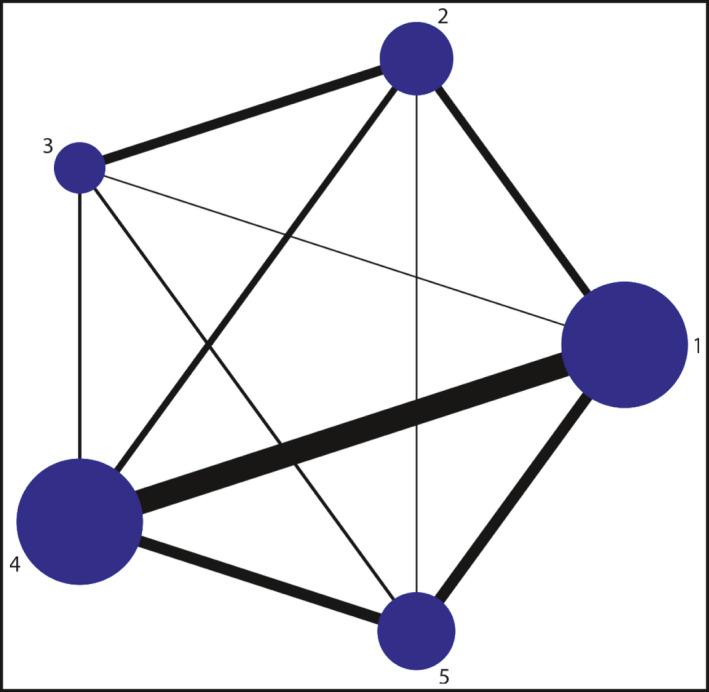
the construction of the network
3.2. Traditional meta‐analyses
The results of traditional meta‐analysis showed that ARB was superior to CCB and BB in reversing LVH under fixed effect model, and the difference was statistically significant. The effect of ACEI on LVMI reduction was significantly better than that of BB. Table 2 presents the results of the meta‐analysis of the data about the regression of LVH between different classes of antihypertension drugs.
Table 2.
Direct comparison results of traditional meta‐analysis
| Number of studies | SMD | I2 | p | Models | |
|---|---|---|---|---|---|
| ACEI vs | |||||
| ARB | 4 | 0.04 (−0.21, 0.28) | 0.00% | .741 | fixed‐effects models |
| BB | 3 | 0.63 (−1.06,−0.20) | 0.00% | .953 | fixed‐effects models |
| CCB | 13 | 0.10 (−0.04, 0.25) | 17.70% | .27 | random‐effects models |
| DIU | 6 | 0.05 (−0.22, 0.33) | 69% | .006 | random‐effects models |
| ARB vs | |||||
| CCB | 4 | −0.82 (−1.22,‐0.42) | 0.00% | .684 | fixed‐effects models |
| BB | 6 | −0.21 (−0.32,−0.10) | 0.00% | .693 | fixed‐effects models |
| DIU | 1 | NA | NA | NA | NA |
| CCB vs | |||||
| BB | 2 | 0.04 (−0.22, 0.29) | 0.00% | .463 | random‐effects models |
| DIU | 7 | −0.16 (−0.44, 0.13) | 28.8% | .230 | random‐effects models |
| BB vs | |||||
| DIU | 2 | 0.20 (−0.68, 0.28) | 61.50% | .107 | fixed‐effects models |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blockers; CCB, calcium channel blocker; DIU, diuretic; NA, not available; SMD, standardized mean difference.
3.3. Bayesian network meta‐analyses
In Network Meta‐Analysis, consistency between direct and indirect comparisons was assessed by calculating inconsistency factors. For the comparison of different types of antihypertensive drugs to reverse LVH, the 95% confidence interval of inconsistency factors contained zero, indicating good consistency. In addition, there was no statistical difference in the consistency test by node‐splitting method (P > .05), which also suggests that there is no inconsistency between direct comparison and indirect comparison. The results of network meta‐analysis showed that ARB could effectively improve LVH in hypertensive patients, and its effect was better than that of CCB (MD −4.07, 95%CI −8.03 to −0.24) and BB (MD −4.57, 95%CI −8.07 to −1.12). ACEI were less effective, and ARB were more effect in reducing LVMI (MD −3.72, 95%CI −7.52 to −0.11). The results of our random‐effects network meta‐analysis for the regression of LVH are summarized in Table 3. The surface under the cumulative ranking for each intervention indicated that the use of ARB was more effective among the six types of antihypertensive drugs. The probabilities of being among the most efficacious treatments were as follows: ARB (97%), ACEI (43%), BB (24%), CCB (33%), and diuretics (53%) (Figure 3).
Table 3.
The results of network meta‐analysis for regression of left ventricular hypertrophy
| ACEI | −3.72 (−7.52, −0.11) | 0.86 (−3.22, 4.94) | 0.33 (−2.34, 2.98) | ‐1.09 (−4.42, 2.43) |
|---|---|---|---|---|
| 3.72 (0.11, 7.52) | ARB | 4.57 (1.12, 8.07) | 4.07 (0.24, 8.03) | 2.62 (−1.64, 7.07) |
| ‐0.86 (−4.94, 3.22) | ‐4.57 (−8.07, −1.12) | BB | ‐0.53 (−4.65, 3.60) | ‐1.93 (−6.31, 2.66) |
| ‐0.33 (−2.98, 2.34) | ‐4.07 (−8.03, −0.24) | 0.53 (−3.60, 4.65) | CCB | ‐1.42 (−4.86, 2.08) |
| 1.09 (−2.43, 4.42) | ‐2.62 (−7.07, 1.64) | 1.93 (−2.66, 6.31) | 1.42 (−2.08, 4.86) | DIU |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta‐blockers; CCB, calcium channel blocker; DIU, diuretic.
Figure 3.
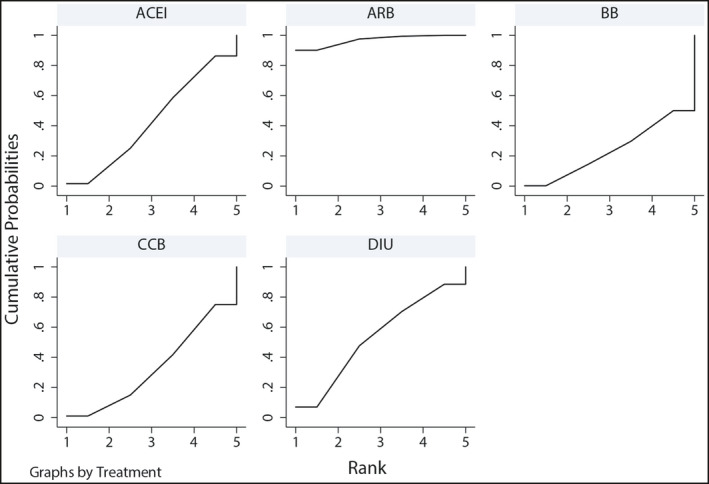
The surface under the cumulative ranking for each intervention
4. DISCUSSION
The results of paired comparison of different types of antihypertensive drugs in the present network meta‐analysis showed that BB and CCB were less effective in reversing LVH than ARB, and matched comparison of the renin‐angiotensin system inhibitors (RASi) showed that ACEI was not effective as ARB in reducing LVMI.
The network meta‐analysis is a comparison of various interventions in the same disease. Compared with the traditional meta‐analysis, it can reduce the bias caused by only analyzing the results of direct comparison. After summarizing and quantifying different intervention measures, the biggest advantage of network meta‐analysis is to sort according to the pros and cons of the outcome indicators, and finally get a relatively good treatment scheme for the same disease, which is more in line with the reality of clinical decision. It has more important application value for clinical decision.
Prevention or reversal of LVH has been shown to reduce the risk of cardiovascular events in hypertensive patients. 59 , 60 Although several clinical trials and meta‐analyses have compared the effects of different classes of antihypertensive drugs on ventricular hypertrophy, the usefulness of the results is limited by their inadequate design and inappropriate methods. 61 , 62 Although meta‐analyses can improve the statistical power and provide more accurate estimates of the effect value, the results depend largely on the criteria for inclusion in the study. 63 , 64 Molecular biology research has shown that LVH in hypertensive patients is a process evolving from quantitative change to qualitative change. 65 , 66 This process includes gene translocation of myosin heavy chain, encoding myosin, membrane protein, and energy metabolism of protein gene shift. 67 , 68 , 69 Brigitte et al have shown that it takes at least 100 days to reverse this process. 70 Therefore, the shorter intervention period in the previous meta‐analysis was insufficient to evaluate the possibility of reversing LVH with various antihypertensive drugs. 61 Unlike previous meta‐analyses, the shortest observation period in our study was three months, and network meta‐analysis suggested that ARB might be the preferred antihypertensive drug to reverse LVH. Moreover, the results of network meta‐analysis suggested that ARNI did not show any advantages in the treatment of LVH in hypertensive patients. Nevertheless, as there are fewer clinical studies on the protective effect of ARNI on target organs of hypertension, larger RCTs are needed to confirm the role of ARNI in reversing LVH.
Notably, this study found that the effect of ACEI on LVH reversal was less effective than that of ARB. The reason underlying these differentials remains unclear, although potential explanations have been suggested. First of all, ACEI block the transformation of angiotensin I into angiotensin II, thus reducing the vessel wall tension and blood volume and achieving the purpose of lowering blood pressure. 71 , 72 On the one hand, ARB can effectively reduce blood pressure by blocking angiotensin (Ang) I receptor, inhibiting aldosterone secretion and eliminating water and sodium retention. On the other hand, ARB can increase the level of endogenous Ang II, that is, increase the level of angiotensin‐converting enzyme (ACE)2 substrate, the homologous enzyme of ACE, thereby activating ACE2‐Ang (1‐7)‐MAS receptor axis to exert cardiac protection. 73 , 74 Secondly, ARB also has vascular‐protective and anti‐inflammatory effects. 75 ARB can enhance pro‐angiogenesis, which includes promoting the production of angiogenic factors and nitric oxide and reducing oxidative stress. 76 Finally, ARB is more prominent than ACEI in inhibiting collagen synthesis. 77 Studies in hypertensive heart failure (HF) rats have shown that ACEI reduces myocardial volume at the early stage of HF and myocardial length at the late stage. ARB is more effective in reducing the diameter of cardiomyocytes in the early and late stages of HF to near normal range. 78
This meta‐analysis provides new clues to support the hypothesis that patients with hypertensive cardiac hypertrophy may obtain better clinical benefits from the use of ARB as compared with other types of antihypertensive drugs. To improve the quality of life and long‐term prognosis of patients with hypertensive cardiac hypertrophy, it is recommended that clinicians choose the optimal antihypertensive drugs to reverse LVH.
5. CONCLUSION
In conclusion, the use of ARB in antihypertensive therapy can achieve better efficacy in reversing LVH in hypertensive patients. There is still a need for larger randomized controlled trials and longer‐term follow‐ups to clarify whether this better effect of ARB in lowering LVMI vs. other antihypertensive drugs could lead to better outcomes.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
All authors fulfill the criteria for authorship. Jing Yu and Jian‐Shu Chen conceived and designed the research. Jian‐Shu Chen, Ying Pei, and Cai‐e Li acquired the data. Jian‐Shu Chen and Qiong‐ying Wang drafted the manuscript and made critical revision of the manuscript for key intellectual content. All authors read and approved the final manuscript.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
The contributions of Jian‐Shu Chen and Ying Pei in this study are consistent.
Funding informationThis study was supported by the National Natural Science Foundation of China (NSFC 81670385), Gansu province health research project (GSWSKY2017‐02), and the Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (CY2017‐QN09).
REFERENCES
- 1. Calvillo L, Gironacci MM, Crotti L, Meroni PL, Parati G. Neuroimmune crosstalk in the pathophysiology of hypertension. Nat Rev Cardiol. 2019;16:476‐490. [DOI] [PubMed] [Google Scholar]
- 2. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Travers JG, Kamal FA, Robbins J, et al. Cardiac fibrosis: the fibroblast awakens. Circ Res. 2016;118:1021‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javier D, Frohlich ED. A translational approach to hypertensive heart disease. Hypertension. 2010;55:1‐8. [DOI] [PubMed] [Google Scholar]
- 5. Yildiz M, Oktay AA, Stewart MH, et al. Left ventricular hypertrophy and hypertension. Prog Cardiovasc Dis. 2020;63:10‐21. [DOI] [PubMed] [Google Scholar]
- 6. Ren J, Kelley RO. Cardiac health in women with metabolic syndrome: clinical aspects and pathophysiology. Obesity. 2012;17:1114‐1123. [DOI] [PubMed] [Google Scholar]
- 7. Park SK, Jung JY, Kang JG, Chung P‐W, Oh C‐M. Left ventricular geometry and risk of incident hypertension. Heart. 2019;105(18):1402‐1407. [DOI] [PubMed] [Google Scholar]
- 8. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953‐2041. [DOI] [PubMed] [Google Scholar]
- 9. Soliman EZ, Byington RP, Bigger JT, et al. Effect of intensive blood pressure lowering on left ventricular hypertrophy in patients with diabetes mellitus: action to control cardiovascular risk in diabetes blood pressure trial. Hypertension. 2015;66:1123‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017;2018(71):1269‐1324. [DOI] [PubMed] [Google Scholar]
- 11. Zhao Y, Heng YU, Zhao XU, et al. The Effects of LCZ696 in Patients with Hypertension Compared with Angiotensin Receptor Blockers: A Meta‐Analysis of Randomized Controlled Trials. J Cardiovasc Pharmacol Ther. 2017;22:447‐457. [DOI] [PubMed] [Google Scholar]
- 12. Li Q, Li L, Wang F, et al. Effect and Safety of LCZ696 in the Treatment of Hypertension: A Meta‐Analysis of 9 RCT Studies. Medicine (Baltimore). 2019;98:e16093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anan F, Takahashi N, Ooie T, et al. Effects of valsartan and perindopril combination therapy on left ventricular hypertrophy and aortic arterial stiffness in patients with essential hypertension. Eur J Clin Pharmacol. 2005;61:353‐359. [DOI] [PubMed] [Google Scholar]
- 14. Azizi M, Perdrix L, Bobrie G, et al. Greater efficacy of aldosterone blockade and diuretic reinforcement vs. dual renin angiotensin blockade for left ventricular mass regression in patients with resistant hypertension. J Hypertens. 2014;32:2038‐2044. [DOI] [PubMed] [Google Scholar]
- 15. Bilge AK, Atilgan D, Tükek T, et al. Effects of amlodipine and fosinopril on heart rate variability and left ventricular mass in mild‐to‐moderate essential hypertension. Int J Clin Pract. 2005;59:306‐310. [DOI] [PubMed] [Google Scholar]
- 16. Fogari R, Mugellini A, Zoppi A, et al. Effect of successful hypertension control by manidipine or lisinopril on albuminuria and left ventricular mass in diabetic hypertensive patients with microalbuminuria. Eur J Clin Pharmacol. 2005;61:483‐490. [DOI] [PubMed] [Google Scholar]
- 17. Grandi AM, Solbiati F, Laurita E, et al. Effects of dual blockade of Renin‐Angiotensin system on concentric left ventricular hypertrophy in essential hypertension: a randomized, controlled pilot study. Am J Hypertens. 2008;21:231‐237. [DOI] [PubMed] [Google Scholar]
- 18. Neutel JM, Smith DHG, Weber MA. Effect of antihypertensive monotherapy and combination therapy on arterial distensibility and left ventricular mass. Am J Hypertens. 2004;17:37‐42. [DOI] [PubMed] [Google Scholar]
- 19. Ogunyankin KO, Day AG. Successful treatment of hypertension accounts for improvements in markers of diastolic function ‐ a pilot study comparing hydrochlorothiazide‐based and amlodipine‐based treatment strategies. Can J Cardiol. 2009;25:e406‐e412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okura T, Miyoshi K‐I, Irita J, et al. Comparison of the effect of combination therapy with an angiotensin II receptor blocker and either a low‐dose diuretic or calcium channel blocker on cardiac hypertrophy in patients with hypertension. Clin Exp Hypertens. 2013;35:563‐569. [DOI] [PubMed] [Google Scholar]
- 21. Scaglione R, Argano C, Di Chiara T, et al. Effect of dual blockade of renin‐angiotensin system on TGFbeta1 and left ventricular structure and function in hypertensive patients. J Hum Hypertens. 2007;21:307‐315. [DOI] [PubMed] [Google Scholar]
- 22. Scognamiglio R, Nosadini R, Marin M, et al. Evaluation of the efficacy and tolerability of nitrendipine in reducing both pressure and left ventricular mass in hypertensive type 2 diabetic patients. Diabetes Care. 1997;20:1290‐1292. [DOI] [PubMed] [Google Scholar]
- 23. Agabiti‐Rosei E, Zulli R, Muiesan ML, et al. Reduction of cardiovascular structural changes by nifedipine GITS in essential hypertensive patients. Blood Press. 1998;7:160‐169. [DOI] [PubMed] [Google Scholar]
- 24. Thürmann PA, Kenedi P, Schmidt A, et al. Influence of the angiotensin II antagonist valsartan on left ventricular hypertrophy in patients with essential hypertension. Circulation. 1998;98:2037‐2042. [DOI] [PubMed] [Google Scholar]
- 25. Martina B, Dieterle T, Weinbacher M, et al. Effects of losartan titrated to Losartan/Hydrochlorothiazide and amlodipine on left ventricular mass in patients with mild‐to‐moderate hypertension. A double‐blind randomized controlled study. Cardiology. 1999;92:110‐114. [DOI] [PubMed] [Google Scholar]
- 26. Kirpizidis HG, Papazachariou GS. Comparative effects of fosinopril and nifedipine on regression of left ventricular hypertrophy in hypertensive patients: a double‐blind study. Cardiovasc Drugs Ther. 1995;9:141‐143. [DOI] [PubMed] [Google Scholar]
- 27. Höglund C, Cifkova R, Mimran A, et al. A comparison of the effects of mibefradil and atenolol on regression of left ventricular hypertrophy in hypertensive patients. Cardiology. 1998;89:263‐270. [DOI] [PubMed] [Google Scholar]
- 28. Topouchian J, Asmar R, Sayegh F, et al. Changes in arterial structure and function under trandolapril‐verapamil combination in hypertension. Stroke. 1999;30:1056‐1064. [DOI] [PubMed] [Google Scholar]
- 29. Radevski I, Skudicky D, Candy G, et al. Antihypertensive monotherapy with nisoldipine CC is superior to enalapril in black patients with severe hypertension. Am J Hypertens. 1999;12:194‐203. [DOI] [PubMed] [Google Scholar]
- 30. Trenkwalder P, Dobrindt R, Aulehner R, et al. Antihypertensive treatment with felodipine but not with a diuretic reduces episodes of myocardial ischaemia in elderly patients with hypertension. Eur Heart J. 1994;15:1673‐1680. [DOI] [PubMed] [Google Scholar]
- 31. Tedesco MA, Ratti G, Aquino D, et al. Effects of losartan on hypertension and left ventricular mass: a long‐term study. J Hum Hypertens. 1998;12:505‐1032. [DOI] [PubMed] [Google Scholar]
- 32. Sihm I, Thygesen K, Krusell LR, et al. Long‐term renal and cardiovascular effects of antihypertensive treatment regimens based upon isradipine, perindopril and thiazide. Blood Press. 2000;9:346‐54. [DOI] [PubMed] [Google Scholar]
- 33. Roman MJ, Alderman MH, Pickering TG, et al. Differential effects of angiotensin converting enzyme inhibition and diuretic therapy on reductions in ambulatory blood pressure, left ventricular mass, and vascular hypertrophy. Am J Hypertens. 1998;11:387‐396. [DOI] [PubMed] [Google Scholar]
- 34. Papademetriou V, Gottdiener JS, Narayan P, et al. Hydrochlorothiazide is superior to isradipine for reduction of left ventricular mass: results of a multicenter trial. The Isradipine Study Group. J Am Coll Cardiol. 1997;30:1802‐1808. [DOI] [PubMed] [Google Scholar]
- 35. Manolis AJ, Beldekos D, Handanis S, et al. Comparison of spirapril, isradipine, or combination in hypertensive patients with left ventricular hypertrophy: effects on LVH regression and arrhythmogenic propensity. Am J Hypertens. 1998;11:640‐648. [DOI] [PubMed] [Google Scholar]
- 36. Devereux RB, Palmieri V, Sharpe N, et al. Effects of Once‐Daily Angiotensin‐Converting Enzyme Inhibition and Calcium Channel Blockade‐Based Antihypertensive Treatment Regimens on Left Ventricular Hypertrophy and Diastolic Filling in Hypertension: The Prospective Randomized Enalapril Study Evaluating Regression of Ventricular Enlargement (PRESERVE) Trial. Circulation. 2001;104:1248‐1254. [DOI] [PubMed] [Google Scholar]
- 37. Terpstra WF, May JF, Smit AJ. Long‐term effects of amlodipine and lisinopril on left ventricular mass and diastolic function in elderly, previously untreated hypertensive patients: the ELVERA trial. J Hypertens. 2001;19:303‐309. [DOI] [PubMed] [Google Scholar]
- 38. Agabiti‐Rosei E, Trimarco B, Muiesan ML, et al. Cardiac structural and functional changes during long‐term antihypertensive treatment with lacidipine and atenolol in the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2005;23:1091‐1098. [DOI] [PubMed] [Google Scholar]
- 39. Sakata K, Yoshida H, Tamekiyo H, et al. Comparative effect of clinidipine and quinapril on left ventricular mass in mild essential hypertension. Drugs Exp Clin Res. 2003;29:117‐123. [PubMed] [Google Scholar]
- 40. Devereux RB, Dahlöf B, Gerdts E, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456‐1462. [DOI] [PubMed] [Google Scholar]
- 41. Gosse P, Sheridan DJ, Zannad F, et al. Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg versus enalapril 20 mg: the LIVE study. J Hypertens. 2000;18:1465‐1475. [DOI] [PubMed] [Google Scholar]
- 42. Cesare Cuspidi M, Muiesan L, Valagussa L, et al. Comparative effects of candesartan and enalapril on left ventricular hypertrophy in patients with essential hypertension: the candesartan assessment in the treatment of cardiac hypertrophy (CATCH) study. J Hypertens. 2002;20:2293‐2300. [DOI] [PubMed] [Google Scholar]
- 43. Nalbantgil S, Yilmaz H, GyFn C, et al. Effects of Valsartan and Enalapril on Regression of Left Ventricular Hypertrophy in Patients with Mild to Moderate Hypertension: A Randomized, Double‐Blind Study. Curr Ther Res Clin Exp. 2000;61:331‐338. [Google Scholar]
- 44. Gaudio C, Ferri FM, Giovannini M, et al. Comparative effects of irbesartan versus amlodipine on left ventricular mass index in hypertensive patients with left ventricular hypertrophy. J Cardiovasc Pharmacol. 2003;42:622‐628. [DOI] [PubMed] [Google Scholar]
- 45. Koldas L, Ayan F, Ikitimur B. Short‐term effects of rilmenidine on left ventricular hypertrophy and systolic and diastolic function in patients with essential hypertension: comparison with an angiotensin converting enzyme inhibitor and a calcium antagonist. Jpn Heart J. 2003;44:693‐704. [DOI] [PubMed] [Google Scholar]
- 46. Galzerano D, Tammaro P, del Viscovo L, et al. Three‐dimensional echocardiographic and magnetic resonance assessment of the effect of telmisartan compared with carvedilol on left ventricular mass a multicenter, randomized, longitudinal study. Am J Hypertens. 2005;18:1563‐1569. [DOI] [PubMed] [Google Scholar]
- 47. Fountoulaki K, Dimopoulos V, Giannakoulis J, et al. Left ventricular mass and mechanics in mild‐to‐moderate hypertension: effect of nebivolol versus telmisartan. Am J Hypertens. 2005;18:171‐177. [DOI] [PubMed] [Google Scholar]
- 48. Schneider MP, Klingbeil AU, Delles C, et al. Effect of irbesartan versus atenolol on left ventricular mass and voltage: results of the CardioVascular Irbesartan Project. Hypertension. 2004;44:61‐66. [DOI] [PubMed] [Google Scholar]
- 49. Dahlof B, Zanchetti A, Diez J, et al. Effects of losartan and atenolol on left ventricular mass and neurohormonal profile in patients with essential hypertension and left ventricular hypertrophy. J Hypertens. 2002;20:1855‐1864. [DOI] [PubMed] [Google Scholar]
- 50. Yoshida C, Goda A, Naito Y, et al. Role of plasma aldosterone concentration in regression of left‐ventricular mass following antihypertensive medication. J Hypertens. 2011;29:357‐363. [DOI] [PubMed] [Google Scholar]
- 51. Dahlöf B, Gosse P, Guéret P, et al. Perindopril/indapamide combination more effective than enalapril in reducing blood pressure and left ventricular mass: the PICXEL study. J Hypertens. 2005;23:2063‐2070. [DOI] [PubMed] [Google Scholar]
- 52. Senior R, Imbs JL, Bory M, et al. Indapamide reduces hypertensive left ventricular hypertrophy: an international multicenter study. J Cardiovasc Pharmacol. 1993;22:S106‐S110. [PubMed] [Google Scholar]
- 53. Ranieri G, Taddei S, Filitti V, et al. The effects of diuretic and beta‐blocker treatment on cardiac and vascular structural changes in untreated essential hypertensive patients. J Hypertens Suppl. 1993;11:S368‐S369. [PubMed] [Google Scholar]
- 54. Schulte KL, Meyer‐Sabellek W, Liederwald K, et al. Relation of regression of left ventricular hypertrophy to changes in ambulatory blood pressure after long‐term therapy with perindopril versus nifedipine. Am J Cardiol. 1992;70:468‐473. [DOI] [PubMed] [Google Scholar]
- 55. Schiffrin EL, Deng LY, Larochelle P. Effects of a beta‐blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension. 1994;23:83‐91. [DOI] [PubMed] [Google Scholar]
- 56. Kuperstein R, Sasson Z. Effects of antihypertensive therapy on glucose and insulin metabolism and on left ventricular mass: A randomized, double‐blind, controlled study of 21 obese hypertensives. Circulation. 2000;102:1802‐1806. [DOI] [PubMed] [Google Scholar]
- 57. Malmqvist K, Kahan T, Isaksson H, et al. Regression of left ventricular mass with captopril and metoprolol, and the effects on glucose and lipid metabolism. Blood Press. 2001;10:101‐110. [DOI] [PubMed] [Google Scholar]
- 58. Yang K, Zhou H, Sun M. Effects of nitrendipine and captopril on the quality of life in hypertensive patients with left ventricular hypertrophy. J Hunan Med Univ. 1995;20:450‐452. [Google Scholar]
- 59. Nwabuo CC, Vasan RS. Pathophysiology of Hypertensive Heart Disease: Beyond Left Ventricular Hypertrophy. Curr Hypertens Rep. 2020;22(11). [DOI] [PubMed] [Google Scholar]
- 60. Kawel‐Boehm N, Kronmal R, Eng J, et al. Left Ventricular Mass at MRI and Long‐term Risk of Cardiovascular Events: The Multi‐Ethnic Study of Atherosclerosis (MESA). Radiology. 2019;293:107‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xing FuWei, Chen J, Zhao BinLiang, Jiang J, Tang A, Chen Y. Real role of β‐blockers in regression of left ventricular mass in hypertension patients. Medicine. 2017;96(10):e6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Salvetti M, Paini A, Bertacchini F, et al. Changes in Left Ventricular Geometry During Antihypertensive Treatment. Pharmacol Res. 2018;134:193‐199. [DOI] [PubMed] [Google Scholar]
- 63. Song F, Xiong T, Parekh‐Bhurke S, et al. Inconsistency Between Direct and Indirect Comparisons of Competing Interventions: Meta‐Epidemiological Study. BMJ. 2011;343:d4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jansen JP, Crawford B, Bergman G, et al. Bayesian Meta‐analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11:956‐964. [DOI] [PubMed] [Google Scholar]
- 65. Budzyń M, Gryszczyńka B, Boruczkowski M, et al. The Potential Role of Circulating Endothelial Cells and Endothelial Progenitor Cells in the Prediction of Left Ventricular Hypertrophy in Hypertensive Patients. Front Physiol. 2019;10:1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Travers JG, Kamal FA, Robbins J, et al. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016;118:1021‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu J, Carretero OA, Liao TD, et al. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Konstantin G, Domenighetti AA, Delbridge LMD, et al. Angiotensin II‐mediated adaptive and maladaptive remodeling of cardiomyocyte excitation‐contraction coupling. Circ Res. 2009;105:42‐50. [DOI] [PubMed] [Google Scholar]
- 69. Majd AG, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chevalier B, Callens‐el Amrani F, Heymes C, et al. Molecular Basis of Regression of Cardiac Hypertrophy. Am J Cardiol. 1994;73:10C‐17C. [DOI] [PubMed] [Google Scholar]
- 71. Daniel H, Zappe DH, Meyer MAR, et al. Changes in aortic pulse wave velocity in hypertensive postmenopausal women: comparison between a calcium channel blocker vs angiotensin receptor blocker regimen. J Clin Hypertens. 2012;14:773‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim SY, Joo SJ, Shin MS, et al. Clinic and home blood pressure lowering effect of an angiotensin receptor blocker, fimasartan, in postmenopausal women with hypertension. Medicine. 2016;95:e3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Serfozo P, Wysocki J, Gulua G, et al. Ang II (Angiotensin II) Conversion to Angiotensin‐(1–7) in the Circulation Is POP (Prolyloligopeptidase)‐Dependent and ACE2 (Angiotensin‐Converting Enzyme 2)‐Independent. Hypertension. 2020;75:173‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Urwyler SA, Ebrahimi F, Burkard T, et al. IL (Interleukin)‐1 Receptor Antagonist Increases Ang (Angiotensin [1–7]) and Decreases Blood Pressure in Obese Individuals. Hypertension. 2020;75(6):1455‐1463. [DOI] [PubMed] [Google Scholar]
- 75. Choe S‐H, Choi E‐Y, Hyeon J‐Y, et al. Telmisartan, an Angiotensin II Receptor Blocker, Attenuates Prevotella Intermedia Lipopolysaccharide‐Induced Production of Nitric Oxide and interleukin‐1β in Murine Macrophages. Int Immunopharmacol. 2019;75:105750. [DOI] [PubMed] [Google Scholar]
- 76. Kilic A, Ustunova S, Usta C, et al. Angiotensin II Type 2 Receptor Blocker PD123319 Has More Beneficial Effects Than Losartan on Ischemia‐Reperfusion Injury and Oxidative Damage in Isolated Rat Heart. Can J Physiol Pharmacol. 2019;97:1124‐1131. [DOI] [PubMed] [Google Scholar]
- 77. Murphy A, LeVatte T, Boudreau C, et al. Angiotensin II Type I Receptor Blockade Is Associated with Decreased Cutaneous Scar Formation in a Rat Model. Plast Reconstr Surg. 2019;144:803e‐813e. [DOI] [PubMed] [Google Scholar]
- 78. Tamura T, Said S, Harris J, Lu W, Gerdes AM. Reverse remodeling of cardiac myocyte hypertrophy in hypertension and failure by targeting of the renin‐angiotensin system. Circulation. 2000;102:253‐259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material


