Abstract
The value of the cardio‐ankle vascular index (CAVI) increases with age. All large‐scale studies of the CAVI have investigated patients <80 years old. Thus, the clinical characteristics of high CAVI in patients aged 80 or more remain unclear. Therefore, we investigated (1) the CAVI in very elderly patients and (2) the determinants of a high CAVI in high‐risk patients, including very elderly patients. The Cardiovascular Prognostic Coupling Study in Japan (Coupling Registry) is a prospective observational study of Japanese outpatients with any cardiovascular risk factors. We enrolled 5109 patients from 30 institutions (average age 68.7 ± 11.4 years, 52.4% males). We investigated the determinants of the CAVI by separating the patients into three groups: 970 middle‐aged (<60 years), 3252 elderly (60‐79 years), and 887 very elderly (≥80 years) patients. The CAVI values of the males were significantly higher those of the females in all age groups (<60 years: 7.81 ± 1.11 vs. 7.38 ± 0.99, P < .001; 60‐79 years: 9.20 ± 1.29 vs. 8.66 ± 1.07, P < .001; ≥80 years: 10.26 ± 1.39 vs. 9.51 ± 1.12, P < .001). In all age groups, the CAVI of the patients with diabetes/glucose tolerance disorder was higher than that of the patients without diabetes/glucose tolerance disorder (<60 years: 7.82 ± 1.22 vs 7.58 ± 1.03, P = .002; 60‐79 years: 9.23 ± 1.20 vs 8.78 ± 1.19, P < .001; ≥80 years: 10.04 ± 1.24 vs 9.75 ± 1.32, P = .002). The determinants of the CAVI in these very elderly patients were age, male sex, low BMI, and mean blood pressure. Diabetes/glucose tolerance disorder and glucose were independently associated with the CAVI in the patients aged <60 years and 60‐79 years, but not in those aged ≥80 years after adjusting for other covariates.
Keywords: cardio‐ankle vascular index, cardiovascular event, cardiovascular risk, registry
1. INTRODUCTION
Arterial stiffness measurement is a common method of assessing atherosclerosis. 1 , 2 Pulse wave velocity (PWV) is widely used as a measurement of arterial stiffness. 3 , 4 However, the value of PWV is strongly affected by blood pressure (BP). 5 The cardio‐ankle vascular index (CAVI), which is consistent with stiffness parameter β, is an index of artery stiffness not affected by BP. 6
The CAVI value has been shown to predict prognosis in patients with coronary artery disease, diabetes, and metabolic syndrome. 7 , 8 , 9 The CAVI value has also been associated with coronary artery disease and carotid arteriosclerosis. 10 , 11 , 12 , 13 Therefore, CAVI measurement is an important screen to identify patients at high risk for cardiovascular (CV) diseases. In recent years, CAVI reference values have been included in the Japanese guidelines for the diagnosis of vascular failure. 14
The CAVI values increase with age, and the CAVI values in males are high than those in females. 6 , 15 , 16 People worldwide are living longer. By 2050, the world's population aged 60 years and older is expected to total 2 billion, and 125 million people are aged 80 years or older today. 17 Because the patients enrolled in past studies of CAVI were aged less than 80 years, the clinical characteristics of high CAVI in patients aged 80 or more remain unclear.
The aim of this study was to examine predictors of CAVI in very elderly patients with CV risk factors.
2. METHODS
2.1. Subjects
The protocol of the Coupling Registry has been registered at the University Hospital Medical Information Network Clinical Trials Registry Web site under the trial number UMIN000018474. 18 Briefly, the Coupling Registry is a nationwide multicenter prospective cohort study to determine CAVI values predictive of cardiovascular events in Japanese patients aged ≥30 years with any of the following cardiovascular risk factors at the clinic or hospital: diabetes, glucose tolerance disorder, dyslipidemia, high‐normal normotension or grade I‐III hypertension (>130/85 mm Hg), current smoking, renal disease (estimated glomerular filtration rate ≤60 or positive proteinuria), medical history of cardiovascular disease (coronary artery disease, cerebrovascular disorder or non‐cardiogenic cerebrovascular disorder, aortic dissection, peripheral artery disease, history of hospitalization by heart failure), atrial fibrillation, metabolic syndrome, chronic obstructive pulmonary disease, and sleep apnea syndrome. Patients with any of the following were excluded: chronic renal failure requiring hemodialysis; other serious illnesses (eg, end‐stage cancer, active connective tissue disease); alcohol or drug addiction; inability to attend hospital visits or provide informed consent; or other factors rendering them inappropriate as judged by the study physician. 18 The ethics committee of the internal review board of the Jichi Medical University School of Medicine approved the protocol. The study protocol was registered on the clinical trials registration site, the University Hospital Medical Information Network Clinical Trials Registry (UMIN‐CTR), under registration number UMIN000018474. Written informed consent was obtained from all patients enrolled in this study.
2.2. Measurement of CAVI
We measured CAVI in 5,109 patients. CAVI was measured using a VaSera VS‐1000 device (Fukuda Denshi, Tokyo) with the patient in the recumbent position and calculated automatically using a VaSera VS‐Series Vascular Screening System (Fukuda Denshi). Measurement of CAVI requires placement of electrocardiogram electrodes on both wrists and a microphone for phonocardiography on the sternum in the second intercostal space. In addition, a BP cuff is wrapped around the four extremities. In this manner, the upper arm and ankle pulse waves, as well as BP, can all be measured using plethysmography. The CAVI value was calculated as the average of the right and left CAVI values.
2.3. Measurement of blood pressure
Clinic BP was measured after at least 5 minutes of rest as the average of two serial measurements 19 taken by physicians or nurses using the same validated methods used in their clinical practice. We obtained systolic and diastolic BP, and pulse pressure was calculated as systolic BP—diastolic BP.
2.4. Age subgroups and body mass index classification
We classified patients into 3 groups by age: <60 years old, 60‐79 years old, and ≥80 years old. We further classified the patients into 4 groups by body mass index (BMI): underweight (BMI < 18.5), normal weight (BMI 18.5‐24.9), overweight (BMI 25‐29.9), and obese (BMI ≥ 30).
2.5. Statistical analysis
Data are shown as the mean ± SD or a percentage. Unpaired t tests were used for the normally distributed data and comparisons between two groups, and an analysis of variance was used for comparisons of more than two groups. Intergroup differences were tested by the Bonferroni test. Multiple linear regression analysis to assess the independent determinants of CAVI was adjusted for the covariates having P values <.1 in univariate analysis: age, sex, BMI, diabetes/glucose tolerance disorder, dyslipidemia, high‐normal normotension or grade I–III hypertension, smoking status, statin use, antihypertensive medication use, heart rate, mean BP, LDL and HDL cholesterol, and glucose. Assessment of the intergroup differences in CAVI and multiple linear regression analysis were conducted for the three age groups defined above. SPSS version 25.0 software (IBM, Armonk, NY) was used for the statistical analysis. A probability value <.05 was considered statistically significant.
3. RESULTS
The average age of patients in the Coupling Study was 68.7 ± 11.4 years, and the percentage of men was 52.4%. Baseline characteristics for each age group are shown in Table 1.
Table 1.
Patient characteristics
|
Age < 60 (N = 970) |
Age 60‐79 (N = 3252) | Age ≥ 80 (N = 887) | P | |
|---|---|---|---|---|
| Age, y | 51.0 ± 6.6 | 69.8 ± 5.4 | 84.2 ± 3.5 | <.001 |
| Men, % | 61.4 | 51.9 | 44.0 | <.001 |
| Body mass index, kg/m2 | 26.5 ± 4.8 | 24.4 ± 3.6 | 23.7 ± 3.6 | <.001 |
| Diabetes, glucose tolerance disorder, % | 27.3 | 34.4 | 30.2 | <.001 |
| Dyslipidemia, % | 51.4 | 59.7 | 53.9 | <.001 |
| High‐normal normotension and grade I‐III hypertension, % | 78.6 | 83.0 | 91.0 | <.001 |
| Current smoker, % | 17.0 | 8.4 | 3.2 | <.001 |
| Renal disease, % | 7.0 | 19.0 | 37.6 | <.001 |
| Past history of cardiovascular disease, % | 17.0 | 24.1 | 29.2 | <.001 |
| Atrial fibrillation, % | 5.1 | 9.4 | 13.3 | <.001 |
| Metabolic syndrome, % | 12.9 | 10.5 | 7.9 | .002 |
| Chronic obstructive pulmonary disease, % | 0.9 | 1.7 | 3.3 | .001 |
| Sleep apnea syndrome, % | 9.6 | 4.2 | 1.6 | <.001 |
| Cardio‐ankle vascular index | 7.6 ± 1.1 | 8.9 ± 1.2 | 9.8 ± 1.3 | <.001 |
| Systolic blood pressure (mm Hg) | 131.7 ± 15.3 | 133.3 ± 16.2 | 136.0 ± 18.9 | <.001 |
| Diastolic blood pressure (mm Hg) | 82.3 ± 10.5 | 76.4 ± 9.9 | 72.3 ± 10.8 | <.001 |
| Mean pressure (mm Hg) | 98.8 ± 11.3 | 95.4 ± 10.7 | 93.6 ± 11.9 | <.001 |
| Pulse rate (bpm) (N = 5055) | 74.0 ± 12.2 | 71.5 ± 12.1 | 74.2 ± 13.6 | <.001 |
| Total cholesterol (N = 4644) | 199.2 ± 36.5 | 189.7 ± 32.9 | 180.2 ± 31.6 | <.001 |
| LDL cholesterol (N = 3696) | 115.4 ± 31.5 | 106.6 ± 27.5 | 97.6 ± 26.7 | <.001 |
| HDL cholesterol (N = 4541) | 56.8 ± 16.0 | 58.8 ± 16.4 | 56.5 ± 15.6 | <.001 |
| Triglyceride (N = 4972) | 148.6 ± 103.0 | 123.8 ± 73.6 | 113.3 ± 55.2 | <.001 |
| Glucose (N = 4923) | 111.2 ± 28.5 | 112.6 ± 32.1 | 110.4 ± 32.9 | .141 |
| HbA1c (N = 4650) | 6.1 ± 0.9 | 6.2 ± 0.8 | 6.1 ± 0.7 | <.001 |
| Uric acid (N = 4935) | 5.9 ± 1.4 | 5.5 ± 1.9 | 5.4 ± 1.4 | <.001 |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
The CAVI values for the males, females, and overall patients in each age group are shown in Figure 1. The CAVI values of the males were significantly higher those of the females in all age groups (<60 years: 7.81 ± 1.11 vs. 7.38 ± 0.99, P < .001; 60‐79 years: 9.20 ± 1.29 vs 8.66 ± 1.07, P < .001; ≥80 years: 10.26 ± 1.39 vs 9.51 ± 1.12, P < .001). The CAVI values were inversely associated with BMI, gradually decreasing between the underweight and normal weight, the normal weight and overweight, and the overweight and obesity groups for all three age groups (Figure 2A, B, and C).
Figure 1.
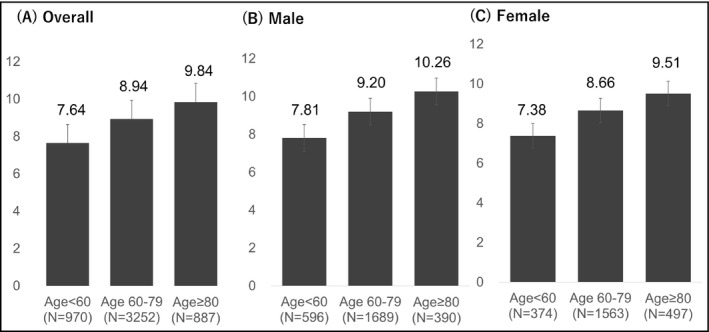
CAVI by age group and sex. A, Overall, B, male, and C, female. CAVI: cardio‐ankle vascular index CAVI
Figure 2.
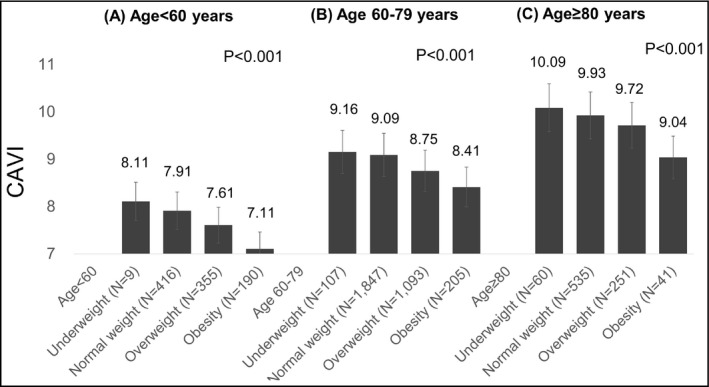
BMI and CAVI by age group. A, Age <60 y, B, age 60‐79 y, C, age ≥80 y. BMI: body mass index; CAVI: cardio‐ankle vascular index CAVI
Figure 3 shows the relationship between the presence of comorbidities and CAVI. In all age groups, the CAVI of the patients with diabetes/glucose tolerance disorder was higher than that of the patients without diabetes/glucose tolerance disorder (<60 years: 7.82 ± 1.22 vs 7.58 ± 1.03, P = .002; 60‐79 years: 9.23 ± 1.20 vs 8.78 ± 1.19, P < .001; ≥80 years: 10.04 ± 1.24 vs 9.75 ± 1.32, P = .002; Figure 3A). The CAVI of patients with dyslipidemia and those without dyslipidemia was similar in the patients aged < 60 years and ≥ 80 years (<60 years: 7.69 ± 1.10 vs 7.59 ± 1.08, P = .153; ≥80 years: 9.76 ± 1.27 vs 9.93 ± 1.33, P = .052; Figure 3B), and the CAVI of the patients with dyslipidemia was slightly lower than that of the patients without dyslipidemia in the patients aged 60‐79 years (8.90 ± 1.20 vs 8.99 ± 1.24, P = .048; Figure 3B). The CAVI of patients with high‐normal normotension or grade I‐III hypertension and without high‐normal normotension or grade I‐III hypertension were similar in all age groups (<60 years: 7.67 ± 1.09 vs 7.56 ± 1.07, P = .199; 60‐79 years: 8.92 ± 1.19 vs 9.01 ± 1.35, P = .145; ≥80 years: 9.81 ± 1.29 vs 10.11 ± 1.44, P = .055; Figure 3C). The CAVI of patients with a current smoking habit was higher than that of patients without a current smoking habit in the patients aged < 60 years and in the patients aged 60‐79 years (<60 years: 7.94 ± 1.18 vs 7.58 ± 1.06, P < .001; 60‐79 years: 9.24 ± 1.35 vs.8.91 ± 1.20, P < .001; Figure 3D).
Figure 3.
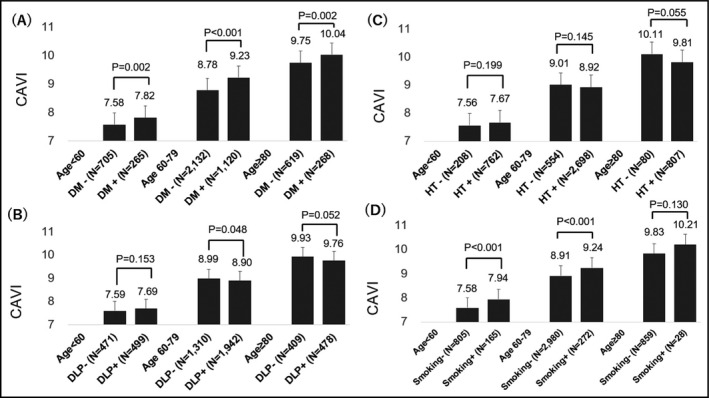
Association of basic parameters by age group. A, Diabetes/glucose tolerance disorder, DM: Diabetes/glucose tolerance disorder. B, Dyslipidemia, DLP: Dyslipidemia. C, High‐normal normotension and grade I‐III hypertension, HT: High‐normal normotension and grade I‐III Hypertension. and D, smoking
In overall group, age, sex, low BMI, and mean BP were independent determinants of high CAVI by multivariate analysis (Table 2). Diabetes/glucose tolerance disorder and glucose were significant determinants of high CAVI in patients aged < 60 years (diabetes/glucose tolerance disorder: β = 0.09, P = .017; glucose: β = 0.08, P = .039) and patients aged 60‐79 years (diabetes/glucose tolerance disorder: β = 0.16, P < .001; glucose: β = 0.08, P < .001), but not in patients aged 80 years or more.
Table 2.
Linear regression analysis of CAVI
| Age < 60 (N = 970) | Age 60‐79 (N = 3252) | Age ≥ 80 (N = 887) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | P | β | P | r | P | β | P | r | P | β | P | |
| Age | 0.45 | <0.001 | 0.39 | <.001 | 0.25 | <.001 | 0.25 | <.001 | 0.14 | <.001 | 0.17 | <.001 |
| Male | 0.18 | <0.001 | 0.18 | <.001 | 0.21 | <.001 | 0.19 | <.001 | 0.29 | <.001 | 0.29 | <.001 |
| Body mass index | −0.28 | <0.001 | −0.21 | <.001 | −0.16 | <.001 | −0.20 | <.001 | −0.17 | <.001 | −0.15 | <.001 |
| Diabetes, glucose tolerance disorder | 0.09 | 0.007 | 0.09 | .017 | 0.19 | <.001 | 0.16 | <.001 | 0.12 | <.001 | 0.06 | .103 |
| Dyslipidemia | 0.05 | 0.157 | −0.03 | .066 | −0.03 | .111 | −0.06 | .059 | 0.01 | .860 | ||
| High‐normal normotension and grade I‐III hypertension | 0.04 | 0.188 | −0.01 | .423 | −0.07 | .031 | −0.05 | .106 | ||||
| Smoking | 0.12 | <0.001 | 0.05 | .136 | 0.07 | <.001 | 0.03 | .139 | 0.03 | .305 | ||
| Statin | 0.06 | 0.074 | 0.01 | .768 | −0.02 | .215 | −0.06 | .059 | 0.00 | 1.000 | ||
| Antihypertensive medication | 0.09 | 0.007 | 0.01 | .855 | −0.01 | .635 | −0.01 | .788 | ||||
| Mean blood pressure | 0.06 | 0.078 | 0.13 | <.001 | 0.08 | <.001 | 0.10 | <.001 | 0.11 | .001 | 0.15 | <.001 |
| Heart rate | −0.03 | 0.394 | 0.07 | <.001 | 0.10 | <.001 | 0.04 | .225 | ||||
| LDL cholesterol | −0.19 | <0.001 | −0.08 | .018 | −0.05 | .023 | 0.00 | .880 | 0.03 | .414 | ||
| HDL cholesterol | 0.03 | 0.441 | −0.08 | <.001 | −0.04 | .061 | −0.01 | .750 | ||||
| Glucose | 0.14 | <0.001 | 0.08 | .039 | 0.20 | <.001 | 0.08 | <.001 | 0.11 | .002 | 0.06 | .141 |
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
We also performed an analysis with patients divided into two groups using age 75 as a cutoff. The CAVI of the patients with diabetes/glucose tolerance disorder was higher than that of the patients without diabetes/glucose tolerance disorder both in patients < 75 years and in those ≥ 75 years (<75 years: 8.83 ± 1.32 vs 8.34 ± 1.23, P < .001; ≥75 years: 9.80 ± 1.24 vs 9.46 ± 1.31, P < .001). The CAVI of patients with a current smoking habit was significantly higher than that of patients without a current smoking habit in the patients in < 75 years (8.69 ± 1.43 vs 8.47 ± 1.26, P = .001), but the difference was not significant in those ≥75 years (9.87 ± 1.43 vs 9.56 ± 1.29, P = .073). After adjusting for covariates, diabetes/glucose tolerance disorder was associated with CAVI in both groups (<75 years: β = 0.13, P < .001; ≥75 years: β = 0.12, P < .001), and mean BP was also associated with CAVI in both groups (<75 years: β = 0.12, P < .001; ≥75 years: β = 0.10, P < .001).
4. DISCUSSION
In the present analysis, CAVI increased according to age even in the very elderly patients, and the CAVI of males was higher than that of females in the very elderly. The determinants of the CAVI in these very elderly patients were age, male sex, low BMI, and mean BP. Diabetes/glucose tolerance disorder and glucose were independently associated with the CAVI in the patients aged <60 years and 60‐79 years, but not in those aged ≥80 years.
Shirai et al reported the CAVI values in 3,259 male and 3,534 female healthy individuals without CV risk factors who were receiving an annual health check. 6 The rate of increase in CAVI was nearly 0.5 per 10 years in men and women, and the CAVI of males was higher than that of females in almost all age groups by 0.2. Wholfahrt et al evaluated CAVI in 1,347 patients aged 25‐65 years (the average age was 44.7 ± 10.7 years) who were free from cardiovascular disease, non‐diabetic, and untreated by antihypertensive or lipid‐lowering drugs. The CAVI of males was significantly higher than that of females (7.4 ± 1.1 vs 7.1 ± 1.0, P < .0001). 15 In each age group, the CAVI of males was significantly higher than that of females, and the association between CAVI and age was linear. 15 Tabara et al evaluated CAVI in 9,501 community residents from 30 to 74 years of age, living independently without physical impairment or dysfunction (53.1 ± 13.3 years). 16 The average CAVI values were 7.79 ± 1.13 in males and 7.19 ± 1.02 in females, and CAVI was significantly higher in men in all age subgroups. 16 However, patients who were aged 80 years or more were not included in those studies. Our present finding of an age‐associated increase in CAVI was concordant with the results of these earlier studies.
In this study, BMI was significantly inversely associated with CAVI. This finding is also concordant with past reports. 16 , 20 , 21 , 22 BaPWV has also been inversely associated with CAVI. 21 CAVI is calculated using the square of PWV, and PWV is depended the length from the origin of the aorta to the ankle. 23 Body mass index is calculated as body weight/height, and thus, BMI might have an inverse effect on CAVI because body length is included in the formula of CAVI. However, not all studies have found a negative association between baPWV and BMI. 22 The formula for CAVI also included blood density, which is dependent on hematocrit and protein. Therefore, we cannot explain why an inverse association was observed between CAVI and BMI in this study. We may have underestimated arteriosclerosis by using CAVI in obese patients.
Diabetes/glucose tolerance disorder and glucose were independent determinants of CAVI in patients < 60 years and 60‐79 years old. The CAVI of diabetic patients was higher by 0.5 than that of non‐diabetic patients in patients 60‐79 years old in this study, and a 0.5 increase in CAVI corresponds to 10 years of age. 6 Although the CAVI of diabetic patients was higher than that of non‐diabetic patients in past reports, 24 , 25 these reports did not include very elderly patients. In this study, the CAVI of diabetic patients was higher than that of non‐diabetic patients even in the very elderly, but diabetes/glucose tolerance disorder was not an independent determinant of CAVI after adjusting for covariates. Lind et al showed that the impact of traditional cardiovascular risk factors including glucose generally declined with aging in a study with a longitudinal design. 26 Advancing age per se might obscure the effect of diabetes on CAVI. Thus, the effects of glucose might differ according to age. The American Diabetes Association recommends avoiding hypoglycemia and excessive treatment in elderly patients with diabetes, aiming for an easier or simplified treatment plan in this age group. 27 We need to carefully decide strategies for diabetes in the elderly.
Mean BP was associated with CAVI even in patients aged 80 years or more. Wen et al investigated 4,659 healthy persons ranging in age from 20 to 75 years and revealed that systolic BP was independently associated with CAVI after 45 years of age. 28 However, the relationship between BP and CAVI in elderly remains unclear. Trials such as the Hypertension in the Very Elderly Trial 29 and SPRINT study 30 showed the benefits of lowering BP even in the elderly. CAVI is not only associated with atherosclerosis, 10 , 11 , 12 , 13 but also predicts future cognitive dysfunction. 31 To prevent CV events and promote maintenance of a healthy lifestyle, the evaluation of CAVI and modification of BP might be useful in the elderly.
The main limitation of the present study was that enrolled patients were limited to Japanese patients with cardiovascular risk. For this reason, the results of this study cannot be applied to the general population. In this study, diabetes/glucose tolerance disorder and glucose were not independently associated with the CAVI in those aged ≥80 years after adjusting for other covariates. This finding may have been due to the small number of patients aged ≥80 years. Overcorrection may have led to a lack of association between diabetes/glucose tolerance, glucose, and CAVI in patients aged ≥80 years. Whether the applicability of CAVI varies with age, including in elderly patients, remains unclear. Further prospective studies are needed to examine this issue, and we are currently conducting Coupling Study with a 7‐year follow‐up to evaluate the relationship between CAVI and CV events, including in the elderly.
5. CONCLUSIONS
The determinants of the CAVI in very elderly patients were age, male sex, low BMI, and mean BP. Diabetes and glucose were independently associated with the CAVI in the patients aged <60 years and 60‐79 years, but not in those aged ≥80 years after adjusting for other covariates.
CONFLICT OF INTEREST
Kazuomi Kario has received research grants from Tanabe Mitsubishi Pharma Corporation. All other authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Kario K, Kabutoya T, and Hoshide S contributed to study design, data collection, organization of the study, drafting of the manuscript, and critical revision for important intellectual content. Fujiwara T, Negishi K, Nishizawa M, Yamamoto M, Yamagiwa K, Kawashima A, Yoshida T, Nakazato J, Matsui Y, Sekizuka H, Abe H, Abe Y, Fujita Y, Sato K, Narita K, Tsuchiya N, Kubota Y, and Hashizume T contributed to data collection. All authors have read and given final approval of the version to be published.
ACKNOWLEDGMENTS
We thank all nurses and physicians of the participating center for their excellent cooperation and help. We also thank Ms Yuri Matsumoto and Ms Yukie Okawara, for providing study management; and Ms Kimiyo Saito, Ms Tomoko Shiga, Ms Chiharu Saito, and Ms Ryoko Nozue for their study coordination and data management.
Kabutoya T, Hoshide S, Fujiwara T, et al. Age‐related difference of the association of cardiovascular risk factors with the cardio‐ankle vascular index in the Cardiovascular Prognostic Coupling Study in Japan (the Coupling Registry). J Clin Hypertens. 2020;22:1208–1215. 10.1111/jch.13896
Funding information
This study was funded by the Fukuda Denshi Co., Ltd.
REFERENCES
- 1. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554‐566. [DOI] [PubMed] [Google Scholar]
- 2. Mattace‐Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657‐663. [DOI] [PubMed] [Google Scholar]
- 3. Reference value for Arterial Stiffness’s Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamashina A, Tomiyama H, Arai T, et al. Brachial‐ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615‐622. [DOI] [PubMed] [Google Scholar]
- 5. Nye ER. The effect of blood pressure alteration on the pulse wave velocity. Br Heart J. 1964;266:261‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shirai K, Hiruta N, Song M, et al. Cardio‐ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18:924‐938. [DOI] [PubMed] [Google Scholar]
- 7. Gohbara M, Iwahashi N, Sano Y, et al. Clinical impact of the cardio‐ankle vascular index for predicting cardiovascular events after acute coronary syndrome. Circ J. 2016;80:1420‐1426. [DOI] [PubMed] [Google Scholar]
- 8. Chung SL, Yang CC, Chen CC, et al. Coronary artery calcium score compared with cardio‐ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb. 2015;22:1255‐1265. [DOI] [PubMed] [Google Scholar]
- 9. Laucevičius A, Ryliškytė L, Balsytė J, et al. Association of cardio‐ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina. 2015;51:152‐158. [DOI] [PubMed] [Google Scholar]
- 10. Horinaka S, Yabe A, Yagi H, et al. Comparison of atherosclerotic indicators between cardio ankle vascular index and brachial ankle pulse wave velocity. Angiology. 2009;60:468‐476. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura K, Tomaru T, Yamamura S, et al. Cardio‐ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J. 2008;72:598‐604. [DOI] [PubMed] [Google Scholar]
- 12. Park JB, Park HE, Choi SY, et al. Relation between cardio‐ankle vascular index and coronary artery calcification or stenosis in asymptomatic subjects. J Atheroscler Thromb. 2013;20:557‐567. [DOI] [PubMed] [Google Scholar]
- 13. Hu H, Cui H, Han W, et al. A cutoff point for arterial stiffness using the cardio‐ankle vascular index based on carotid arteriosclerosis. Hypertens Res. 2013;36:334‐341. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka A, Tomiyama H, Maruhashi T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72:1060‐1071. [DOI] [PubMed] [Google Scholar]
- 15. Wohlfahrt P, Cífková R, Movsisyan N, et al. Reference values of cardio‐ankle vascular index in a random sample of a white population. J Hypertens. 2017;35:2238‐2244. [DOI] [PubMed] [Google Scholar]
- 16. Tabara Y, Setoh K, Kawaguchi T, et al. Factors affecting longitudinal changes in cardio‐ankle vascular index in a large general population: the Nagahama study. J Hypertens. 2018;36:1147‐1153. [DOI] [PubMed] [Google Scholar]
- 17. The World Health Organization: Ageing and health. https://www.who.int/news‐room/fact‐sheets/detail/ageing‐and‐health
- 18. Kario K, Kabutoya T, Fujiwara T, et al. Rationale, design, and baseline characteristics of the cardiovascular prognostic coupling study in Japan (the Coupling Registry). J Clin Hypertens (Greenwich). 2020;22(3):465‐474. 10.1111/jch.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253‐390. [DOI] [PubMed] [Google Scholar]
- 20. Nagayama D, Imamura H, Sato Y, et al. Inverse relationship of cardioankle vascular index with BMI in healthy Japanese subjects: a cross‐sectional study. Vasc Health Risk Manag. 2017;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Momin M, Li JP, Zhang Y, et al. Body mass index is inversely associated with arterial stiffness in Chinese adults with primary hypertension: results from the China Stroke Primary Prevention Trial (CSPPT). Clin Exp Hypertens. 2017;39(5):394‐401. [DOI] [PubMed] [Google Scholar]
- 22. Gomez‐Marcos MA, Gomez‐Sanchez L, Patino‐Alonso MC, et al. A body shape index and vascular structure and function in Spanish adults (MARK study): A cross‐sectional study. Medicine (Baltimore). 2018;97(47):e13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shirai K, Utino J, Otsuka K, et al. A novel blood pressure independent arterial wall stiffness parameter; Cardio‐ankle vascular index (CAVI). J Atheroscler Thromb. 2006;13:101‐107. [DOI] [PubMed] [Google Scholar]
- 24. Namekata T, Suzuki K, Ishizuka N, et al. Establishing baseline criteria of cardio‐ankle vascular index as a new indicator of arteriosclerosis: a cross‐sectional study. BMC Cardiovasc Disord. 2011;11:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang H, Liu J, Zhao H, et al. Arterial stiffness evaluation by cardio‐ankle vascular index in hypertension and diabetes mellitus subjects. J Am Soc Hypertens. 2013;7:426‐431. [DOI] [PubMed] [Google Scholar]
- 26. Lind L, Sundström J, Ärnlöv J, et al. Impact of Aging on the Strength of Cardiovascular Risk Factors: A Longitudinal Study Over 40 Years. J Am Heart Assoc. 2018;7(1):e007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Diabetes Association . Standards of medical care in diabetes‐2019. Diabetes Care. 2019;42:S1‐S193. [DOI] [PubMed] [Google Scholar]
- 28. Wen W, Luo R, Tang X, et al. Age‐related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis. 2015;238:147‐152. [DOI] [PubMed] [Google Scholar]
- 29. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887‐1898. [DOI] [PubMed] [Google Scholar]
- 30. SPRINT Research Group , Wright JT Jr, Williamson JD, et al. A Randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yukutake T, Yamada M, Fukutani N, et al. Arterial stiffness predicts cognitive decline in Japanese community‐dwelling elderly subjects: a one‐year follow‐up study. J Atheroscler Thromb. 2015;22:637‐644. [DOI] [PubMed] [Google Scholar]


