Abstract
Vascular biomarkers, including the cardio‐ankle vascular index (CAVI), are increasingly being recognized as important indicators of cardiovascular risk. CAVI has been shown to have good discriminative ability for detecting new‐onset hypertension, but results of studies investigating cardiovascular risk prediction are inconsistent. Furthermore, there is a lack of data on the prognostic value of changes in CAVI over time. The Cardiovascular Prognostic Coupling study was designed to determine the impact of baseline CAVI and changes in CAVI on cardiovascular events in a Japanese cohort. The design of the ongoing, multicenter, prospective, observational registry and baseline characteristics of the enrolled population are reported. Eligible consecutive patients were aged ≥30 years, had ≥1 cardiovascular risk factor, and were being treated according to relevant Japanese guidelines. The primary outcome is time to onset of a major cardiovascular event (a composite of cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, stroke of unknown etiology, myocardial infarction, cardiovascular intervention for angina pectoris, and sudden death). Screening and enrollment occurred over a period of 3 years, followed by ≥7 years of follow‐up, with CAVI determined annually. A total of 5279 patients were registered, of whom 5109 had baseline data available and will be included in future analyses. Mean CAVI at baseline was 8.8 ± 1.4. The proportion of patients with CAVI of <8, 8‐10 or >10 was 25.3%, 57.0%, and 17.7%, respectively. Data from this registry should provide information on the significance of baseline CAVI and change in CAVI as indicators of cardiovascular prognosis in a representative patient population.
Keywords: cardio‐ankle vascular index, cardiovascular death, cardiovascular risk, registry, stroke
1. INTRODUCTION
We recently proposed a novel disease entity, the systemic hemodynamic atherothrombotic syndrome (SHATS), which is characterized by a vicious cycle between hemodynamic stress and vascular disease, and is a risk factor for cardiovascular events and organ damage.1, 2, 3 Pressure and vascular biomarkers make up two core biomarkers for SHATS. Pressure biomarkers include variability in visit‐to‐visit clinic, home and ambulatory blood pressure (BP) readings, morning and/or nighttime BP surge, and central pressure. Vascular biomarkers are the cardio‐ankle vascular index (CAVI), ankle‐branchial index (ABI), pulse wave velocity (PWV), flow‐mediated dilation (FMD), arterial waveform, and aortic stiffness evaluated by aortic magnetic resonance imaging (MRI).
Pressure biomarkers of SHATS can be detected using home BP monitoring (HBPM), ambulatory BP monitoring (ABPM), and the active standing test. A number of studies have investigated associations between pressure biomarkers and cardiovascular risk. Firstly, cardiovascular risk in individuals with white‐coat hypertension appears only to be elevated in the presence of coexisting risk factors, whereas all patients with masked hypertension are at increased risk of target organ damage and cardiovascular events.4 In another study, mean and maximum systolic BP (SBP) values in patients with one cardiovascular risk factor were significantly associated with markers of target organ damage, and the association between maximum SBP and carotid intima‐media thickness was significantly stronger than that between mean SBP and carotid intima‐media thickness.5
Studies such as these have contributed to a better understanding of the importance of BP control for cardiovascular organ protection. However, there is currently less understanding about the relationship between vascular biomarkers and cardiovascular damage. CAVI has been shown to have good discriminative ability for detecting new‐onset hypertension in a study of Japanese adults.6 In addition, a systematic review reported modest associations between CAVI and incident cardiovascular disease events (but not all‐cause mortality).7 However, the systematic review was based on cross‐sectional and short‐term studies, and there are currently no longitudinal studies investigating the association between CAVI and cardiovascular disease.
The Cardiovascular Prognostic COUPLING Study in Japan was designed to clarify the relationship between BP and vascular properties in hypertensive patients and to investigate the relationship between vascular properties and the onset of cardiovascular events in patients at high risk of cardiovascular disease. Specifically, the impact of baseline CAVI and changes in CAVI over time on cardiovascular events in a nationwide general practitioner‐based cohort is being assessed. This paper described the study design and baseline characteristics of the enrolled population.
2. METHODS
2.1. Study design
The nationwide Cardiovascular Prognostic Coupling study is an ongoing, multicenter, prospective, observational registry. The study protocol was submitted to and approved by the ethics committee of the internal review board of the Jichi Medical University School of Medicine and the independent ethics committees at each study institution. This study was registered at http://www.umin.ac.jp/ctr/ (Trial registration reference number: UMIN000018474). This study is coordinated Community Medicine Cardiovascular Research Asia IT Network Center, Division of Cardiovascular Medicine, Jichi Medical University School of Medicine. Fukuda Denshi Co., Ltd. is co‐investigator. Written informed consent was obtained from all patients prior to enrollment in the study, and patients were made aware of their right to withdraw from the study at any time and the measures in place for protection of privacy.
2.2. Patients
Consecutive patients aged ≥30 years with at least one cardiovascular risk factor (Table 1) were recruited by 67 doctors at 30 medical institutions throughout Japan from July 2015 to September 2018. Patients with any of the following were excluded: chronic renal failure requiring hemodialysis; other serious illnesses (eg, end‐stage cancer, active connective tissue disease); alcohol or drug addiction; inability to attend hospital visits or provide informed consent; or judged as inappropriate by the study physician.
Table 1.
Cardiovascular risk factor inclusion criteria
| Cardiovascular risk factors |
|---|
| Diabetes or glucose tolerance disorder |
| Dyslipidemia |
| High‐normal normotension and grade I‐III hypertension (blood pressure >130/85 mm Hg) |
| Current smoker |
| Renal disease (estimated glomerular filtration rate ≥60 mL/min/1.73 m2, or positive proteinuria) |
| History of cardiovascular disease (coronary artery disease, cerebrovascular or non‐cardiogenic cerebrovascular disorder, aortic dissection, peripheral artery disease, hospitalization for heart failure) |
| Atrial fibrillation |
| Metabolic syndrome |
| Chronic obstructive pulmonary disease |
| Sleep apnea syndrome |
2.3. Outcomes
The primary outcome is time to onset of a major cardiovascular event (a composite of cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, stroke of unknown etiology, myocardial infarction, cardiovascular intervention for angina pectoris, and sudden death). Key secondary outcomes are the individual component events of the composite primary endpoint. Other secondary and additional outcomes are also being investigated (Table 2).
Table 2.
Outcomes
| Primary outcomes |
| A composite of cerebral infarction, cerebral hemorrhage, cardiogenic stroke, subarachnoid hemorrhage, stroke of unknown etiology, myocardial infarction, cardiovascular intervention for angina pectoris, and sudden death |
| Secondary outcomes |
| Each cardiovascular event of the primary outcome (ie, cerebral infarction, cerebral hemorrhage, cardiogenic stroke, subarachnoid hemorrhage, stroke of unknown etiology, myocardial infarction, cardiovascular intervention for angina pectoris, and sudden death) |
| Any of the following events: hospitalized heart failure; aortic dissection; peripheral artery disease; end‐stage renal insufficiency; doubling of serum creatinine values; new‐onset atrial fibrillation; dementia; requirement for nursing care; death from any cause |
| Change in clinic blood pressure |
| Change in cardio‐ankle vascular index or ankle‐branchial index |
| Development of left ventricular hypertrophy (by echocardiography or cardiac magnetic resonance imaging) |
| Adverse events |
| Other outcomes |
| Home blood pressure |
| 24‐h ambulatory blood pressure |
| Findings of echocardiography |
| Findings of carotid echography |
| Cardiac and aortic findings of magnetic resonance imaging |
| Flow‐mediated dilatation |
| Oxygen saturation during sleep (pulse oximetry) |
| Lung function of pulmonary function testing |
2.4. Assessments
The study outline is shown in Table 3. Briefly, screening and enrollment occurred over a period of 3 years and then patients are being followed up for at least 7 years. Throughout the study, all patients are receiving standard therapy based on the relevant Japanese guidelines. The occurrence of major cardiovascular events is being monitored continually during follow‐up. CAVI, ABI, pulse waveform, electrocardiogram, clinic BP, and blood and urine laboratory testing are being evaluated annually. Special blood tests for determining N‐terminal pro‐B‐type natriuretic peptide, troponin T, calciprotein particle, and growth differentiation factor 15 are performed after 3 and 7 years of follow‐up. All data are collected electronically and transferred to a central electronic data capture system via the Internet. CAVI and ABI are measured using the cuff‐oscillometric method (Vasera‐1500 or 3000; Fukuda Denshi, Co., Ltd.).8
Table 3.
Outline of coupling registry study assessments
| Screening and enrollment | Follow‐up for 7 y | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 y | 2 y | 3 y | 4 y | 5 y | 6 y | 7 y | ||
| Patient background and baseline data | ○ | |||||||
| Primary outcome: time to onset of major CV events (composite) | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Secondary outcomes: time to onset of each CV event | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| Secondary key outcomes | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| CAVI | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| ABI | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Pulse waveform | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| ECG | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Clinic blood pressure | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Blood and urine laboratory testing | ○ | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
| Special blood testa | ○ | ○ | ○ | |||||
Abbreviations: ABI, ankle‐brachial index; CAVI, cardio‐ankle vascular index; CV, cardiovascular; ECG, electrocardiogram.
To measure N‐terminal pro‐brain natriuretic peptide, troponin T, calciprotein particle and growth differentiation factor 15.
Cardio‐ankle vascular index is measured after a few minutes of rest in a supine position. Cuffs were attached to the brachia and ankles, and pulse volume waveforms at four extremities were simultaneously recorded using a plethysmographic sensor connected to the cuffs. Measurements were recorded for maximum 16 seconds under compression of 50 mm Hg. BP at four extremities were then measured by the cuff‐oscillometric method. CAVI was calculated using the following formula,9 where Psys = SBP, P dia = DBP, ρ = blood density, haPWV is PWV from the origin of the aorta to tibial artery at the ankle through the femoral artery, and a and b are constants to convert the values of CAVI:
2.5. Sample size
Sample size calculations were made based on data from the Japan Morning Surge‐Home Blood Pressure (J‐HOP) study.10 J‐HOP was a nationwide practice‐based study that included 4310 patients with a history of, or risk factors for, cardiovascular disease, or both (mean age, 65 years; 79% using antihypertensive medication). During a mean follow‐up of 4 years (16 929 person‐years), 74 stroke and 77 coronary artery disease events occurred. On that basis, it was assumed that 91 stroke and 87 coronary artery disease events would occur in a sample of 5000 patients with a mean follow‐up of 4 years (20 000 person‐years).
2.6. Statistical analyses
Categorical variables are presented as number and percentage. Continuous variables are expressed as mean ± standard deviation (SD). Changes from baseline will be assessed using a by paired t test. Time to onset of primary and secondary outcome cardiovascular events will be estimated using Kaplan‐Meier analysis. The relationship between time to onset of cardiovascular events and each variable of interest (change in clinic BP, change in CAVI or ABI, development of left ventricular hypertrophy) will be analyzed using a Cox proportional hazard regression model. In all analyses, a two‐sided P‐value of <.05 is considered statistically significant. All statistical analyses are being performed using SAS software (ver 9.4; SAS Institute Inc).
3. RESULTS
3.1. Subjects
A total of 5279 patients were registered, of whom 5109 had baseline data available and will be included in future analyses (Table 4). The most common cardiovascular risk factor was high‐normal BP or grade I‐III hypertension (>80% of patients), and 17.4% of patients (20.4% of women and 14.6% of men) were aged ≥80 years (Table 4).
Table 4.
Patient demographic and clinical characteristics at baseline
| Variables | Patients (n = 5109) |
|---|---|
| Male (patients) | 52.4% |
| Age (y) | 68.7 ± 11.4 |
| Age ≥80 y (patients) | 17.4% |
| Body mass index (kg/m2) | 24.7 ± 4.0 |
| Current smoker (patients) | 9.1% |
| History of cardiovascular diseasea (patients) | 23.6% |
| Complications (patients) | |
| Diabetes, glucose tolerance disorder | 32.4% |
| Dyslipidemia | 57.1% |
| High‐normal normotension or grade I‐III hypertension | 83.5% |
| Renal disease (eGFR ≥60 mL/min/1.73 m2 or positive proteinuria) | 19.9% |
| Atrial fibrillation | 9.3% |
| Metabolic syndrome | 10.5% |
| Chronic obstructive pulmonary disease | 1.8% |
| Sleep apnea syndrome | 4.8% |
| Main concomitant drugs (patients) | |
| Antihypertensives | 83.4% |
| Statins | 46.8% |
| Aspirin | 16.1% |
| Clinic blood pressure, mm Hg | |
| Systolic | 133.5 ± 16.6 |
| Diastolic | 76.8 ± 10.6 |
| Cardio‐ankle vascular index | 8.8 ± 1.4 |
| Ankle‐branchial index | 1.1 ± 0.1 |
Values are presented as mean ± SD, or percentage of patients.
Abbreviation: eGFR, estimated glomerular filtration rate.
Stroke, percutaneous coronary revascularization or myocardial infarction.
3.2. Baseline CAVI
Mean CAVI at baseline was 8.8 ± 1.4. CAVI showed a normal distribution, with the majority of patients having a value of 8‐10 (Figure 1). The proportion of patients with CAVI of <8, 8‐10, or >10 was 25.3%, 57.0%, and 17.7%, respectively. Mean CAVI values in men were significantly higher than those in women from age 40 years onwards, and the rate of increase in CAVI as age increased was significantly greater in men than in women (interaction P < .001; Figure 2).
Figure 1.
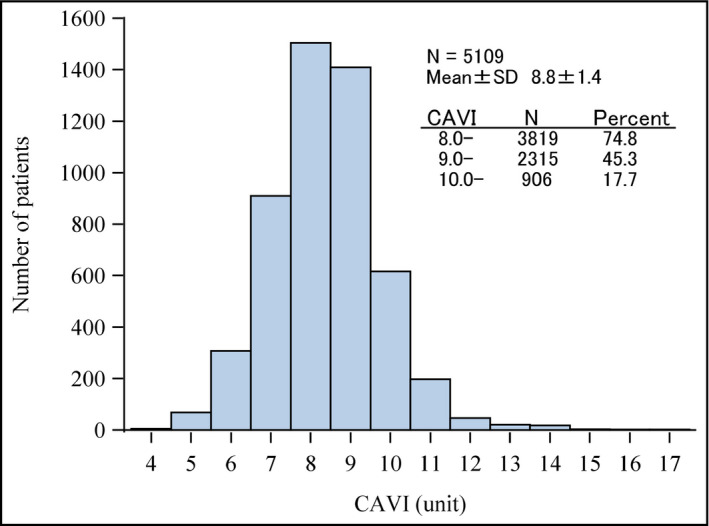
Distribution of baseline cardio‐ankle vascular index (CAVI) values in the population at baseline
Figure 2.
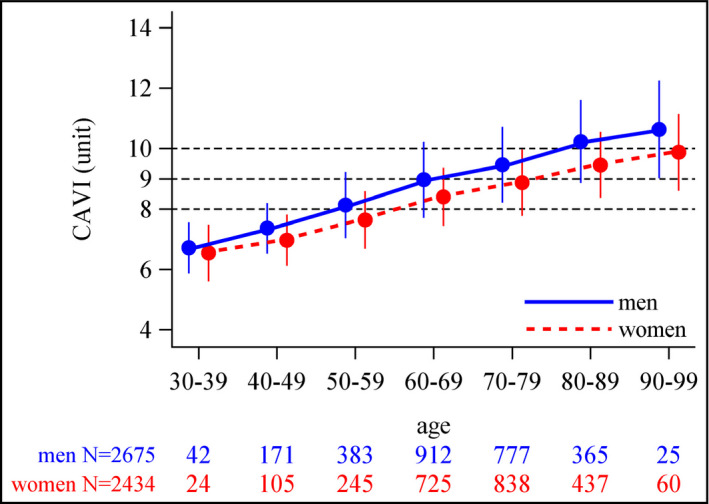
Mean baseline cardio‐ankle vascular index (CAVI) values in 10‐y age categories by patient sex (values are mean ± SD)
4. DISCUSSION
The CAVI is a new measure of arterial stiffness that reflects stiffness from the ascending aorta to the ankle arteries. CAVI is largely independent of heart rate and BP, and is a method that is reproducible and easy to use.11, 12 Both CAVI13, 14 and the ABI15, 16 have been widely used to evaluate arterial stiffening and arterial stenosis/obstruction, and both indices are considered a useful part of strategies to for prevent macroangiopathies.
A number of studies have investigated the association between CAVI and the occurrence of cardiovascular events, and these are summarized in Table 5. All previous studies apart from one17 had a cross‐sectional design. In general, higher baseline CAVI predicted future cardiovascular events,17, 18, 19, 20, 21, 22 but this was not a consistent finding across studies,23, 24 and CAVI may not be a good predictor of cardiovascular events in patients with CKD.25 Data did show that CAVI has a strong relationship with age, sex, and arterial stiffness. However, the relationship between change in CAVI over time (rather than baseline CAVI) and the occurrence of cardiovascular events is currently unclear.
Table 5.
Summary of studies investigating the association between cardio‐ankle vascular index (CAVI) and cardiovascular (CV) outcomes
| Study | Subjects (n) | Follow‐up | Baseline data | CV outcomes |
|---|---|---|---|---|
| Kato et al, 201223 | HD pts (n = 135) | 5.3 y |
Mean age: 60 ± 11 y Male: 67.4% Time on HD: 110 ± 93 mo CAVI: 9.7 ± 3.0 |
In a Cox proportional hazard analysis, CAVI tertile was not significantly associated with CV mortality. HR (95% CI) for CV mortality in CAVI tertiles:
|
| Otsuka et al, 201417 | Newly‐diagnosed CAD (n = 211) | 2.9 y |
Mean age: 65 ± 10 y Male: 56% CAVI: 10.05 ± 0.78 and 9.87 ± 0.65 in pt subgroups who went on to have improved or persistently impaired CAVI at 6 mo, respectively |
In a Cox proportional hazards model, persistently impaired CAVI at 6 mo was a significant independent predictor of CV events (cardiac death, non‐fatal MI, unstable angina, coronary revascularization, stroke) vs improved CAVI at 6 mo:
|
| Chung, 201518 | T2DM (n = 626) | 4.1 y |
Mean age: 64 y (range 37‐90) Male: 46% CAVI: 8.8 ± 1.4 |
In a logistic regression analysis, CAVI of ≥9.0 vs <9.0 was a significant predictor of CV events (PCI, CABG, coronary revascularization, ACS, ischemic stroke, death):
|
| Sato‐Asahara et al, 201519 | Obese pts (n = 425) | 5 y |
Mean age: 51.5 ± 14.1 y Male: 44.5% CAVI: 7.6 ± 1.5 |
In a step‐wise multivariate Cox analysis adjusted for age and sex, CAVI was a significant predictor of CV events (PCI, MI, stroke, atherosclerosis):
|
| Laucevicius et al, 201520 | MS without overt atherosclerosis (n = 2106) | 3.8 y |
Mean age: 53.8 ± 6.2 y Male: 38% CAVI: 7.92 ± 1.43 |
Cox proportional hazard regression analysis showed that each SD increase in CAVI increased the risk of CV events (MI, stroke or TIA, sudden cardiac death) by 26%:
This relationship was no longer statistically significant in the model adjusted for significant variables on univariate analysis. Kaplan‐Meier analysis showed that CAVI above the median was significantly associated with better CV event‐free survival (P = .038) |
| Sato et al, 201621 | Outputs with metabolic disorders (n = 1003) | 6.7 y |
Mean age: 62.5 ± 11.2 y Male: 51.2% CAVI: 9.25 ± 1.61 |
Cox proportional hazards regression analysis showed that CAVI was independently associated with future CV event risk (acute MI, unstable angina pectoris, stable angina pectoris):
|
| Gohbara et al, 201622 | ACS (n = 288) | 1.25 y |
Low CAVI group (≤8.325): Mean age: 58 ± 11 y Male: 87% High CAVI group (>8.325): Mean age: 71 ± 9 y Male: 78% |
Multivariate Cox proportional hazards analysis for CV events (CV death, non‐fatal MI, non‐fatal ischemic stroke) in the high CAVI vs low CAVI group:
|
| Kusunose et al, 201624 | Pts with ≥ 2 CV risk factors (n = 114) | 4.25 y |
Mean age: 69 ± 11 y Male: 78% CAVI: 8.5 ± 1.5 |
CAVI was not a significant predictor of CV events (cardiac death, non‐fatal MI/coronary revascularization, acute pulmonary edema, stroke) on univariable Cox proportional hazard analysis:
|
| Furusawa et al, 201925 | Asymptomatic pre‐dialysis CKD (n = 218) | 3.4 y |
Mean age: 68 ± 12 y Male: 70% CAVI: 9.1 ± 1.3 |
CAVI was not a significant predictor of CV events (CV death, MI, PCI, CABG, heart failure, cerebral infarction) on univariate Cox regression analysis:
|
|
CAVI‐J study (NCT01859897); Ongoing |
Pts with CV risk factors (n = 3000) | 5 y | Not yet reported | Primary CV endpoints: cardiac death, non‐fatal MI, stroke |
| Coupling study (UMIN000018474); Ongoing | Pts with CV risk factors | 7 y |
Mean age: 68.7 ± 11.4 y Male: 52.4% CAVI: 8.8 ± 1.4 |
Primary CV endpoints: a composite of cerebral infarction, cerebral hemorrhage, subarachnoid hemorrhage, stroke of unknown etiology, MI, CV intervention for angina pectoris, and sudden death |
Abbreviations: ACI, aortic calcification index; ACS, acute coronary syndrome; CABG, coronary artery bypass graft; CAD, coronary artery disease; CAVI, cardio‐ankle vascular index; CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; HD, hemodialysis; HR, hazard ratio; MI, myocardial infarction; mo, months; MS, metabolic syndrome; OR, odds ratio; PCI, percutaneous coronary intervention; pts, patients; SD, standard deviation; T2DM, type 2 diabetes mellitus; TIA, transient ischemic attack; y, years.
The Coupling registry is a prospective, large‐scale, and longitudinal study with repeated measurement of CAVI in high cardiovascular risk patients. It will provide data to allow determination of the effect of changes in CAVI, as well as baseline CAVI, on the cardiovascular event rate and its relative impact on cardiovascular events compared with baseline CAVI. Another study is also underway looking at the usefulness of CAVI for predicting cardiovascular events in Japan (the CAVI‐J study; NCT01859897). CAVI‐J will provide complementary data to the Coupling study, facilitating a more comprehensive picture of the association between CAVI and cardiovascular events.
Data from the Coupling registry have already been used to investigate the relationship between CAVI and brachial‐ankle pulse wave velocity (baPWV) and determined CAVI cutoff values that equate to baPWV values of 14 and 18 m/s.26 There was a positive and statistically significant association between CAVI and baPWV (r = .50, P < .001). Average baPWV in low‐risk patients (CAVI <8.303, n = 642) was 14.97 ± 2.91 m/s, in medium‐risk patients (CAVI 8.303‐9.058, n = 408) was 16.12 ± 2.80 m/s, and in high‐risk patients (CAVI ≥9.059, n = 687) was 18.40 ± 3.51 m/s.26 A CAVI value of 8.303 corresponded to a baPWV cutoff of 14 m/s, and CAVI 9.059 corresponded to a baPWV cutoff of 18 m/s.26
Asian populations have unique characteristics associated with the risk and incidence of cardiovascular disease compared with Western populations. Effective management of hypertension is particularly important in Asians because in many parts of the region the prevalence of stroke events is higher than that of coronary events, whereas the opposite is the case in Western populations.27 Furthermore, the risk of cardiovascular events with increasing BP increases more steeply in Asian vs Western populations.28 Therefore, the goal for the Coupling registry was to include a wide range of patients with a variety of cardiovascular risk factors. The current study includes a relatively high proportion of very elderly patients (17.4% were aged ≥80 years), reflecting the rapidly aging demographic in Asia. The mean age of patients enrolled in the registry was 68.7 years, similar to two previous CAVI studies24, 25 but higher than in others17, 18, 19, 20, 21, 23 (Table 5). Other features of the registry population, including the proportions of patients with hypertension, dyslipidemia, diabetes mellitus, and/or a history of cardiovascular disease, suggest that this is a high cardiovascular risk group.
The Framingham risk score is the most commonly used model for predicting the 10‐year incidence of cardiovascular events in the general population. This takes into account age, sex, BP, smoking habit, total or low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, and the presence/absence of diabetes mellitus.29, 30 The Framingham score is useful for encouraging lifestyle modification and promotes early prevention in the general population.31, 32 The addition of the ankle‐brachial BP index (ABI) to the Framingham risk score has been shown to significantly improve prognostic power.24 A baPWV of 14 m/s corresponds to a moderate risk of cardiovascular events based on the Framingham risk score.33 In analyses of Coupling registry data so far, this would be equivalent to a CAVI value of 8.303. Further analysis will provide additional information on the prognostic significance of this CAVI value in a representative patient population, and future studies could provide information about whether adding CAVI to the Framingham score might also increase the accuracy of cardiovascular risk prediction.
5. CONCLUSIONS
The design details and baseline characteristics of patients enrolled in the Coupling registry show that the study population is representative of routine clinical practice in Japan. The results of the anticipated analyses should provide robust and useful information on the significance of both baseline CAVI and change in CAVI over time as indicators of cardiovascular prognosis.
CONFLICT OF INTEREST
Kazuomi Kario has received research grants from Tanabe Mitsubishi Pharma Corporation. All other authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Kario K supervised the conduct of the study and data analysis, and had the primary responsibility of writing this paper. Kabutoya T, Fujiwara T, Negishi K, Nishizawa M, Yamamoto M, Yamagiwa K, Kawashima A, Yoshida T, Nakazato J, Matsui Y, Sekizuka H, Abe H, Abe Y, Fujita Y, Sato K, Narita K, Tsuchiya N, Kubota Y, Hashizume T, and Hoshide S collected data. Kabutoya T, Fujiwara T, Negishi K, Nishizawa M, Yamamoto M, Yamagiwa K, Kawashima A, Yoshida T, Nakazato J, Matsui Y, Sekizuka H, Abe H, Abe Y, Fujita Y, Sato K, Narita K, Tsuchiya N, Kubota Y, Hashizume T, and Hoshide S reviewed/edited the manuscript.
PARTICIPANTS AND PARTICIPATING CENTERS
Kazuomi Kario, Jichi Medical University School of Medicine and Washiya Hospital; Satoshi Hoshide, Jichi Medical University School of Medicine and Washiya Hospital; Tomoyuki Kabutoya, Jichi Medical University School of Medicine; Masahisa Shimpo, Jichi Medical University School of Medicine; Yasushi Imai, Jichi Medical University School of Medicine; Takahide Kohro, Jichi Medical University School of Medicine; Makoto Kuroo, Jichi Medical University School of Medicine; Kazuo Eguchi, Jichi Medical University School of Medicine and International University of Health and Welfare Hospital; Hiroshi Funayama, Jichi Medical University School of Medicine; Kenji Harada, Jichi Medical University School of Medicine; Ken Kono, Jichi Medical University School of Medicine; Kenta Okada, Jichi Medical University School of Medicine; Sachiyo Ogata, Jichi Medical University School of Medicine; Toshinobu Saito, Jichi Medical University School of Medicine; Takahiro Komori, Jichi Medical University School of Medicine and Yuki Hospital; Mutsuko Sarukawa, Jichi Medical University School of Medicine; Masao Takahashi, Jichi Medical University School of Medicine; Tomonori Watanabe, Jichi Medical University School of Medicine; Hiroyuki Mizuno, Jichi Medical University School of Medicine and Washiya Hospital; Hayato Shimizu, Jichi Medical University School of Medicine; Mizuri Taki, Jichi Medical University School of Medicine; Ayako Yokota, Jichi Medical University School of Medicine; Hiroaki Watanabe, Jichi Medical University School of Medicine; Kana Kubota, Jichi Medical University School of Medicine; Yukako Ogoyama, Jichi Medical University School of Medicine; Yusuke Ishiyama, Jichi Medical University School of Medicine; Keita Negishi, Jichi Medical University School of Medicine, Washiya Hospital and JCHO Utsunomiya Hospital; Satoshi Niijima, Jichi Medical University School of Medicine; Hajime Shinohara, Jichi Medical University School of Medicine; Kazuyo Ishibashi, Jichi Medical University School of Medicine; Yasuhiro Yokoyama, Jichi Medical University School of Medicine; Takeshi Fujiwara, Jichi Medical University School of Medicine and Higashiagatsuma Town National Health Insurance Clinic; Yumiko Fujita, Fujita Neurosurgery Clinic; Tetsuro Yoshida, Onga Nakama Medical Association Onga Hospital; Tetsuro Matsuda, Imabetsu Town National Health Insurance Imabetsu Clinic; Kaku Ryuichiro, Kotake Town Hospital; Mitsuyoshi Yamamoto, Kotake Town Hospital; Yuki Imaizumi, Kotake Town Hospital and Iizuka City Hospital; Masafumi Nishizawa, Minamisanriku Hospital; Akihiro Kawashima, Kawashima Cardiology Clinic; Shojiro Ishibashi, Ishibashi Internal Medicine Clinic; Suguru Horiguchi, Higashiagatsuma Town National Health Insurance Clinic; Yoshio Matsui, Iwakuni Medical Center Medical Association Hospital; Yoshiaki Kubota, Kubota Clinic; Miki Horigome, Saku Central Hospital Advanced Care Center; Jun Nakazato, Okinawa Chubu Hospital; Takahiro Shinohara, Suo Oshima, Municipal Towa Hospital; Meduki Masahiro, Suo Oshima Municipal Towa Hospital; Kayo Yamagiwa, Yamagiwa Clinic; Hideyasu Abe, Abe Internal Medicine Clinic; Yasuhisa Abe, Abe Internal Medicine Clinic; Toshikazu Hashidume, National Hospital Organization Minami Wakayama Medical Center; Norihiro Tsuchiya, Omotesando Clinic; Hiromitsu Sekizuka, Fujitsu Clinic; Takeshi Murakami, Munakata City National Health Insurance Oshima Clinic; Takahiro Tsuji, Munakata City National Health Insurance Oshima Clinic; Shunsuke Nakajima, Munakata City National Health Insurance Oshima Clinic; Takako Soma, Oma Hospital; Yasufumi Matsuoka, Oma Hospital; Takahiro Horano, Oma Hospital; Kei Sato, Yatsushiro City Shibaru Clinic; Kosuke Maehara, Yatsushiro City Shibaru Clinic; Akihiro Saitsu, Yatsushiro City Shibaru Clinic; Keisuke Narita, Karatsu City Madarashima Clinic; Shota Ikeda, Karatsu City Madarashima Clinic; Shinichi Toriumi, Tochigi Medical Center Shimotsuga; Yoshioki Nishimura, Shin‐Oyama Municipal Hospital.
ACKNOWLEDGMENTS
We thank all nurses and physicians of the participating center for their excellent cooperation and help. We also thank Ms Yuri Matsumoto and Ms Yukie Okawara, for providing study management; and Ms Kimiyo Saito, Ms Tomoko Shiga, Ms Chiharu Saito, and Ms Ryoko Nozue, for their study coordination and data management. Medical writing assistance was provided by Nicola Ryan, independent medical writer.
Kario K, Kabutoya T, Fujiwara T, et al. Rationale, design, and baseline characteristics of the Cardiovascular Prognostic COUPLING Study in Japan (the COUPLING Registry). J Clin Hypertens. 2020;22:465–474. 10.1111/jch.13764
Funding information
This study was funded by the Fukuda Denshi Co., Ltd.
REFERENCES
- 1. Kario K. Orthostatic hypertension‐a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726‐738. [DOI] [PubMed] [Google Scholar]
- 2. Kario K. Morning surge in blood pressure: a phenotype of systemic hemodynamic atherothrombotic syndrome. Am J Hypertens. 2015;28:7‐9. [DOI] [PubMed] [Google Scholar]
- 3. Kario K. Hemodynamic arteriosclerotic syndrome ‐ a vicious cycle of hemodynamic stress and vascular disease. J Clin Hypertens (Greenwich). 2018;20:1073‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kario K, Thijs L, Staessen JA. Blood pressure measurement and treatment decisions: masked and white coat hypertension. Circ Res. 2019;124:990‐1008. [DOI] [PubMed] [Google Scholar]
- 5. Kaihara T, Hoshide S, Tomitani N, Kanegae H, Kario K. Maximum home systolic blood pressure is a marker of carotid atherosclerosis. Clin Exp Hypertens. 2019;41:774‐778. [DOI] [PubMed] [Google Scholar]
- 6. Kario K, Kanegae H, Oikawa T, Suzuki K. Hypertension is predicted by both large and small artery disease. Hypertension. 2019;73:75‐83. [DOI] [PubMed] [Google Scholar]
- 7. Matsushita K, Ding N, Kim ED, et al. Cardio‐ankle vascular index and cardiovascular disease: systematic review and meta‐analysis of prospective and cross‐sectional studies. J Clin Hypertens (Greenwich). 2019;21:16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shirai K, Hiruta N, Song M, et al. Cardio‐ankle vascular index (CAVI) as a novel indicator of arterial stiffness: theory, evidence and perspectives. J Atheroscler Thromb. 2011;18:924‐938. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi K, Yamamoto T, Tsuda S, et al. Coefficients in the CAVI equation and the comparison between CAVI with and without the coefficients using clinical data. J Atheroscler Thromb. 2019;26:465‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoshide S, Kario K, Yano Y, et al. Association of morning and evening blood pressure at home with asymptomatic organ damage in the J‐HOP Study. Am J Hypertens. 2014;27:939‐947. [DOI] [PubMed] [Google Scholar]
- 11. Asmar R. Principles and usefulness of the cardio‐ankle vascular index (CAVI): a new global arterial stiffness index. Eur Heart J Suppl. 2017;19:B4‐B10. [Google Scholar]
- 12. Hayashi K, Yamamoto T, Takahara A, Shirai K. Clinical assessment of arterial stiffness with cardio‐ankle vascular index. J Hypertens. 2015;33(9):1742‐1757; discussion 57. [DOI] [PubMed] [Google Scholar]
- 13. Shirai K, Saiki A, Nagayama D, Tatsuno I, Shimizu K, Takahashi M. The role of monitoring arterial stiffness with cardio‐ankle vascular index in the control of lifestyle‐related diseases. Pulse (Basel). 2015;3:118‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horinaka S, Yagi H, Ishimura K, et al. Cardio‐ankle vascular index (CAVI) correlates with aortic stiffness in the thoracic aorta using ECG‐gated multi‐detector row computed tomography. Atherosclerosis. 2014;235:239‐245. [DOI] [PubMed] [Google Scholar]
- 15. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45(1):S5‐S67. [DOI] [PubMed] [Google Scholar]
- 16. Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 17. Otsuka K, Fukuda S, Shimada K, et al. Serial assessment of arterial stiffness by cardio‐ankle vascular index for prediction of future cardiovascular events in patients with coronary artery disease. Hypertens Res. 2014;37:1014‐1020. [DOI] [PubMed] [Google Scholar]
- 18. Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary artery calcium score compared with cardio‐ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb. 2015;22:1255‐1265. [DOI] [PubMed] [Google Scholar]
- 19. Satoh‐Asahara N, Kotani K, Yamakage H, et al. Cardio‐ankle vascular index predicts for the incidence of cardiovascular events in obese patients: a multicenter prospective cohort study (Japan Obesity and Metabolic Syndrome Study: JOMS). Atherosclerosis. 2015;242:461‐468. [DOI] [PubMed] [Google Scholar]
- 20. Laucevicius A, Ryliskyte L, Balsyte J, et al. Association of cardio‐ankle vascular index with cardiovascular risk factors and cardiovascular events in metabolic syndrome patients. Medicina. 2015;51:152‐158. [DOI] [PubMed] [Google Scholar]
- 21. Sato Y, Nagayama D, Saiki A, et al. Cardio‐ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb. 2016;23:596‐605. [DOI] [PubMed] [Google Scholar]
- 22. Gohbara M, Iwahashi N, Sano Y, et al. Clinical impact of the cardio‐ankle vascular index for predicting cardiovascular events after acute coronary syndrome. Circ J. 2016;80:1420‐1426. [DOI] [PubMed] [Google Scholar]
- 23. Kato A, Takita T, Furuhashi M, Maruyama Y, Miyajima H, Kumagai H. Brachial‐ankle pulse wave velocity and the cardio‐ankle vascular index as a predictor of cardiovascular outcomes in patients on regular hemodialysis. Ther Apher Dial. 2012;16:232‐241. [DOI] [PubMed] [Google Scholar]
- 24. Kusunose K, Sato M, Yamada H, et al. Prognostic implications of non‐invasive vascular function tests in high‐risk atherosclerosis patients. Circ J. 2016;80:1034‐1040. [DOI] [PubMed] [Google Scholar]
- 25. Furusawa K, Takeshita K, Suzuki S, et al. Assessment of abdominal aortic calcification by computed tomography for prediction of latent left ventricular stiffness and future cardiovascular risk in pre‐dialysis patients with chronic kidney disease: A single center cross‐sectional study. Int J Med Sci. 2019;16:939‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kabutoya T, Kario K. Comparative assessment of cutoffs for the cardio‐ankle vascular index and brachial‐ankle pulse wave velocity in a nationwide registry: a cardiovascular prognostic coupling study. Pulse (Basel). 2019;6:131‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueshima H, Sekikawa A, Miura K, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702‐2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Perkovic V, Huxley R, Wu Y, Prabhakaran D, MacMahon S. The burden of blood pressure‐related disease: a neglected priority for global health. Hypertension. 2007;50:991‐997. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM, Pasternak R, Greenland P, Smith S Jr, Fuster V. Assessment of cardiovascular risk by use of multiple‐risk‐factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Circulation. 1999;100:1481‐1492. [DOI] [PubMed] [Google Scholar]
- 30. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837‐1847. [DOI] [PubMed] [Google Scholar]
- 31. Brindle P, Beswick A, Fahey T, Ebrahim S. Accuracy and impact of risk assessment in the primary prevention of cardiovascular disease: a systematic review. Heart. 2006;92:1752‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Okwuosa TM, Greenland P, Ning H, et al. Distribution of coronary artery calcium scores by Framingham 10‐year risk strata in the MESA (Multi‐Ethnic Study of Atherosclerosis) potential implications for coronary risk assessment. J Am Coll Cardiol. 2011;57:1838‐1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamashina A, Tomiyama H, Arai T, et al. Brachial‐ankle pulse wave velocity as a marker of atherosclerotic vascular damage and cardiovascular risk. Hypertens Res. 2003;26:615‐622. [DOI] [PubMed] [Google Scholar]


