Abstract
Hypertension and dyslipidemia are important risk factors for cardiovascular disease. However, the clinical outcomes of fixed‐dose combination (FDC) versus free‐equivalent combination (FEC) of amlodipine and atorvastatin in the treatment of concurrent hypertension and dyslipidemia remain unknown. In this study, we included patients with newly diagnosed hypertension and dyslipidemia, without previously established cardiovascular disease, and treated with either FDC or FEC of amlodipine and atorvastatin were identified from the National Health Insurance Research Database of Taiwan and follow‐up for 5 years. By using 1:1 propensity score matching, a total of 1756 patients were enrolled in this study. The composite of major adverse cardiovascular events, including all‐cause mortality, myocardial infarction (MI), stroke, and coronary revascularization, occurred more frequently in the FEC group than in the FDC group (hazard ratio, 1.88; 95% confidence interval [CI], 1.42 to 2.5). Although the all‐cause mortality did not differ (hazard ratio, 0.46; 95% CI, 0.36 to 1.59), the FEC group developed increased MI, stroke, and coronary revascularization (hazard ratio, 2.87; 95% CI, 1.07 to 7.68; hazard ratio, 1.97; 95% CI, 1.41 to 2.74; and hazard ratio, 2.44; 95% CI, 1.26 to 4.69, respectively). Furthermore, as an unexpected result, a higher risk to develop new‐onset diabetes mellitus was observed with FEC regimens (hazard ratio, 2.19; 95% CI, 1.6 to 3.0). In conclusion, although the all‐cause mortality did not differ between the two groups, the FDC regimen of amlodipine and atorvastatin improved clinical outcomes when compared to FEC in patients with newly diagnosed hypertension and dyslipidemia.
Keywords: clinical outcome, dyslipidemia, fixed‐dose combination, hypertension, new‐onset diabetes mellitus
1. INTRODUCTION
Cardiovascular disease (CVD) is caused by several factors and its risk factors rarely occur alone. 1 , 2 , 3 The combination of certain risk factors such as hypertension and dyslipidemia can act multiplicatively or synergistically to increase the risk of CVD events. 4 , 5 Additionally, the relationship between these two risk factors is an important modifiable element for CVD. Therefore, this synergistic relationship is recognized by most major clinical guidelines used to aid the management of symptomatic patients or those at risk for CVD since they recommend a strategy of treating these risk factors simultaneously rather than in isolation. 6 , 7 , 8
Previously, several studies have suggested that, in hypertensive patients, fixed‐dose combination (FDC) is more effective to control blood pressure than free‐equivalent combination (FEC) or monotherapy. 9 , 10 , 11 Better medication compliance with FDC regimens may significantly reduce the major adverse cardiovascular events (MACE) and health care costs, 12 , 13 which was also recommended by the current major guidelines. 14 , 15 However, studies comparing the efficacy and interaction between FDC and FEC in two different diseases, such as hypertension and dyslipidemia, are rare. 16 , 17 , 18 Furthermore, in these studies, only drug adherence or laboratory efficacy was compared, with clinical outcomes remaining unassessed between these two treatment strategies in patients with concomitant hypertension and dyslipidemia.
In the present study, we aimed to analyze the clinical outcomes of FDC versus FEC regimes of amlodipine and atorvastatin in the primary prevention of cardiovascular events in patients with concurrent hypertension and dyslipidemia. Simultaneously, drug adherence was also evaluated in these two different strategies.
2. METHOD
2.1. Data source
The data included in this study were obtained from the National Health Insurance Research Database (NHIRD) of Taiwan. The National Health Insurance (NHI) program, a state‐operated universal health insurance program, implemented from 1995, covering over 99% of the entire Taiwanese population. The NHIRD contains both inpatient and outpatient registries from all medical facilities contracted with the NHI Administration and provides comprehensive medication, procedure, and the established diagnoses of patients, classified into one principal and four secondary International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes. The Bureau of NHI encrypted all personal identifiers before the release of information to the researchers. Confidentiality was addressed by following the data processing regulations set by the Bureau of NHI. The Institutional Review Board of the Chang Gung Memorial Hospital in Linkou approved this study (approval number: 201701147B0).
2.2. Study cohort and design
Two study cohorts of patients with newly diagnosed hypertension (ICD‐9‐CM, 401.×) from January 2008 to December 2012 were generated from the NHIRD. The first cohort consisted of patients receiving the FDC regimen of amlodipine and atorvastatin, while the second cohort received the FEC with the same medications (Figure 1). The date of the first prescription of the studied medications was defined as the index date, and 6 months preceding the index date was defined as the baseline period. Hypertensive patients who received any FDC regimen other than amlodipine/atorvastatin or FEC regimens other than these two drugs during the baseline period were excluded from this study. To avoid the clinical effects of different durations of hypertension, we only enrolled newly diagnosed hypertension during this study period. In this study, the only available daily dosage of the FDC regimen was amlodipine 5 mg plus atorvastatin 10 mg. In the FEC group, nonequivalent dosages of both drugs were also excluded. To estimate the frequency of newly onset MACE in this population without established cardiovascular diseases, we excluded patients with a previous diagnosis of coronary artery disease (CAD), myocardial infarction (MI), stroke, end‐stage renal disease, cirrhosis, peripheral artery disease, or heart failure before or during the baseline period. Other exclusion criteria were age <18 years, previously diagnosed hypertension, or study agents prescribed less than 6 months. Additionally, we performed propensity score matching to avoid selection biases resulting from the nonrandom assignment. The variables used in the matching process were sex, age, diabetes mellitus (DM) (ICD‐9‐CM, 250), chronic kidney disease (CKD) (ICD‐9‐CM, 585), Charlson Comorbidity Index (CCI), and baseline concomitant medications including antiplatelet agents, angiotensin‐converting enzyme inhibitors (ACEI), angiotensin‐receptor blockers (ARB), beta‐blockers, diuretics, oral hypoglycemic agents, insulin, and other anti‐hypertensive drugs. The FDC group was matched at a 1:1 ratio to the FEC group. Medication adherence was assessed by using the proportion of days covered (PDC) according to the insurance claims for the medications, which is defined as the total number of days covered by the study drugs divided by the total number of days in the study period. 12 All patients were followed up for 5 years or until the development of end points, whichever was first.
Figure 1.
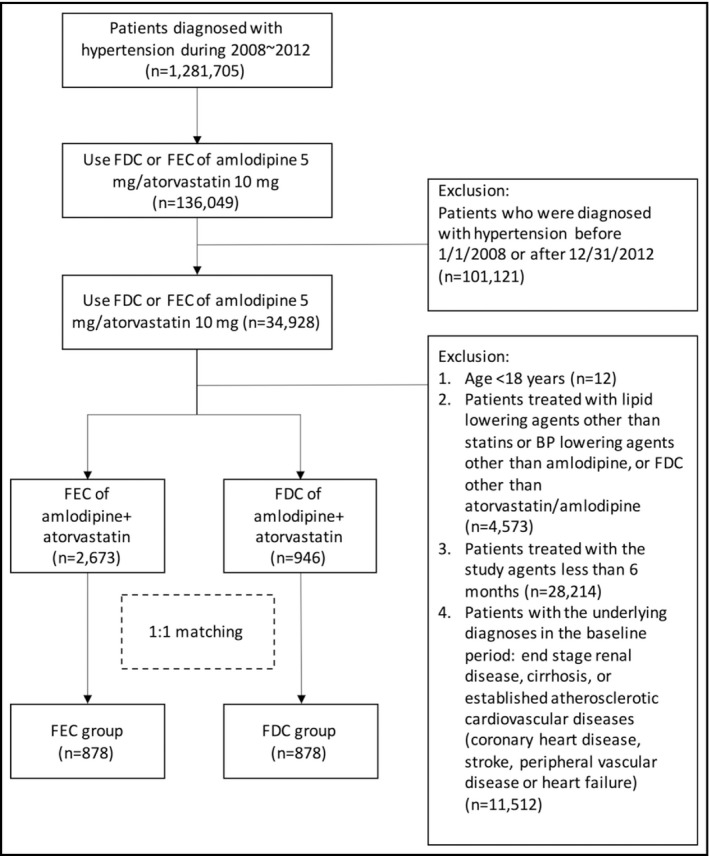
Patient enrollment. FDC, fixed‐dose combination; FEC, free‐equivalent combination
2.3. Study end points
The primary end point was the composition of MACE, including all‐cause mortality, MI (ICD‐9‐CM, 410‐410.9), stroke (ICD‐9‐CM, 430‐437), percutaneous coronary intervention (PCI) (ICD‐9‐CM, 36.0‐36.03 and 36.05‐36.09), or coronary artery bypass grafting surgery (CABG) (ICD‐9‐CM, 36.1‐36.99 and V45.81). Mortality was identified by using death certificate data files. The secondary end points included the components of the primary end point, new‐onset diabetes mellitus (NODM), hospitalization for CAD, and newly initiated hemodialysis. All these end points were based on the morbidity‐driven ICD‐9‐CM coding.
2.4. Statistics
Continuous variables were compared by using Student's t test, and categorical variables were analyzed by the chi‐square test. Data are presented as means and standard deviations or medians and percentages. A Cox proportional hazard model was used for a time to event analysis. Univariable and multivariable logistic regression analyses were used to identify independent predictors for primary end point. All analyses were conducted by using SAS Statistical Software, version 9.3 (SAS Institute Inc.) and R: A language and environment for statistical computing, version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). P values <.05 were considered statistically significant.
2.5. Privacy and confidentiality
The NHIRD deleted all identifiable information, and we further protected all patient information. Only the researchers and the authorized research participants had access to the dataset. All the database information was saved and locked in a safe place.
3. RESULTS
A total of 1 281 705 patients diagnosed with hypertension were identified in NHIRD from January 2008 to December 2012, but only 136 049 participants were treated daily with either FDC or FEC regimens composed of amlodipine 5 mg and atorvastatin 10 mg. After exclusion, 3619 newly diagnosed hypertensive patients were included for further propensity score matching, including 2673 patients in the FEC group and 946 patients in the FDC group (Figure 1). After matching, 1756 patients were enrolled in the present study, including 878 in each group. No significant inter‐group differences were observed in sex, age, type 2 DM, CKD, CCI, and baseline concomitant medications (Table 1).
Table 1.
Patient demographic characteristics
| Before match | After match | |||||
|---|---|---|---|---|---|---|
| FEC | FDC | P‐value | FEC | FDC | P‐value | |
| N | 2673 | 946 | 878 | 878 | ||
| Male (%) | 1269 (47.5) | 482 (51.0) | .07 | 428 (48.7) | 453 (51.6) | .25 |
| Age (mean [sd]) | 58.05 (11.74) | 58.60 (11.48) | .21 | 58.01 (11.66) | 58.36 (11.48) | .53 |
| Type 2 diabetes mellitus (%) | 929 (34.8) | 481 (50.8) | <.001 | 405 (46.1) | 423 (48.2) | .42 |
| Chronic kidney disease (%) | 65 (2.4) | 17 (1.8) | .32 | 16 (1.8) | 14 (1.6) | .85 |
| Charlson comorbidity index (mean [sd]) | 0.66 (1.16) | 1.28 (1.30) | <.001 | 1.12 (1.42) | 1.15 (1.21) | .56 |
| Baseline concomitant medications | ||||||
| Antiplatelet agents (%) | 280 (10.5) | 189 (20.0) | <.001 | 151 (17.2) | 155 (17.7) | .85 |
| ACE inhibitors (%) | 408 (15.3) | 210 (22.2) | <.001 | 185 (21.1) | 188 (21.4) | .91 |
| ARBs (%) | 596 (22.3) | 453 (47.9) | <.001 | 404 (46.0) | 391 (44.5) | .57 |
| Beta‐blockers (%) | 646 (24.2) | 347 (36.7) | <.001 | 306 (34.9) | 304 (34.6) | .96 |
| Diuretics (%) | 334 (12.5) | 174 (18.4) | <.001 | 144 (16.4) | 152 (17.3) | .66 |
| Oral hypoglycemic agents (%) | 799 (29.9) | 433 (45.8) | <.001 | 364 (41.5) | 377 (42.9) | .56 |
| Insulin (%) | 147 (5.5) | 102 (10.8) | <.001 | 84 (9.6) | 82 (9.3) | .94 |
| Other anti‐HTN agents (%) | 65 (2.4) | 43 (4.5) | .002 | 34 (3.9) | 38 (4.3) | .72 |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; FDC, fixed‐dose combination; FEC, free‐equivalent combination; HTN, hypertension.
Medication adherence as assessed by PDC was better in the FDC group than in the FEC group (0.49 ± 0.26 vs 0.32 ± 0.3, P < .001). The Cox proportional hazards model revealed significantly enhanced MACE in the FEC group than in the FDC group (hazard ratio, 1.88; 95% confidence interval [CI], 1.42 to 2.5; P < .001) within 5 years (Figure 2). A difference in favor of the FDC group was seen early in this analysis. Regarding each component of MACE, a higher incidence of MI, stroke, and revascularization (PCI or CABG) were observed in the FEC group than in the FDC group (hazard ratio, 2.87; 95% CI, 1.07 to 7.68; P = .04; hazard ratio, 1.97; 95% CI, 1.41 to 2.74; P < .001; and hazard ratio, 2.44; 95% CI, 1.26 to 4.69; P = .008, respectively) (Figure 3). However, all‐cause mortality was not different between the two groups (hazard ratio, 0.76; 95% CI, 0.36 to 1.59; P = .46). Compared with the FDC group, a greater number of patients developed NODM in the FEC group (hazard ratio, 2.19; 95% CI, 1.6 to 3.0; P < .001) during the follow‐up period (Figure 4). Similarly, more patients in the FEC groups experienced CAD hospitalization and newly initiated hemodialysis (hazard ratio, 2.0; 95% CI, 1.56 to 2.57; P < .001 and hazard ratio, 3.34; 95% CI, 1.67 to 6.66; P = .001, respectively).
Figure 2.
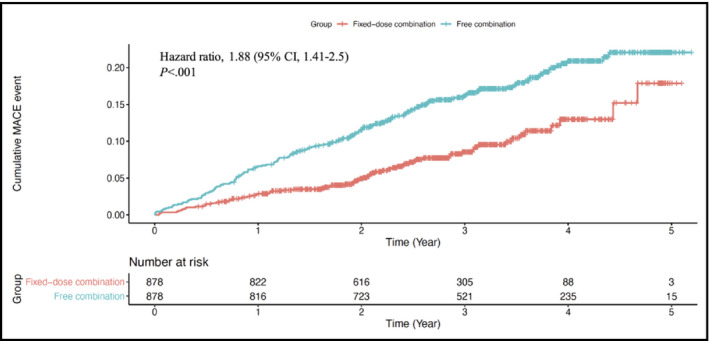
Cumulative events of MACE (primary end point) in FDC (red line) and FEC (blue line) groups of amlodipine 5 mg/atorvastatin 10 mg. FDC, fixed‐dose combination; FEC, free‐equivalent combination; MACE, major adverse cardiovascular event
Figure 3.
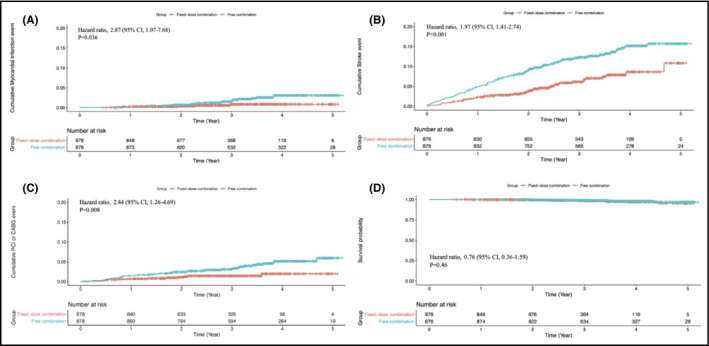
Comparison of the components of the primary end point in FDC (red line) versus FEC (blue line) of amlodipine/atorvastatin: A, myocardial infarction; B, stroke; C, coronary revascularization; and D, survival probability. FDC, fixed‐dose combination; FEC, free‐equivalent combination
Figure 4.
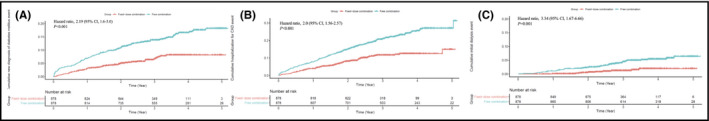
Comparison of secondary end points of FDC (red line) versus FEC (blue line) of amlodipine/atorvastatin: A, newly onset diabetes mellitus; B, hospitalization for coronary artery disease; and C, newly initiated hemodialysis. FDC, fixed‐dose combination; FEC, free‐equivalent combination
In univariable and multivariable analyses, FEC group, male gender, age, concomitant use of antiplatelet agents, beta‐blockers, and diuretics were positive predictors for primary end points, while concomitant use of ARBs negatively predicted MACE (Table 2).
Table 2.
Univariable and multivariable logistic analyses to predict major adverse cardiovascular events
| Risk factor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P‐value | aHR (95% CI) | P‐value | |
| FEC Group | 1.883 (1.418, 2.501) | <.0001 | 2.048 (1.596, 2.627) | <.0001 |
| Male | 1.411 (1.085, 1.836) | .0102 | 1.606 (1.355, 1.903) | <.0001 |
| Age | 1.029 (1.018, 1.041) | <.0001 | 1.041 (1.034, 1.049) | <.0001 |
| Type2 DM a | 1.235 (0.952, 1.604) | .1121 | ||
| CKD | 1.920 (0.853, 4.321) | .1151 | ||
| CCI Score | 1.013 (0.918, 1.119) | .7949 | ||
| Antiplatelet agents | 1.595 (1.182, 2.154) | .0023 | 1.372 (1.100, 1.711) | .005 |
| ACE inhibitors | 1.033 (0.749, 1.425) | .841 | ||
| ARBs | 0.971 (0.747, 1.262) | .8251 | 0.817 (0.668, 0.998) | .048 |
| Beta‐blockers | 0.998 (0.758, 1.314) | .9883 | 1.342 (1.116, 1.614) | .0018 |
| Diuretics | 1.644 (1.214, 2.228) | .0013 | 1.307 (1.047, 1.633) | .0181 |
| Other anti‐HTN agents | 1.162 (0.616, 2.191) | .6426 | ||
Abbreviations: ACE, angiotensin‐converting enzyme; aHR, adjusted hazard ratio; ARBs, angiotensin‐receptor blockers; CCI, Charlson comorbidity index; CI, confidence interval; CKD, chronic kidney disease; DM, diabetes mellitus; FEC, free‐equivalent combination; HR, hazard ratio; HTN, hypertension.
Glucose‐lowering agents were not included in this analysis because of collinearity with DM.
4. DISCUSSION
This nationwide population‐based cohort study is the first study to compare the clinical outcomes of FDC versus FEC of amlodipine and atorvastatin in patients with concomitant hypertension and dyslipidemia without previous established cardiovascular diseases. During 5 years of follow‐up, we observed that the FDC treatment strategy was more effective in reducing the risk of MACE than the FEC strategy. Meanwhile, compared to FDC, FEC group was also an independent predictor for MACE by multivariable analysis. Additionally, FDC was also superior to FEC in reducing the incidence of MI, stroke, NODM, CAD hospitalization, and newly initiated hemodialysis. The benefit of the FDC treatment strategy, which was apparent early in these outcomes except in MI, was observed in newly diagnosed hypertensive patients with a higher proportional incidence of DM and receiving ACEI, ARB, beta‐blockers, and oral hypoglycemic agents.
Previous studies have evaluated the efficacy and compliance of combination regimens of anti‐hypertension/lipid‐lowering. 16 , 17 , 18 In a randomized, multi‐center, double‐blind, placebo‐controlled study comparing the efficacy and tolerability of triple combination of amlodipine/losartan/rosuvastatin (A/L/R) to either losartan/rosuvastatin (L/R) or amlodipine/losartan (A/L) double combination in patients with hypertension and dyslipidemia, 16 the low‐density lipoprotein cholesterol (LDL‐C) level was reportedly lower in the A/L/R group than in the A/L group after 8 weeks of treatment. In addition, the mean reduction in sitting diastolic blood pressure was significantly greater in the A/L/R group than in the L/R group, with no clinically significant tolerability issue reported throughout the study. Two other studies both demonstrated that the FDC of amlodipine/atorvastatin can help to improve drug adherence versus the two‐pill calcium channel blocker (CCB) + statin regimen. 17 , 18 Furthermore, such an FDC of amlodipine/atorvastatin for LDL‐C reduction was also cost‐effective compared with the two‐pill regimen. 13 However, none of the above studies demonstrated the beneficial clinical outcomes of FDC regimens.
Adherence is a substantial factor governing the outcome of medical treatment, especially in chronic diseases. 19 Furthermore, nonadherence was also confirmed as an important contributor to the higher hospitalization rate and health care cost. 20 Currently, FDC is widely used in several chronic diseases such as hypertension, DM, and pulmonary tuberculosis to simplify treatment regimens, improving medication adherence and clinical outcomes. 10 , 12 , 21 , 22 , 23 , 24 In our previous study, compared to the free combination of CCB/ARB, the FDC of amlodipine/valsartan improves MACE‐free survival, medication compliance, hospitalization rates, and also decreases total health care costs. 12 The effect of FDC in the treatment of type 2 DM has been addressed in a systemic review including 10 studies, 2 of which were prospective, 1 was observational, and 7 were randomized, double‐blinded, parallel studies. 21 The authors concluded that the FDCs of various oral hypoglycemic agents significantly reduce glycated hemoglobin and fasting plasma glucose values, improve adherence, and reduce serious adverse drug reactions in diabetic patients. On the other hand, two previous meta‐analyses on anti‐tuberculosis treatments have shown no clinical benefit of FDC in terms of acquired drug resistance, culture conversion, treatment failure, or relapse when compared with separate drug formulations. 22 , 24 The authors explained that these results could be generated due to infrequent outcomes.
Although the all‐cause mortality rate did not differ between the FDC and FEC groups in the current study, similar results have been reported in previous studies. 25 , 26 A population‐based, retrospective cohort study demonstrated that the FDC of blood pressure‐lowering medications among hypertensive patients was not associated with lower mortality when compared with the multi‐pill group by on‐treatment analysis, despite the superior adherence recorded. 25 In another comprehensive review of randomized controlled trials regarding the FDC of blood pressure‐lowering and lipid‐lowering for the prevention of atherosclerotic CVD, compared with the comparator groups such as placebo, usual care, or active drug treatment, the effects of the FDC treatment on mortality did not differ from these groups (RR, 1.1; 95% CI, 0.64 to 1.89). 26 However, superior drug adherence may have the potential to reduce mortality in some specific populations. In patients with documented CVD, the lower adherence to statin therapy has been associated with a greater mortality risk. 27 In another health care database study from Sweden, lower refill adherence to lipid‐lowering medications resulted in higher CVD mortality among patients with type 2 DM. 28 Similarly, in newly diagnosed type 2 DM patients, lower anti‐diabetic medication adherence has been associated with higher long‐term all‐cause mortality. 29 In the current study, the difference in mortality was insignificant between the FDC and FEC groups may be explained by the low event rates in both groups, which could be attributed to the limited sample size, limited 4‐year follow‐up period, and the primary preventive nature of the intervention.
In a previous collaborative meta‐analysis assessing randomized statin trials, statin use was associated with a 9% increased risk of developing NODM, 30 which was positively correlated with the strength of the statin 31 ; however, the risk was reportedly low in absolute terms and when compared with the reduction in coronary events. Furthermore, the underlying mechanisms of statin‐induced NODM are not precisely known, and several possibilities have been proposed. 32 Notably, the beneficial effects of statins on CVD outweigh its risk of NODM development, with no neutralizing factor for such a risk documented in the literature. In the current study, we demonstrated the unexpected finding that in the FEC group of atorvastatin and amlodipine, the risk of NODM was more than twice when compared with the FDC group. The risk of NODM in anti‐hypertensive drug therapy, including CCBs, has been evaluated in previous meta‐analyses. 33 , 34 , 35 Compared to diuretics and beta‐blockers, CCBs are associated with reduced odds of developing NODM among hypertensive patients. 33 , 34 , 35 In contrast, treatment with CCBs is associated with a higher risk of developing NODM when compared with both ACEI and ARB treatment. 33 , 34 However, the use of ARB or ACEI in addition to CCB has demonstrated comparable incidences of NODM when compared to CCB monotherapy. 36 The drug‐drug interaction between CCBs and statins on the development of NODM remains unclear and is yet to be evaluated. In a previous study, combined treatment with amlodipine and atorvastatin improved endothelial function and inflammation as reflected by lower circulating levels of intercellular adhesion molecule‐1 and tumor necrosis factor‐α. 37 Similarly, oral administration of atorvastatin combined with amlodipine effectively prevents both endothelial dysfunction and elevated blood pressure in insulin‐resistant rats. 38 Furthermore, combination therapy with amlodipine and atorvastatin, but not individual monotherapy, suppresses angiotensin II‐induced abdominal aortic aneurysm formation in mice in vivo, by involving the inhibition of Rho‐kinase. 39 In the current study, medication adherence was superior in the FDC group than in the FEC group. One possibility was that the higher risk of developing NODM due to higher atorvastatin treatment in the FDC group could be counterbalanced by higher amlodipine administration. However, some confounding factors which may affect the development of NODM were not included in our database and could not be corrected by the matching such as body mass index, metabolic profiles, and socioeconomic status. The underlying molecular mechanism of such a phenomenon remains unclear and further interventional studies are needed to elucidate whether amlodipine could attenuate or neutralize the NODM risk of atorvastatin.
4.1. Study limitation
This study was based on a large administrative database and carried several limitations. First, we had no personal data such as family history, lifestyle, smoking, laboratory data, body weight, or blood pressure records. Therefore, the efficacy of blood pressure‐ or cholesterol‐lowering, as well as the critical link between medication compliance and patient outcomes, could not be estimated in this study. Second, although PDC has been widely used in studies of pharmacy claims datasets, 12 this surrogate marker of medication compliance does not ensure that the patients consumed the medications accordingly. Thus, medication compliance could be overestimated. Third, we used propensity score matching to balance the potential differences between two study groups; however, some parameters were not considered and may have confounded the study results, which is an inherent limitation of retrospective studies. Finally, we only enrolled hypertensive patients without established CVD so that these results could not be extrapolated to patients with documented atherosclerotic CVD as secondary prevention.
5. CONCLUSION
In this retrospective claims database study, although the all‐cause mortality did not differ, the FDC regimen of amlodipine and atorvastatin improved compliance and clinical outcomes, including MACE, MI, stroke, revascularization (PCI/CABG), hospitalization for CAD, NODM, and newly initiated hemodialysis when compared to an FEC regimen with the same medications in patients with newly diagnosed hypertension and dyslipidemia, with no previous CVD.
CONFLICT OF INTEREST
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ACKNOWLEDGMENT
This research is supported by the Chang Gung Memorial Hospital grant (CORPG3G0761)
Lin C‐P, Tung Y‐C, Hsiao F‐C, et al. Fixed‐dose combination of amlodipine and atorvastatin improves clinical outcomes in patients with concomitant hypertension and dyslipidemia. J Clin Hypertens. 2020;22:1846–1853. 10.1111/jch.14016
REFERENCES
- 1. Zanchetti A. The hypertensive patient with multiple risk factors: is treatment really so difficult? Am J Hypertens. 1997;10(10 Pt 2):223S‐229S. [DOI] [PubMed] [Google Scholar]
- 2. Gu D, Gupta A, Muntner P, et al. Prevalence of cardiovascular disease risk factor clustering among the adult population of China: results from the International Collaborative Study of Cardiovascular Disease in Asia (InterAsia). Circulation. 2005;112(5):658‐665. [DOI] [PubMed] [Google Scholar]
- 3. Asmar R, Vol S, Pannier B, Brisac AM, Tichet J, El Hasnaoui A. High blood pressure and associated cardiovascular risk factors in France. J Hypertens. 2001;19(10):1727‐1732. [DOI] [PubMed] [Google Scholar]
- 4. Kannel WB. Fifty years of Framingham Study contributions to understanding hypertension. J Hum Hypertens. 2000;14(2):83‐90. [DOI] [PubMed] [Google Scholar]
- 5. Thomas F, Bean K, Guize L, Quentzel S, Argyriadis P, Benetos A. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young (<55 years) men and women. Eur Heart J. 2002;23(7):528‐535. [DOI] [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206‐1252. [DOI] [PubMed] [Google Scholar]
- 7. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and Other Societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315‐2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of cardiology and the European Society of hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953‐2041. [DOI] [PubMed] [Google Scholar]
- 9. Struijker‐Boudier HA, Ambrosioni E, Holzgreve H, et al. The need for combination antihypertensive therapy to reach target blood pressures: what has been learned from clinical practice and morbidity‐mortality trials? Int J Clin Pract. 2007;61(9):1592‐1602. [DOI] [PubMed] [Google Scholar]
- 10. Simons LA, Chung E, Ortiz M. Long‐term persistence with single‐pill, fixed‐dose combination therapy versus two pills of amlodipine and perindopril for hypertension: Australian experience. Curr Med Res Opin. 2017;33(10):1783‐1787. [DOI] [PubMed] [Google Scholar]
- 11. Egan BM, Bandyopadhyay D, Shaftman SR, Wagner CS, Zhao Y, Yu‐Isenberg KS. Initial monotherapy and combination therapy and hypertension control the first year. Hypertension. 2012;59(6):1124‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tung YC, Lin YS, Wu LS, Chang CJ, Chu PH. Clinical outcomes and healthcare costs in hypertensive patients treated with a fixed‐dose combination of amlodipine/valsartan. J Clin Hypertens (Greenwich). 2015;17(1):51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Park JH, Lee YH, Ko SK, Cha BS. Cost‐effectiveness analysis of low density lipoprotein cholesterol‐lowering therapy in hypertensive patients with type 2 diabetes in Korea: single‐pill regimen (amlodipine/atorvastatin) versus double‐pill regimen (amlodipine+atorvastatin). Epidemiol Health. 2015;37:e2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 15. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2018;71(19):e127‐e248. [DOI] [PubMed] [Google Scholar]
- 16. Lee HY, Kim SY, Choi KJ, et al. A randomized, multicenter, double‐blind, placebo‐controlled study to evaluate the efficacy and the tolerability of a triple combination of amlodipine/losartan/rosuvastatin in patients with comorbid essential hypertension and hyperlipidemia. Clin Ther. 2017;39(12):2366‐2379. [DOI] [PubMed] [Google Scholar]
- 17. Hussein MA, Chapman RH, Benner JS, et al. Does a single‐pill antihypertensive/lipid‐lowering regimen improve adherence in US managed care enrolees? A non‐randomized, observational, retrospective study. Am J Cardiovasc Drugs. 2010;10(3):193‐202. [DOI] [PubMed] [Google Scholar]
- 18. Patel BV, Leslie RS, Thiebaud P, et al. Adherence with single‐pill amlodipine/atorvastatin vs a two‐pill regimen. Vasc Health Risk Manag. 2008;4(3):673‐681. [PMC free article] [PubMed] [Google Scholar]
- 19. DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta‐analysis. Med Care. 2002;40(9):794‐811. [DOI] [PubMed] [Google Scholar]
- 20. Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521‐530. [DOI] [PubMed] [Google Scholar]
- 21. Vijayakumar TM, Jayram J, Meghana Cheekireddy V, Himaja D, Dharma Teja Y, Narayanasamy D. Safety, efficacy, and bioavailability of fixed‐dose combinations in Type 2 diabetes mellitus: a systematic updated review. Curr Ther Res Clin Exp. 2017;84:4‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lima GC, Silva EV, Magalhaes PO, Naves JS. Efficacy and safety of a four‐drug fixed‐dose combination regimen versus separate drugs for treatment of pulmonary tuberculosis: a systematic review and meta‐analysis. Braz J Microbiol. 2017;48(2):198‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris SB. The power of two: an update on fixed‐dose combinations for type 2 diabetes. Expert Rev Clin Pharmacol. 2016;9(11):1453‐1462. [DOI] [PubMed] [Google Scholar]
- 24. Albanna AS, Smith BM, Cowan D, Menzies D. Fixed‐dose combination antituberculosis therapy: a systematic review and meta‐analysis. Eur Respir J. 2013;42(3):721‐732. [DOI] [PubMed] [Google Scholar]
- 25. Verma AA, Khuu W, Tadrous M, Gomes T, Mamdani MM. Fixed‐dose combination antihypertensive medications, adherence, and clinical outcomes: a population‐based retrospective cohort study. PLoS Medicine. 2018;15(6):e1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bahiru E, de Cates AN, Farr MR, et al. Fixed‐dose combination therapy for the prevention of atherosclerotic cardiovascular diseases. Cochrane Database Syst Rev. 2017;3:CD009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez F, Maron DJ, Knowles JW, Virani SS, Lin S, Heidenreich PA. Association of statin adherence with mortality in patients with atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4(3):206‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karlsson SA, Eliasson B, Franzen S, Miftaraj M, Svensson AM, Andersson SK. Risk of cardiovascular event and mortality in relation to refill and guideline adherence to lipid‐lowering medications among patients with type 2 diabetes mellitus in Sweden. BMJ Open Diabetes Res Care. 2019;7(1):e000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim YY, Lee JS, Kang HJ, Park SM. Effect of medication adherence on long‐term all‐cause‐mortality and hospitalization for cardiovascular disease in 65,067 newly diagnosed type 2 diabetes patients. Sci Rep. 2018;8(1):12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375(9716):735‐742. [DOI] [PubMed] [Google Scholar]
- 31. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305(24):2556‐2564. [DOI] [PubMed] [Google Scholar]
- 32. Paseban M, Butler AE, Sahebkar A. Mechanisms of statin‐induced new‐onset diabetes. J Cell Physiol. 2019;234(8):12551‐12561. [DOI] [PubMed] [Google Scholar]
- 33. Noto H, Goto A, Tsujimoto T, Noda M. Effect of calcium channel blockers on incidence of diabetes: a meta‐analysis. Diabetes Metab Syndr Obes. 2013;6:257‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Z, Li Y, Liu Y, Xu W, Wang Q. Comparative risk of new‐onset diabetes mellitus for antihypertensive drugs: a network meta‐analysis. J Clin Hypertens (Greenwich). 2017;19(12):1348‐1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuti EL, Baker WL, White CM. The development of new‐onset type 2 diabetes associated with choosing a calcium channel blocker compared to a diuretic or beta‐blocker. Curr Med Res Opin. 2007;23(6):1239‐1244. [DOI] [PubMed] [Google Scholar]
- 36. Kim YH, Her AY, Rha SW, et al. Calcium channel blocker monotherapy versus combination with renin‐angiotensin system inhibitors on the development of new‐onset diabetes mellitus in hypertensive Korean patients. J Geriatr Cardiol. 2019;16(6):439‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang Z, Chen C, Li S, Kong F, Shan P, Huang W. Combined treatment with amlodipine and atorvastatin calcium reduces circulating levels of intercellular adhesion molecule‐1 and tumor necrosis factor‐alpha in hypertensive patients with prediabetes. Front Aging Neurosci. 2016;8:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okamura T, Tawa M, Geddawy A, et al. Effects of atorvastatin, amlodipine, and their combination on vascular dysfunction in insulin‐resistant rats. J Pharmacol Sci. 2014;124(1):76‐85. [DOI] [PubMed] [Google Scholar]
- 39. Takahashi K, Matsumoto Y, Do.e Z, et al. Combination therapy with atorvastatin and amlodipine suppresses angiotensin II‐induced aortic aneurysm formation. PLoS One. 2013;8(8):e72558. [DOI] [PMC free article] [PubMed] [Google Scholar]


