Abstract
The prognostic value of arterial stiffness in Korean hypertensive patients has not been specified. This study was performed to evaluate the prognostic value of brachial‐ankle pulse wave velocity (baPWV) in Korean hypertensive patients and to propose a cutoff value of baPWV predicting future cardiovascular events. A total of 2561 hypertensive patients without documented cardiovascular disease (mean age, 63 years; 47% females) who underwent baPWV measurement were retrospectively analyzed. Composite events of cardiac death, non‐fatal myocardial infarction, coronary revascularization, and ischemic stroke were assessed during the clinical follow‐up period. During a median follow‐up period of 4.14 years (interquartile range, 2.18‐5.48 years), there were 69 cases of composite events (2.7%). In receiver operating characteristic curve analysis, the best cutoff value of baPWV predicting composite events was 1630 cm/s (sensitivity, 79.7%; specificity, 49.1%; area under curve, 0.674; 95% confidence interval [CI], 0.613‐0.735; P < .001). In multivariable Cox regression analysis, higher baPWV (≥1630 cm/s) was independently associated with higher risk of the occurrence of composite events even after controlling for potential confounders (hazard ratio, 2.83; 95% confidence interval, 1.49‐5.36; P = .001). In conclusion, increased baPWV in Korean hypertensive patients was associated with an increased risk of future cardiovascular events. In addition, we suggested 1630 cm as a cutoff value of baPWV for predicting cardiovascular events, which will be helpful in guiding the treatment strategy of Korean hypertensive patients.
Keywords: arterial stiffness, brachial‐ankle pulse wave velocity, cutoff value, hypertension, prognosis
1. INTRODUCTION
Hypertension is a disease that has a high prevalence and is closely related to the occurrence of cardiovascular disease, which poses a huge burden on mankind. 1 , 2 Therefore, early detection and active treatment of hypertension are very important for improving patient’ prognosis. 3 Additionally, it would be beneficial to find factors that can predict future cardiovascular events and use them for risk classification in hypertensive patients.
A long‐lasting increase in blood pressure causes histological changes in the arterial wall, which increases arterial stiffness. 4 Measurement of arterial stiffness is clinically important, since arterial stiffness is associated with patients’ future cardiovascular events, independent of traditional cardiovascular risk factors. 5 , 6 , 7 , 8 Recent guidelines recommend the measurement of arterial stiffness in the initial evaluation of hypertensive patients, 9 , 10 , 11 and several studies have reported that arterial stiffness is an important factor determining the prognosis of hypertensive patients. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 There are various methods for measuring arterial stiffness; however, pulse wave velocity (PWV) is non‐invasive and relatively simple to measure, making it widely used clinically or for research purposes. 20 The cutoff values of PWV in the prediction of cardiovascular events in hypertensive patients have been presented in a few studies. 12 , 18 Suggesting this cutoff value is very important, because it provides an opportunity for clinicians facing patients to make better use of PWV. The cutoff value depends on PWV measurement methods and races and patient characteristics. Although PWV has been increasingly used in Korea, studies on the usefulness of PWV for predicting the prognosis of hypertensive patients have not been conducted. Therefore, the purpose of this study was to evaluate the prognostic value of PWV in Korean hypertensive patients and to propose a cutoff value of PWV predicting future cardiovascular events.
2. METHODS
2.1. Study population
This is a single‐center and retrospective study, performed at a general hospital of a big city (Seoul, Korea). Between January 2008 and June 2018, hypertensive patients aged between 19 and 90 years who underwent the measurement of brachial‐ankle pulse wave velocity (baPWV) were eligible to this study. Measurement of baPWV was performed as part of a general cardiovascular examination in our hospital. Initially, 3527 patients were screened, but those with following conditions were excluded: (a) prior history of documented cardiovascular disease including coronary revascularization and myocardial infarction, and stroke; (b) ankle‐brachial index < 0.9 or > 1.4; (c) uncontrolled arrhythmia; (d) significant valvular heart disease greater than mild degree of regurgitation or stenosis; and (e) presence of pericardial effusion. Finally, 2561 hypertensive patients were analyzed in this study. The study protocol was approved by the Institutional Review Board of Boramae Medical Center (Seoul, Korea), and informed consent was waived due to its retrospective study design and the routine nature of information collected.
2.2. Clinical data collection
Baseline clinical data were obtained based on when the patient was first tested for baPWV. Body mass index was calculated as body weight divided by height in square meters (kg/m2). Body mass index ≥ 25 kg/m2 was considered as having obesity. 21 Hypertension was defined on the basis of (a) previous diagnosis of hypertension, (b) current anti‐hypertensive medications, or (c) systolic/diastolic blood pressure ≥140/90 mm Hg. Diabetes mellitus was defined on the basis of (a) previous diagnosis of diabetes mellitus, (b) current anti‐diabetic medications, or (c) fasting blood glucose level ≥126 mg/dL in repeated tests. Dyslipidemia was defined on the basis of (a) previous diagnosis of dyslipidemia, (b) current anti‐dyslipidemic medications, or (c) low‐density lipoprotein cholesterol >130 mg/dL. A person who smoked regularly within the last year was defined as a smoker. After overnight fasting, blood levels of following laboratory parameters were obtained: white blood cell count, hemoglobin, creatinine, glucose, glycated hemoglobin, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol triglyceride, and C‐reactive protein. Estimated glomerular filtration rate was calculated using Modification of Diet in Renal Disease (MDRD) Study equation. 22 Information on concomitant cardiovascular medications including calcium channel blocker, beta blocker, renin‐angiotensin system blocker, diuretics, and statin was also obtained.
2.3. baPWV measurement
The baPWV measurement method has been published elsewhere. 23 , 24 Briefly, the test subjects restricted smoking, drinking, and caffeine‐containing beverages on the day of baPWV measurement, but maintained medications that were normally taken on a regular basis. The patient lay still in a quiet room for at least 5 minutes before the examination. For baPWV measurement, a commercially available volume‐plethysmographic apparatus (VP‐1000; Colin Co., Ltd., Komaki, Japan) was used. Electrocardiographic electrodes were applied to both wrists, phonocardiographic electrodes were placed on the edge of the sternum to detect heart sounds, and pneumatic cuffs were wrapped on both upper arms and ankles. PWV was calculated by dividing distance by transit time. The distance between measurement points of baPWV was estimated by subject height. Transit time was calculated from the start point of the brachial pulse wave to the start of the ankle pulse wave. The average value of left and right baPWV measurements was used for the study. The coefficient of variance for interobserver reliability of baPWV was 5.1% in our laboratory. 24
2.4. Assessment of cardiovascular events
The primary end point of this study was the composite of cardiac death, non‐fatal myocardial infarction, coronary revascularization, and ischemic stroke. Cardiac death was defined as death caused by acute myocardial infarction, fatal ventricular arrhythmia, and pump failure. Unexplained sudden death was also considered as cardiac death. Non‐fatal myocardial infarction was defined on the basis of electrocardiographic findings, elevated cardiac enzyme, and coronary angiography results. Coronary revascularization indicated percutaneous coronary intervention and coronary bypass surgery. Ischemic stroke was defined on the basis of neurologic signs or symptoms with documented imaging studies. For patients who were unable to follow‐up for more than 6 months on medical records, we attempted to obtain the information on the occurrence of cardiovascular events as accurately as possible by using telephone contact and national death data.
2.5. Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as n (%). Student’s t test was used for the comparisons of continuous variable, and chi‐square test was used for the comparison of categorical variables between patients with and without events. Receiver operating characteristic (ROC) curve analysis was performed to obtain cutoff value of baPWV in the prediction of composite events. Cox proportional hazard analysis was performed to determine independent associations between baPWV and composite events. The following clinical covariates were considered as potential confounders and controlled during multivariable analysis: age, sex, body mass index, heart rate, diabetes mellitus, dyslipidemia, cigarette smoking, and cardiovascular medications including beta blocker, renin‐angiotensin system blocker, and statin. The baPWV measurements were categorized into 2 groups according to the cutoff value obtained from ROC curve analysis and into 3 groups according to tertiles during multivariable analysis. Kaplan‐Meier survival curves were generated with log‐rank comparison to show differences in event‐free survival rates according to baPWV values. A P value of < .05 was considered statistically significant. All statistical analyses were conducted using SPSS version 21.0 (IBM Crop., Armonk, NY, USA).
3. RESULTS
Table 1 shows the baseline clinical characteristics of the study subjects. Mean age was 62.7 ± 11.7 years, and about half (46.9%) were female. The prevalence of obesity, diabetes mellitus, dyslipidemia, and current cigarette smoking was 51.5%, 35.0%, 65.7%, and 15.9%, respectively. Major laboratory results were within normal limits except elevated blood levels of glucose and C‐reactive protein. The proportion of subjects taking calcium channel blocker, beta blocker, renin‐angiotensin system blocker, diuretics, and statin were 38.2%, 31.2%, 50.1%, 2.8%, and 57.3%, respectively.
Table 1.
Baseline clinical characteristics of study patients
| Characteristic |
Total (n = 2561) |
Event (+) (n = 69) |
Event (−) (n = 2492) |
P value |
|---|---|---|---|---|
| Age, y | 62.7 ± 11.7 | 65.8 ± 9.3 | 62.6 ± 11.8 | .007 |
| Female sex | 1202 (46.9) | 26 (37.7) | 1176 (47.2) | .118 |
| BMI, kg/m2 | 25.4 ± 3.4 | 26.0 ± 3.5 | 25.4 ± 3.4 | .147 |
| SBP, mm Hg | 138 ± 19 | 136 ± 20 | 138 ± 19 | .540 |
| DBP, mm Hg | 81.2 ± 12.1 | 79.5 ± 10.8 | 81.3 ± 12.1 | .231 |
| Heart rate, per min | 70.2 ± 12.5 | 69.7 ± 11.5 | 70.2 ± 12.5 | .724 |
| Cardiovascular risk factors | ||||
| Obesity (BMI ≥ 25 kg/m2) | 1320 (51.5) | 41 (59.4) | 1279 (51.3) | .185 |
| Diabetes mellitus | 896 (35.0) | 39 (56.5) | 857 (34.4) | <.001 |
| Dyslipidemia | 1682 (65.7) | 57 (82.6) | 1625 (65.2) | .003 |
| Cigarette smoking | 406 (15.9) | 16 (23.2) | 390 (15.7) | .091 |
| Laboratory findings | ||||
| WBC, per microliter | 7191 ± 3647 | 7557 ± 2711 | 7180 ± 3669 | .412 |
| Hemoglobin, g/dL | 13.3 ± 1.9 | 12.9 ± 2.5 | 13.3 ± 1.9 | .191 |
| GFR, mL/min/1.73 m2 | 80.7 ± 30.1 | 74.5 ± 36.8 | 80.9 ± 29.9 | .159 |
| Fasting glucose, mg/dL | 124 ± 43 | 133 ± 53 | 123 ± 42 | .146 |
| Glycated hemoglobin, % | 6.44 ± 1.12 | 6.89 ± 1.21 | 6.43 ± 1.11 | .021 |
| Total cholesterol, mg/dL | 155 ± 39 | 142 ± 36 | 155 ± 39 | .006 |
| LDL cholesterol, mg/dL | 87.8 ± 32.8 | 75.5 ± 33.6 | 88.1 ± 32.7 | .003 |
| HDL cholesterol, mg/dL | 48.9 ± 12.9 | 47.5 ± 13.9 | 48.9 ± 12.9 | .400 |
| Triglycerides, mg/dL | 129 ± 76 | 126 ± 61 | 129 ± 76 | .719 |
| C‐reactive protein, mg/dL | 1.17 ± 3.66 | 1.92 ± 4.39 | 1.15 ± 3.64 | .212 |
| Concomitant medications | ||||
| Calcium channel blocker | 979 (38.2) | 26 (37.7) | 953 (38.2) | .925 |
| Beta blocker | 800 (31.2) | 29 (42.0) | 771 (30.9) | .050 |
| RAS blocker | 1284 (50.1) | 34 (39.3) | 1250 (50.2) | .885 |
| Diuretics | 71 (2.8) | 0 | 71 (2.8) | .262 |
| Statin | 1467 (57.3) | 52 (75.4) | 1415 (56.8) | .002 |
Numbers are expressed as mean ± standard deviation or n (%).
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; RAS, renin‐angiotensin system; SBP, systolic blood pressure; WBC, white blood cell.
During a median follow‐up period of 4.14 years (interquartile range, 2.18‐5.48 years), there were 69 cases of composite events (2.7%), including 6 cases of cardiac death (0.2%), 2 cases of non‐fatal myocardial infarction (0.1%), 38 cases of coronary revascularization (1.5%), and 24 cases of ischemic stroke (0.9%). There were 38 cases (1.5%) of all‐cause death. Comparisons of baseline clinical characteristics between patients with and without events are also demonstrated in Table 1. Patients with events were older had more cardiovascular risk factors including diabetes mellitus and dyslipidemia than those without. Beta blocker and statin were more frequently prescribed in patients with events than those without. In ROC curve analysis, the best cutoff value of baPWV predicting composite events was 1630 cm/s (sensitivity, 79.7%; specificity, 49.1%; area under curve, 0.674; 95% confidence interval [CI], 0.613‐0.735; P < .001) (Figure 1). There was a significant difference in event‐free survival rates between subjects with baPWV < 1630 cm/s and ≥1630 cm/s, which was demonstrated in the Kaplan‐Meier survival curve (log‐rank P < .001) (Figure 2). In univariable Cox regression analysis, both above cutoff value (≥1630 cm/s) and higher tertile of baPWV (middle and highest tertile compared to lowest tertile) were associated with increased risk of clinical events (P < .05). In multivariable Cox regression analysis, higher baPWV (≥1630 cm/s) was independently associated with higher risk of the occurrence of composite events even after controlling for potential confounders (hazard ratio [HR], 2.83; 95% CI, 1.49‐5.36; P = .001). Compared to the lowest tertile, middle tertile (HR, 2.40; 95% CI, 1.08‐5.33; P = .032) and the highest tertile (HR, 3.81; 95% CI, 1.68‐8.60; P = .001) of baPWV were significantly associated with higher incidence of composite events in the same multivariable model (Table 2). The Kaplan‐Meier survival curves show a significant difference in event‐free survival rates according to baPWV tertiles (log‐rank P < .001) (Figure 3).
Figure 1.
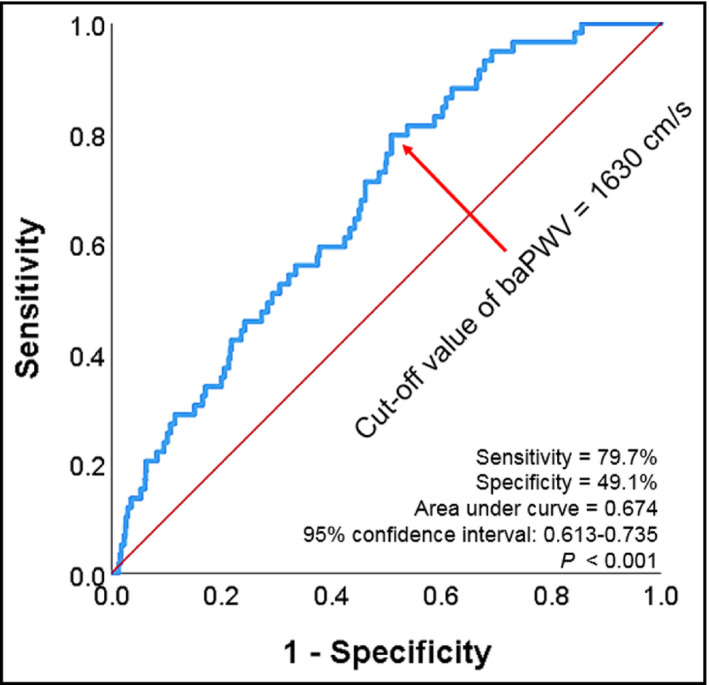
Receiver operating characteristic curve analysis showing the cutoff value of baPWV in the prediction of composite events. baPWV, brachial‐ankle pulse wave velocity
Figure 2.
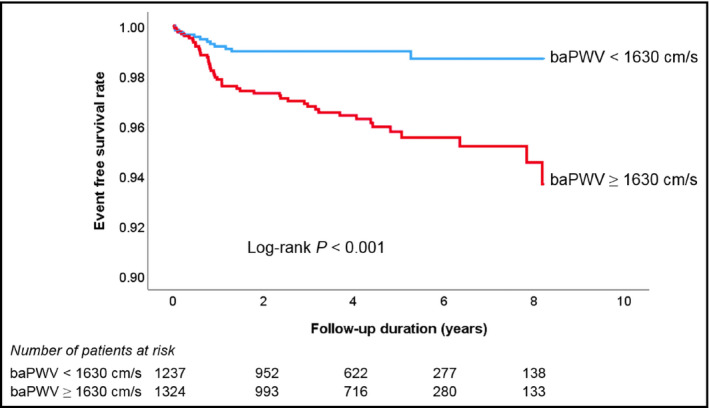
Kaplan‐Meier survival curves showing event‐free survival rates according to the baPWV cutoff value. baPWV, brachial‐ankle pulse wave velocity
Table 2.
Independent association of baPWV with clinical outcomes
| Variable | Univariable | Multivariable* | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Cutoff value of baPWV | ||||
| baPWV ≥ 1630 cm/s | 2.87 (1.66‐4.96) | <.001 | 2.83 (1.49‐5.36) | .001 |
| Tertile of baPWV | ||||
| Lowest tertile (814 ~ 1525 cm/s) | 1 | 1 | ||
| Middle tertile (1526 ~ 1800 cm/s) | 2.39 (1.14‐5.00) | .021 | 2.40 (1.08‐5.33) | .032 |
| Highest tertile (1801 ~ 4672 cm/s) | 3.66 (1.81‐7.40) | <.001 | 3.81 (1.68‐8.60) | .001 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CI, confidence interval; HR, hazard ratio.
Following clinical covariates were controlled as potential confounders: age, sex, body mass index, heart rate, diabetes mellitus, dyslipidemia, cigarette smoking, and cardiovascular medications including beta blocker, renin‐angiotensin system blocker, and statin.
Figure 3.
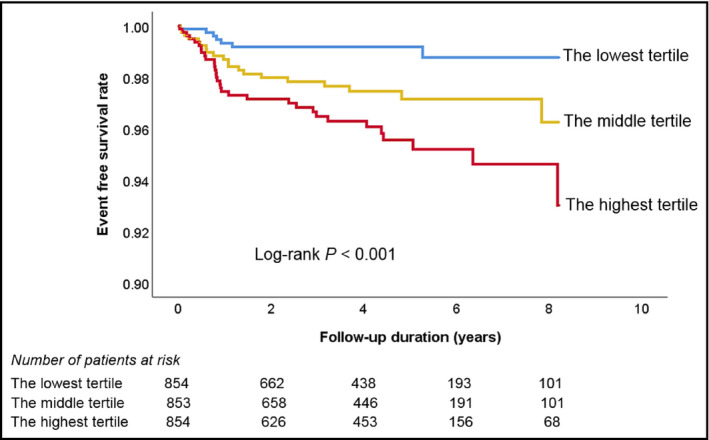
Kaplan‐Meier survival curves showing event‐free survival rates according to the tertiles of baPWV value. baPWV, brachial‐ankle pulse wave velocity
4. DISCUSSION
Our study showed that higher baseline baPWV value was associated with the development of cardiovascular events even after controlling for important clinical risk factors in hypertensive patients without documented cardiovascular disease. We also proposed that a baPWV value of 1630 cm/s was the cutoff value predicting cardiovascular events in hypertensive patients.
4.1. The role of arterial stiffness in hypertensive patients
Many studies have been conducted on the important role of arterial stiffness in hypertensive patients, mainly in Europe and Japan. In Europe, a study of 1980 patients with essential hypertension showed that an increase in cfPWV of 5 m/s was associated with 1.4‐fold increase risk of cardiovascular mortality. 17 As a result of analyzing the same cohort data, an increase in 1 SD in carotid‐femoral PWV (cfPWV) (4 m/s) increased the risk of stroke by 1.72 times, independent of other cardiovascular risk factors. 19 The same cohort study also showed that baseline cfPWV was significantly associated with the occurrence of coronary events even after controlling for the Framingham risk score (relative risk, 1.34 for 3.5 m/s). 16 Kawai et al 13 investigated 338 Japanese subjects with essential hypertension and showed that those having upper one‐fourth of baPWV (>1888 cm/s) had more cardiovascular events, stroke, and death. Another small observational study of 531 Japanese hypertensive subjects demonstrated that high cfPWV (>10.1 m/s) was associated with the development of stroke and cardiovascular disease. 15 Another recent observational study of 662 Japanese patients with essential hypertension confirmed that high baPWV (≥1750 cm/s) compared with low baPWV (<1750 cm/s) was associated with a significantly increased risk of cardiovascular events (HR [hazard ration], 2.97). 14 Consistent with these findings, our result also showed increased the risk of cardiovascular events in hypertensive patients with higher baPWV. Taken together, arterial stiffness may be an important prognostic factor predicting future cardiovascular events in hypertensive patients.
4.2. Cutoff value of PWV in hypertensive patients
Information on the cutoff value of baPWV is useful for both research and clinical purposes. Two Japanese studies have proposed the cutoff value of baPWV for predicting future cardiovascular events in hypertensive patients: 1750 cm/s in one study 18 and 1830 cm/s in the other study. 12 These values are somewhat higher than 1630 cm/s suggested in our study. Differences among studies are probably due to differences in races and study end points. Our study included cardiac death, myocardial infarction, revascularization, and stroke, while other studies included ischemic heart disease and stroke, 12 or cardiovascular disease, stroke, and total death. 18 In one study, the clinical follow‐up period was longer than in our study and only patients with recent cardiovascular disease were excluded from the study. 18 Therefore, the patients were more likely to be at higher risk and the cardiovascular events were more frequent than in our study. 18 The other study was a meta‐analysis from 8 different studies. 12
4.3. cfPWV vs baPWV
It has been suggested that cfPWV measurement is the gold standard non‐invasive method for measuring arterial stiffness. 25 Measurement of cfPWV was developed earlier, 26 and its clinical data have been accumulated mainly in Western countries. Also, cfPWV is theoretically more reasonable, because it contains only elastic arteries between the carotid and femoral arteries. However, cfPWV measurement has several shortcomings. When measuring cfPWV, it requires skill to find exact locations of the carotid and femoral arteries. In addition, it causes some discomfort to the patient in the process of finding these blood vessels. 27 , 28 Another non‐invasive method of measuring arterial stiffness is baPWV. Although it was developed later than cfPWV, and its measurement includes part of the muscular arteries of the arms and legs, baPWV has been widely used especially in Asian countries, because baPWV measurement is easy and simple by just wrapping blood pressure cuffs to both arms and ankles. 29 , 30 Moreover, a number of clinical studies have been demonstrated the usefulness of baPWV in Asian countries. 8 , 30 Recently, there have been some reports showing that the baPWV better reflects the blood pressure condition or cardiac afterload compared to the cfPWV. 31 , 32
4.4. Clinical implications
In patients with hypertension, arterial stiffness is related to target organ damage and clinical outcomes, so it can be helpful for risk stratification and determination of treatment strategy. The recent hypertension guidelines recommend the measurement of arterial stiffness in the evaluation of hypertensive patients. 9 , 10 , 11 Based on the meta‐analysis, 12 current Japanese hypertension guideline used a cutoff level of baPWV ≥ 1800 cm/s in risk assessment of hypertensive patients. 11 Although no Korean data are available, recent Korean hypertension guidelines also applied this cutoff value of baPWV to Koreans for classifying patient risk. 9 Our study of a large number of hypertensive Koreans showed that baPWV ≥ 1630 cm/s is the cutoff value that best predicts future cardiovascular events. We believe that the results of this study will be of great help in risk stratification and treatment of Korean hypertensive patients using baPWV.
4.5. Study limitations
This study has several limitations. First, although we attempted to collect as much information as possible about the occurrence of cardiovascular events, some cardiovascular events may not have been recorded. Therefore, there might be a possibility that incidence rates of cardiovascular events might be underestimated. However, even considering the clinical follow‐up period, the incidence of cardiovascular events in our study was similar when compared with Asian data. 33 Second, the diagnosis of hypertension was based on office blood pressure in our study. If home or ambulatory blood pressure monitoring were used, the diagnosis of hypertension could have been more accurate. Third, this study was conducted in one hospital and may have some potential bias. Lastly, our subjects are all Koreans, which may make it difficult to generalize our research results to other races.
5. CONCLUSIONS
Increased baPWV in Korean hypertensive patients was associated with an increased risk of future cardiovascular events. Given the non‐invasiveness and simplicity of baPWV measurement, baPWV could be used as an important risk stratification tool in Korean hypertensive patients. In addition, we suggested 1630 cm as a cutoff value of baPWV for predicting cardiovascular events, which will be helpful in guiding the treatment strategy of Korean hypertensive patients.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest associated with this manuscript.
AUTHORS’ CONTRIBUTIONS
Hack‐Lyoung Kim designed the research. Hack‐Lyoung Kim, Woo‐Hyun Lim, and Jae‐Bin Seo analyzed data. Hack‐Lyoung Kim wrote the paper. Sang‐Hyun Kim, Zoo‐Hee Zo, and Myung‐A Kim contributed to data collection. All authors reviewed/edited the manuscript important intellectual content, and read and approved the final manuscript.
Kim H‐L, Lim W‐H, Seo J‐B, Kim S‐H, Zo Z‐H, Kim M‐A. Prediction of cardiovascular events using brachial‐ankle pulse wave velocity in hypertensive patients. J Clin Hypertens. 2020;22:1659–1665. 10.1111/jch.13992
REFERENCES
- 1. NCD Risk Factor Collaboration (NCD‐RisC) . Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population‐based measurement studies with 19.1 million participants. Lancet. 2017;389:37‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life‐years lost, and age‐specific associations in 1.25 million people. Lancet. 2014;383:1899‐1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. J Hypertens. 2014;32:2285‐2295. [DOI] [PubMed] [Google Scholar]
- 4. Safar ME, Asmar R, Benetos A, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72:796‐805. [DOI] [PubMed] [Google Scholar]
- 5. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664‐670. [DOI] [PubMed] [Google Scholar]
- 7. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 9. Lee HY, Shin J, Kim GH, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II‐diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021‐3104. [DOI] [PubMed] [Google Scholar]
- 11. Umemura S, Arima H, Arima S, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens Res. 2019;42:1235‐1481. [DOI] [PubMed] [Google Scholar]
- 12. Ohkuma T, Tomiyama H, Ninomiya T, et al. Proposed cutoff value of brachial‐ankle pulse wave velocity for the management of hypertension. Circ J. 2017;81:1540‐1542. [DOI] [PubMed] [Google Scholar]
- 13. Kawai T, Ohishi M, Onishi M, et al. Prognostic impact of regional arterial stiffness in hypertensive patients. Heart Vessels. 2015;30:338‐346. [DOI] [PubMed] [Google Scholar]
- 14. Munakata M, Konno S, Miura Y, Yoshinaga K. Prognostic significance of the brachial‐ankle pulse wave velocity in patients with essential hypertension: final results of the J‐TOPP study. Hypertens Res. 2012;35:839‐842. [DOI] [PubMed] [Google Scholar]
- 15. Ohishi M, Tatara Y, Ito N, et al. The combination of chronic kidney disease and increased arterial stiffness is a predictor for stroke and cardiovascular disease in hypertensive patients. Hypertens Res. 2011;34:1209‐1215. [DOI] [PubMed] [Google Scholar]
- 16. Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10‐5. [DOI] [PubMed] [Google Scholar]
- 17. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236‐1241. [DOI] [PubMed] [Google Scholar]
- 18. Kawai T, Ohishi M, Onishi M, et al. Cut‐off value of brachial‐ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013;20:391‐400. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203‐1206. [DOI] [PubMed] [Google Scholar]
- 20. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511‐1522. [DOI] [PubMed] [Google Scholar]
- 21. Seo MH, Lee WY, Kim SS, et al. 2018 Korean Society for the Study of Obesity Guideline for the Management of Obesity in Korea. J Obes Metab Syndr. 2019;28:40‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee CS, Cha RH, Lim YH, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25:1616‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hwang IC, Jin KN, Kim HL et al Additional prognostic value of brachial‐ankle pulse wave velocity to coronary computed tomography angiography in patients with suspected coronary artery disease. Atherosclerosis. 2018;268:127‐137. [DOI] [PubMed] [Google Scholar]
- 24. Lee HS, Kim HL, Kim H, et al. Incremental prognostic value of brachial‐ankle pulse wave velocity to single‐photon emission computed tomography in patients with suspected coronary artery disease. J Atheroscler Thromb. 2015;22:1040‐1050. [DOI] [PubMed] [Google Scholar]
- 25. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445‐448. [DOI] [PubMed] [Google Scholar]
- 26. Nielsen BL, Nielsen JS, Roin J, Fabricius J. Carotid‐femoral pulse wave velocity. J Am Geriatr Soc. 1968;16:658‐665. [DOI] [PubMed] [Google Scholar]
- 27. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vlachopoulos C, Xaplanteris P, Aboyans V, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507‐532. [DOI] [PubMed] [Google Scholar]
- 29. Munakata M. Brachial‐ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10:49‐57. [DOI] [PubMed] [Google Scholar]
- 30. Kim HL, Kim SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med. 2019;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beck DT, Martin JS, Casey DP, Braith RW. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am J Hypertens. 2013;26:1093‐1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park KH, Park WJ, Kim MK, et al. Noninvasive brachial‐ankle pulse wave velocity in hypertensive patients with left ventricular hypertrophy. Am J Hypertens. 2010;23:269‐274. [DOI] [PubMed] [Google Scholar]
- 33. Lawes CM, Bennett DA, Parag V, et al. Blood pressure indices and cardiovascular disease in the Asia Pacific region: a pooled analysis. Hypertension. 2003;42:69‐75. [DOI] [PubMed] [Google Scholar]


