Abstract
An elevated heart rate increases the risk of cardiovascular disease, but the relationship between resting heart rate (RHR) and the risk of heart failure (HF) in hypertensive patients is unclear. This study was performed to assess the relationship between elevated RHR and incident HF in hypertensive patients. In total, 16 286 hypertensive patients from the Kailuan cohort were enrolled and underwent three physical examinations. According to mean RHR based on quartile, the hypertensive patients were divided into four groups: Q1 (mean RHR ≤ 69 bpm), Q2 (69 bpm < mean RHR ≤ 74 bpm), Q3 (74 bpm < mean RHR ≤ 79 bpm), and Q4 (mean RHR > 79 bpm). The cumulative mortality rate was analyzed by using the Kaplan–Meier method, with comparisons among RHR quartiles. Cox proportional hazards regression models and restricted cubic spline models were established to evaluate the association between RHR and risk of incident HF. After adjustment for confounders, the hazard ratio (HR) for HF was 1.97(95% CI: 1.28‐3.04, P < .001) in the fourth quartile compared to the first quartile. Each 1‐standard deviation [10 (beats/min)] increase in RHR was associated with a 40% increase in the risk of incident HF. Restricted cubic spline models presented a linear relationship between RHR and incident HF. Our study suggests that elevated RHR is associated with an enhanced risk of HF in hypertensive patients.
Keywords: heart failure, heart rate, hypertension
1. INTRODUCTION
Resting heart rate (RHR) is an easily accessible clinical indicator that is closely related to the activity of the sympathetic nervous system. 1 Previous studies have shown that elevated RHR is associated with enhanced risks of cardiovascular diseases and all‐cause mortality in the general population, as well as in patients with coronary heart disease (CVD) or heart failure (HF). 2 , 3 , 4 , 5 In addition, the CRUSADE study reported that reduction of heart rate may improve outcomes in patients with coronary heart disease or HF. 6 To the best of our knowledge, there have been few studies regarding the impact of heart rate on HF in hypertensive patients. Hypertension is a major risk factor for HF; additionally, most HF patients have history of hypertension. 7 The VALUE trial showed that elevated RHR was related to an enhanced risk of HF in treated hypertensive patients, including those with well‐controlled blood pressure. 8 However, the relationship between RHR and new‐onset HF in patients with hypertension has not been investigated in an Asian population. Therefore, we explored the association between RHR and the risk of HF in data from the prospective Kailuan Cohort Study (clinical trial no. ChiCTR‐TNC‐11001489).
2. METHODS
2.1. Population
The present study was based on the Kailuan Cohort Study, a prospective cohort study that was carried out from June 2006 to October 2007 and included 101 510 participants aged 18‐98 years in the Kailuan community in Tangshan, China. Follow‐up assessments were performed at 2‐year intervals. The details of the Kailuan Cohort Study have been described previously. 9 , 10 , 11 , 12 The present study included participants who underwent biennial check‐up examinations in 2006/2007, 2008/2009, and 2009/2010, all of whom exhibited hypertension (blood pressure [BP] ≥140/90 mm Hg, current use of antihypertensive therapy, or physician diagnosis of hypertension). The exclusion criteria were as follows: (a) arrhythmia (eg, atrial fibrillation, atrial flutter, atrial premature beat, ventricular ectopic beats, and/or atrioventricular block); (b) use of β‐adrenergic blocking agents; (c) a history of cardiovascular diseases (eg, HF, myocardial infarction, or stroke).
2.2. Data collection
Information regarding demographic variables (eg, age, sex, history of antihypertensive or hypoglycemic drug use, smoking status, and drinking status) was collected via questionnaires; measurements of height, weight, and biochemical parameters were described previously. 13 , 14 BP was measured at each patient's right upper arm by trained stuff using a corrected mercury sphygmomanometer with an appropriately sized cuff, after the patient had rested in the sitting position for at least 5 minutes. Three consecutive measurements were performed at measurement intervals of 1‐2 min; the mean value of the three measurements was recorded for analysis. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine Equation. 15
2.3. Assessment of mean RHR
RHR was measured by means of a 10‐s 12‐lead electrocardiogram (ECG9130P, NIHON KOHDEN CORP, Japan) at baseline, after the patient had rested in the supine position for at least 5 minutes. The number of R–R intervals (number of QRS complexes – 1) was divided by the time difference between the first and last beat; the results were converted to beats per minute (bpm), as previously reported. 16 The following equations were utilized to calculate the mean RHR:
The abbreviations RHR2006, RHR2008, and RHR2010 indicate the RHR at the first (2006/2007), second (2007/2008), and third (2009/2010) examinations. Mean RHR values were grouped into quartiles as follows: Q1 (mean RHR ≤ 69 bpm), Q2(69 bpm < mean RHR ≤ 74 bpm), Q3(74 bpm < mean RHR ≤ 79 bpm), and Q4(mean RHR > 79 bpm).
2.4. Definitions
Patients who smoked were regarded as those who had smoked at least one cigarette per day in the past year. Patients who drank alcohol were regarded as those who had an average daily wine (ie, alcohol content 50%) intake of approximately 100 mL in the past year. Physical exercise was defined as aerobic exercise > 3 sessions per week, >30 minutes per session. Histories of hypertension, diabetes, heart failure, stroke, and myocardial infarction were ascertained by self‐reported physician diagnosis. Hypertension was defined as self‐reported use of antihypertensive medication, history of hypertension, systolic BP ≥ 140 mm Hg, or diastolic BP ≥ 90 mm Hg. Diabetes mellitus was defined as a fasting blood glucose level of ≥ 7.0 mmol/L, history of diabetes, and/or use of antidiabetic drugs. Obesity was defined as BMI ≥ 28 kg/m2. 17 Age was recorded as the patient's age at the date of the first physical examination (ie, in 2006/2007).
To further explore the relationship between RHR and HF in patients with well‐controlled BP, patients were divided into two groups—BP‐controlled (mean BP < 140/90 mm Hg) and BP‐uncontrolled (mean BP ≥ 140/90 mm Hg)—according to the average BP among the three examinations (ie, 2006/2007, 2008/2009, and 2009/2010 examinations); the effect of RHR on incident HF was compared between the two groups.
2.5. Follow‐up and primary outcomes
The participants were followed from the 2009/2010 examination until December 31, 2016, or until the diagnosis of HF. The method for diagnosis of heart failure was described previously. 18 Incident HF was defined in accordance with the criteria of the European Society of Cardiology 19 on the basis of clinical symptoms, echocardiography, chest X‐ray, and electrocardiography. The diagnosis of HF was also confirmed based on an annual review of each patient's medical records by a team of experienced cardiologists.
2.6. Statistics
Continuous variables are shown as mean ± standard deviation; categorical variables are shown as percentages. Characteristics were compared among the four groups by using analysis of variance (ANOVA) for continuous variables and the chi‐squared test for categorical variables. Kaplan–Meier analysis and the log‐rank test were used to compare the cumulative incidence of HF among the four groups. The hazard ratio (HR) and 95% confidence interval (95% CI) for HF were calculated by using multivariate Cox regression and restricted cubic spline models. To exclude the effects of antihypertensive drugs on the results, a sensitivity analysis was conducted by excluding participants who received antihypertensive drugs. To address the possibility of reverse causation, the first 1 year of follow‐up was excluded and the analyses were repeated. The analyses for HF were repeated within different subgroups, according to age (<45, 45‐65, and ≥ 65 years), blood pressure control (<140/90 mm Hg and ≥ 140/90 mm Hg), smoking status, and drinking status. All statistical analyses were performed using SAS statistical software, version 9.3 (SAS Institute).
3. RESULTS
3.1. Baseline characteristics
A total of 16 286 patients were included in our study (13 575 men [83.35%] and 2711 women [16.65%]) (Figure 1). The patients’ clinical characteristics are shown in Table 1. The patients were stratified into quartiles based on mean RHR, as described in the Methods section. We found that systolic blood pressure (SBP), diastolic blood pressure (DBP), low‐density lipoprotein cholesterol (LDL), triglycerides (TGs), and prevalence of diabetes all increased as RHR increased (P < .001). The baseline characteristics of the BP‐controlled and BP‐uncontrolled groups are shown in Supplemental Table S1. Echocardiographic indexes of heart failure patients are shown in Supplemental Table S2.
Figure 1.
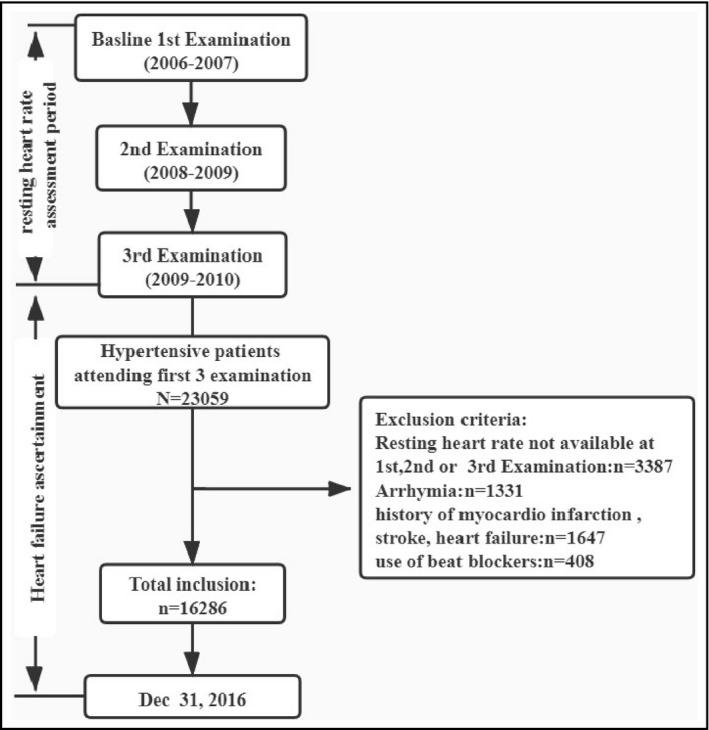
Flowchart of Kailuan Cohort Study
Table 1.
Baseline characteristics of the participants according to resting heart rate
|
Q1 N = 3993 |
Q2 N = 4044 |
Q3 N = 4220 |
Q4 N = 4029 |
P | |
|---|---|---|---|---|---|
| Sex(%) | 82.72 | 81.87 | 83.46 | 85.36 | <.01 |
| Age (years) | 53.93 ± 10.56 | 51.87 ± 10.66 | 51.08 ± 10.65 | 49.99 ± 10.67 | <.01 |
| RHR (beats/min) | 65.37 ± 3.58 | 71.94 ± 1.33 | 76.72 ± 1.57 | 86.09 ± 5.84 | <.01 |
| SBP (mmHg) | 140.99 ± 14.38 | 141.56 ± 14.35 | 143.04 ± 14.26 | 145.48 ± 14.92 | <.01 |
| DBP (mmHg) | 88.50 ± 7.53 | 90.12 ± 7.69 | 91.20 ± 8.05 | 92.69 ± 8.47 | <.01 |
| BMI (kg/m2) | 26.07 ± 3.26 | 26.12 ± 3.39 | 26.14 ± 3.45 | 26.03 ± 3.56 | .44 |
| TG (mmol/L) | 1.78 ± 1.34 | 1.89 ± 1.48 | 2.00 ± 1.60 | 49.99 ± 10.68 | <.01 |
| LDL (mmol/L) | 2.33 ± 1.06 | 2.34 ± 0.95 | 2.34 ± 0.94 | 2.50 ± 0.91 | <.01 |
| HDL (mmol/L) | 1.57 ± 0.41 | 1.58 ± 0.41 | 1.58 ± 0.41 | 1.62 ± 0.42 | <.01 |
| FBG (mmol/L) | 5.33 ± 1.33 | 5.46 ± 1.56 | 5.66 ± 1.82 | 5.98 ± 2.07 | <.01 |
| Diabetes (%) | 10.37 | 12.14 | 14.81 | 20.33 | <.01 |
| Use of antihypertensive (%) | 35.64 | 33.53 | 34.08 | 36.19 | <.05 |
| Smoke (%) | 36.19 | 36.98 | 37.60 | 43.14 | <.01 |
| Drink (%) | 39.98 | 37.89 | 38.44 | 43.60 | <.01 |
| Physical exercise (%) | 20.57 | 17.29 | 14.01 | 12.86 | <.01 |
Q1, mean RHR ≤ 69 bpm; Q2, 69 bpm < mean RHR ≤ 74 bpm; Q3, 74 bpm < mean RHR ≤ 79 bpm; Q4, mean RHR > 79 bpm.
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; RHR, resting heart rate; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride.
3.2. Incidence of incident HF according to RHR
During 5.86 ± 0.86 years of follow‐up in this study, 196 patients were diagnosed with new‐onset HF. The numbers of patients with incident HF and corresponding cumulative incidence in each subgroup were 38 (0.95%), 46 (1.14%), 55 (1.30%), 57 (1.41%), respectively. Log‐rank test analysis revealed that the respective incidences of HF were 1.62, 1.94, 2.21, and 2.42 per 1000 person‐years in groups Q1, Q2, Q3, and Q4 (Figure 2).
Figure 2.

Hazard ratios and 95% CIs of heart failure according to resting heart rate. Model 1 includes adjustments for age and sex. Model 2 includes adjustments for age, sex, SBP, DBP, HDL, LDL, smoking status, drinking status, physical exercise, BMI, diabetes status, use of antihypertensive agents, and eGFR. Abbreviations: bpm, beats per minute; CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; eGFR, estimated glomerular filtration rate
3.3. Relationship between RHR and risk of HF
Figure 2 presents the results of Cox survival analyses regarding the relationship between RHR and the risk of incident HF. After adjustments for age, sex, SBP, DBP, high‐density lipoprotein cholesterol (HDL), LDL, BMI, smoking status, drinking status, diabetes status, and use of antihypertensive drugs, the HR for the risk of incident HF in the Q4 group was 1.97 (95% CI: 1.28‐3.04, P < .001), compared with the Q1 group. The predictive values of RHR for HF in the major subgroups are shown in Figure 3. In analyses adjusted for multiple risk factors, there was a linear association between RHR and HF. Similar associations were also observed when patients were grouped according to age, BP control, obesity status, smoking status, and drinking status (Figure 3).
Figure 3.

Subgroup analyses of association between resting heart rate and heart failure stratified by age, sex, diabetes, blood pressure control. Hazard ratios and 95% CIs of heart failure by resting heart rate. HR is calculated for a per‐10 bpm increase in RHR. Adjusted for age, sex, low‐density lipoprotein, higher‐density lipoprotein, physical activity, drinking, smoking, body mass index, systolic blood pressure, history of diabetes, and antihypertensive treatment
The association between RHR and HF was modeled using multivariable‐adjusted spline regression models with the reference point set at 79 bpm and the results revealed a significant linear dose‐response relationship between heart rate and HF, such that an RHR ≥ 79 bpm was associated with an elevated risk of HF (Figure 4).
Figure 4.

Cubic spline graph of the adjusted HR and 95% CI for the association between RHR and HF. Note: The adjusted cubic spline model demonstrates the relationship between RHR and incident HF when a resting heart rate of 79 bpm is the reference. The red line represents hazard ratio and blue lines represent the upper and lower 95% confidence limits
3.4. Sensitivity analysis
Given that antihypertensive drugs influence RHR, we excluded patients who were taking antihypertensive drugs. In sensitivity analyses, the significant relationship between RHR and HF remained, despite adjustments for potential confounders including age, sex, SBP, DBP, HDL, LDL, BMI, smoking status, drinking status, diabetes status, and use of antihypertensive drugs. Individuals in the Q4 group had a 2.13‐fold greater risk (95% CI: 1.10‐4.11, P < .05) of HF. Similar results were observed after exclusion of the first 1 year of follow‐up (Figure 2, Supplemental Table S3).
4. DISCUSSION
To the best of our knowledge, this is the first prospective study to investigate the relationship between RHR and the risk of HF among hypertensive patients in China. We found that elevated RHR was predictive of an enhanced risk of HF in hypertensive patients during a median follow‐up period of 6.06 years. In addition, we found that the risk of HF increased with increasing RHR in untreated hypertensive patients.
Heart rate is a known prognostic marker of cardiovascular diseases. A previous study showed that, in treated hypertensive patients, the risk of new HF increased by 1.24‐fold for each 10‐bpm increment in baseline HR. 8 In a similar study, Okin et al 20 showed that each 10‐bpm increase in RHR was associated with a 45% increase in the risk of new HF. Our results were consistent with the findings of those two previous studies, in that elevated RHR was identified as a risk factor for HF in hypertensive patients. Moreover, we found that elevated RHR had a greater impact on HF in hypertensive patients in the BP‐controlled group, compared with the BP‐uncontrolled group, which indicated that RHR may be a residual risk factor for HF in hypertensive patients who have well‐controlled blood pressure. In addition, we evaluated the effect of RHR on the risk of new HF in hypertensive patients without treatment, which revealed similar results. Additionally, each 10‐bpm elevation of RHR in younger patients (<45 years) was associated with an increased risk of HF, in comparison of middle‐aged (45‐60 years) and elderly (≥60 years) patients, which indicated that young patients with an elevated heart rate may be at greater risk of incident HF. Our findings highlight the importance of controlling heart rate at a young age. Notably, we also found that patients with RHR > 79 bpm had an enhanced risk of new‐onset HF. Therefore, we speculate that RHR > 79 bpm may be a suitable threshold for therapeutic intervention in patients with hypertension in China.
Currently, heart rate management in hypertensive patients is supported by the 2018 ESC/ESH guidelines for the management of arterial hypertension, which suggests that RHR > 80 bpm is a risk factor for poor cardiovascular disease prognosis in hypertensive patients. 6 However, the 2018 Guidelines for Prevention and Treatment of Hypertension in China did not recommend elevated RHR as a cardiovascular prognosis risk factor in hypertensive patients. 21 The findings of the BEAUTIFUL 22 and SHIFT 23 studies have shown that lowering HR can effectively reduce the risk of poor cardiovascular outcomes in patients with coronary artery disease or HF. However, it is unclear whether a therapeutic reduction of heart rate can improve cardiovascular prognosis in hypertensive patients. Therefore, the effect of RHR intervention on HF in hypertensive patients should be confirmed by randomized controlled clinical trials.
The mechanism underlying the impact of RHR on HF in hypertensive patients remains unclear. There are several possible explanations. First, RHR is an indicator of autonomic nervous function; thus, elevated RHR may be related to sympathetic hyperexcitability. Sympathetic excitation increases the epinephrine level and causes elevations of blood pressure, blood fat, and blood sugar, which can lead to the development of coronary atherosclerosis and increase the risk of HF. 24 , 25 , 26 , 27 Second, long‐term elevation of heart rate can cause greater shear stress, which induces endothelial gene expression and affects cell signaling pathways, leading to vascular oxidative stress, endothelial dysfunction, and vascular wall structural changes. 28 , 29 , 30 , 31 Third, an elevated heart rate has been shown to amplify the effects of inflammation on the progression of arteriosclerosis, by means of increasing the levels of inflammatory factors such as chemoattractants and cytokines. 32
The strength of the present study is that it constitutes the first prospective cohort study regarding the relationship between RHR and incident HF in Chinese hypertensive patients with a long‐term follow‐up period. The results were based on repeated measurements of RHR, whereas most previous studies used single measurements of RHR. However, this study also had several limitations. First, there may have been potential confounders that were not considered in the analysis. Second, this study used an observational design. However, considering the large sample size and long‐term follow‐up duration, the findings of this study provide a basis for management of heart rate in hypertensive patients. Third, our study included more men than women. However, the sex distribution in our study was similar to that of the original Kailuan Cohort Study.
5. CONCLUSION
Elevated RHR is an independent risk factor for HF in Chinese hypertensive patients. Further prospective drug intervention studies are needed to investigate the effect of RHR reduction on the risk of HF.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
MaoXiang Zhao had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. MaoXiang Zhao, Yanming Chen, Miao Wang, Chi Wang, Siyu Yao, Yao Li, Sijin Zhang, and Cuijuan Yun involved in concept and design. MaoXiang Zhao, Shouling Wu, and Hao Xue involved in acquisition, analysis, or interpretation of data. MaoXiang Zhao, Yanming Chen, Shouling Wu, and Hao Xue drafted the manuscript. All authors made critical revision of the manuscript for important intellectual content. MaoXiang Zhao made statistical analysis. Shouling Wu and Hao Xue supervised the study.
ETHICAL APPROVAL
IRB: This was approved by the Medical Ethics Committee of Kailuan General Hospital (approval no. [2006]5).
Supporting information
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
We thank all the members of the Kailuan Study Group for their contribution and the participants who contributed their data. We thank Ryan Chastain‐Gross, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Zhao M, Chen Y, Wang M, et al. Relationship between resting heart rate and incident heart failure in patients with hypertension: The Kailuan Cohort Study in China. J Clin Hypertens 2020;22:2325–2331. 10.1111/jch.14062
Trials registry number: ChiCTR‐TNC‐11001489.
Contributor Information
Shouling Wu, Email: xuehaoxh301@163.com, Email: drwusl@163.com.
Hao Xue, Email: xuehaoxh301@163.com.
REFERENCES
- 1. Lahiri M.K., Kannankeril P.J., Goldberger J.J.. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51(18):1725‐1733. [DOI] [PubMed] [Google Scholar]
- 2. Pfister R., Michels G., Sharp S.J., Luben R., Wareham N.J., Khaw K.T.. Resting heart rate and incident heart failure in apparently healthy men and women in the EPIC‐Norfolk study. Eur J Heart Fail. 2012;14(10):1163‐1170. [DOI] [PubMed] [Google Scholar]
- 3. Rautaharju P.M., Prineas R.J., Wood J., Zhang Z.M., Crow R., Heiss G.. Electrocardiographic predictors of new‐onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol. 2007;100(9):1437‐1441. [DOI] [PubMed] [Google Scholar]
- 4. Diaz A., Bourassa M.G., Guertin M.C., Tardif J.C.. Long‐term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967‐974. [DOI] [PubMed] [Google Scholar]
- 5. Mauss O., Klingenheben T., Ptaszynski P., Hohnloser S.H.. Bedside risk stratification after acute myocardial infarction: prospective evaluation of the use of heart rate and left ventricular function. J Electrocardiol. 2005;38(2):106‐112. [DOI] [PubMed] [Google Scholar]
- 6. Bangalore S., Messerli F.H., Ou F.S., et al. The association of admission heart rate and in‐hospital cardiovascular events in patients with non‐ST‐segment elevation acute coronary syndromes: results from 135 164 patients in the CRUSADE quality improvement initiative. Eur Heart J. 2010;31(5):552‐560. [DOI] [PubMed] [Google Scholar]
- 7. Williams B., Mancia G., Spiering W., et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G Ital Cardiol (Rome). 2018;19(11):3‐73. [DOI] [PubMed] [Google Scholar]
- 8. Julius S., Palatini P., Kjeldsen S.E., et al. Usefulness of heart rate to predict cardiac events in treated patients with high‐risk systemic hypertension. Am J Cardiol. 2012;109(5):685‐692. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Q., Zhou Y., Gao X., et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44(9):2451‐2456. [DOI] [PubMed] [Google Scholar]
- 10. Liu X., Cui L., Wang A., et al. Cumulative exposure to ideal cardiovascular health and incident diabetes in a Chinese population: the Kailuan study. J Am Heart Assoc. 2016;5(9):e004132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu J., Chen S., Zhou Y., et al. Non‐high‐density lipoprotein cholesterol on the risks of stroke: a result from the Kailuan study. PLoS One. 2013;8(9):e74634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dai H., Lu Y., Song L., et al. Visit‐to‐visit Variability of Blood Pressure and Risk of Stroke: Results of the Kailuan Cohort Study. Sci Rep. 2017;7(1):285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y., Jin C., Xing A., et al. Serum uric acid levels and the risk of impaired fasting glucose: a prospective study in adults of north China. PLoS One. 2013;8(12):e84712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang A., Wu J., Zhou Y., et al. Measures of adiposity and risk of stroke in China: a result from the Kailuan study. PLoS One. 2013;8(4):e61665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey A.S., Stevens L.A., Schmid C.H., et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang A., Liu X., Guo X., et al. Resting heart rate and risk of hypertension: Results of the Kailuan cohort study. J Hypertens. 2014;32(8):1600‐1605. [DOI] [PubMed] [Google Scholar]
- 17. Zhou B.F.. Cooperative Meta‐Analysis Group of the Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut‐of points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15:83‐96. [PubMed] [Google Scholar]
- 18. Zhijun W.U., Cheng J., Anand V., et al. Longitudinal Patterns of Blood Pressure, Incident Cardiovascular Events, and All‐Cause Mortality in Normotensive Diabetic People. Hypertension. 2016;68:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swedberg K., Cleland J., Dargie H., et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(11):1115‐1140. [DOI] [PubMed] [Google Scholar]
- 20. Okin P.M., Kjeldsen S.E., Julius S., Hille D.A., Dahlöf B., Devereux R.B.. Effect of changing heart rate during treatment of hypertension on incidence of heart failure. Am J Cardiol. 2012;109(5):699‐704. [DOI] [PubMed] [Google Scholar]
- 21. Writing Group of 2018 Chinese Guidelines for the Management of Hypertension . Guidelines for the prevention and treatment of hypertension in China 2018. Prev Treat Cardio Cerebral Vasc Dis. 2019;19(1):1‐45. [Google Scholar]
- 22. Fox K., Ford I., Steg P.G., Tendera M., Robertson M., Ferrari R.. Heart rate as a prognostic risk factor in patients with coronary artery disease and left‐ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomized controlled trial. Lancet. 2008;372(9641):817‐821. [DOI] [PubMed] [Google Scholar]
- 23. Karl S., Michel K., Michael B., et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo‐controlled study. Lancet. 2010;376(9758):2069‐2070. [DOI] [PubMed] [Google Scholar]
- 24. Jamerson K.A., Julius S., Gudbrandsson T., Andersson O., Brant D.O.. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21(5):618‐623. [DOI] [PubMed] [Google Scholar]
- 25. Palatini P., Mos L., Santonastaso M., et al. Resting heart rate as a predictor of body weight gain in the early stage of hypertension. Obesity. 2011;19(3):618‐623. [DOI] [PubMed] [Google Scholar]
- 26. Reaven G.M., Lithell H., Landsberg L.. Hypertension and associated metabolic abnormalities–the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334(6):374‐381. [DOI] [PubMed] [Google Scholar]
- 27. Aune D., Sen A., ó'Hartaigh B., et al. Resting heart rate and the risk of cardiovascular disease, total cancer, and all‐cause mortality ‐ A systematic review and dose‐response meta‐analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017;27:504‐517. [DOI] [PubMed] [Google Scholar]
- 28. Palatini P.. Role of elevated heart rate in the development of cardiovascular disease in hypertension. Hypertension. 2011;58(5):745‐750. [DOI] [PubMed] [Google Scholar]
- 29. Shimazu T.. Innervation of the liver and glucoregulation: roles of the hypothalamus and autonomic nerves. Nutrition. 1996;12(1):65‐66. [DOI] [PubMed] [Google Scholar]
- 30. Nonogaki K.. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533‐549. [DOI] [PubMed] [Google Scholar]
- 31. Böhm M., Reil J.C., Deedwania P., Kim J.B., Borer J.S.. Resting heart rate: risk indicator and emerging risk factor in cardiovascular disease. Am J Med. 2015;128:219‐228. [DOI] [PubMed] [Google Scholar]
- 32. O Hartaigh B., Bosch J.A., Carroll D., et al. Evidence of a synergistic association between heart rate, inflammation, and cardiovascular mortality in patients undergoing coronary angiography. Eur Heart J. 2013;34:932‐941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2
Table S3


