Abstract
Drug‐induced hypertension is one of the commonest causes of secondary hypertension. In the last few years, secondary hypertension due to tyrosine kinase inhibitors, from the vascular endothelial growth factor class, has been recognized to be an important cause of hypertension, as well as proteinuria, and occasionally kidney dysfunction in some cases. Less well‐recognized is that BCR‐ABL tyrosine kinase inhibitors also have adverse vascular effects. These manifest as vascular stenoses in large vessels, which may sometimes cause renal artery stenosis and subsequent hypertension. We describe a case report which presented as classical bilateral renal artery stenosis, and responded to revascularization. Increased awareness of these effects, as well as research into the pathogenesis, may provide more insight into vascular biology.
Keywords: BCR‐ABL mutation, drug‐induced hypertension, hypertension, renovascular hypertension, tyrosine kinase inhibitors
1. INTRODUCTION
Tyrosine kinase inhibitors (TKIs) that inhibit vascular endothelial growth factor (VEGF) have been implicated in development of hypertension, proteinuria, and kidney injury.1 TKIs that inhibit BCR‐ABL for the treatment of chronic myeloid leukemia (CML) have also been associated with adverse effects including peripheral edema, pleural effusions, pulmonary arterial hypertension, and vascular events.2 Vascular adverse events (VAEs) and peripheral arterial occlusive disease (PAOD) have been more commonly observed with second/third‐generation TKIs (nilotinib, dasatinib, and ponatinib).3, 4, 5 We present a patient with CML receiving ponatinib who developed renovascular hypertension due to bilateral renal artery stenosis, which responded to revascularization.
2. CASE PRESENTATION
A 55‐year‐old man of East Asian ethnicity had been diagnosed with CML in 2007, subsequently developed the T315I mutation and was initiated on ponatinib 45 mg daily in July 2011 via an expanded access program. He achieved major molecular remission with the targeted therapy. Ponatinib was decreased to 15 mg in October 2013 in response to an FDA safety review reporting a cumulative risk of serious arterial thrombosis of 11% from phase I and II trials to date. He was also evaluated for stem cell transplantation, but had no available donors, hence continued on Ponatinib. The patient was an ex‐smoker, having quit in 2006 after a 12 pack‐year history, and in 2014 was found to have hypertriglyceridemia and started on fenofibrate. He was also diagnosed with hypertension in September 2014, 3 years after starting ponatinib. He was initially started on candesartan 8 mg orally. Given inadequate blood pressure (BP) response, the dose was gradually increased, and in the summer of 2015, he was receiving candesartan 32 mg in a fixed‐dose combination with hydrochlorothiazide 12.5 mg daily. He was referred to nephrology in August 2015 after his serum creatinine was found to be elevated to 230 µmol/L (2.61 mg/dL) from a baseline of 121 µmol/L (1.37 mg/dL) in 2014. The urinalysis was bland, and a vasculitis workup was unremarkable. With candesartan/hydrochlorothiazide, the BP was 130/78 mm Hg. Since the creatinine was elevated, BP medications were switched in October 2015 to amlodipine 10 mg daily, with prompt improvement in creatinine to 116 µmol/L (1.31 mg/dL). However, the BP remained uncontrolled, needing additional anti‐hypertensive agents. In January 2016, BP was 168/83 despite amlodipine 10 mg, hydrochlorothiazide 25 mg, and bisoprolol 5 mg daily. To rule out renovascular disease, he underwent abdominal computed tomography (CT) angiogram, which revealed extensive stenoses of the arterial bed, including celiac, superior and inferior mesenteric, and both renal arteries (see Figure 1).
Figure 1.
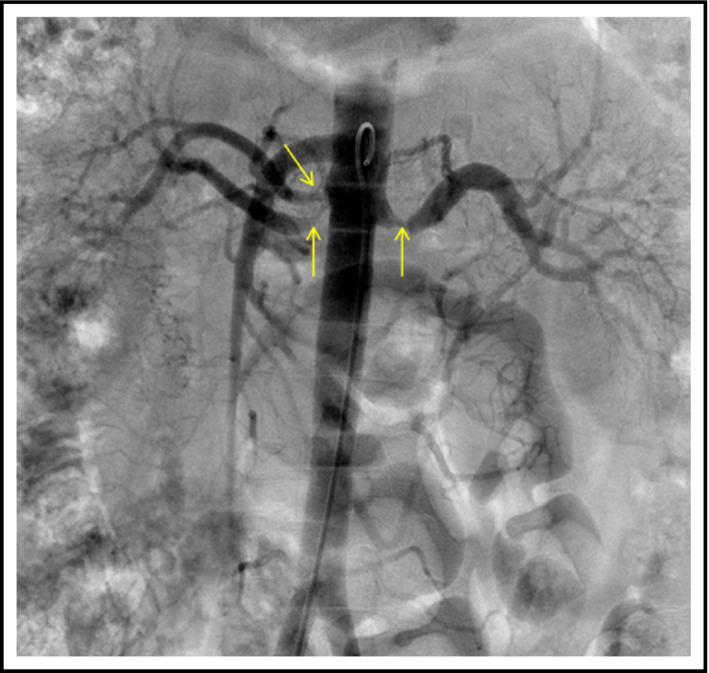
Bilateral renal artery stenosis
He was referred for further evaluation to the renal hypertension referral clinic. On five BP‐lowering drugs (amlodipine 10 mg daily, hydrochlorothiazide 25 mg daily, bisoprolol 5 mg daily, spironolactone 25 mg daily, and hydralazine 10 mg three times daily), 24‐hours ambulatory BP testing revealed daytime systolic hypertension (153/79), nighttime systolic and diastolic hypertension (136/75) with overall average BP 150/78. On physical examination, cardiac and respiratory examinations were unremarkable with no carotid bruits and normal jugular venous pressure and no signs of overt volume overload. There was a clear epigastric bruit and no peripheral edema.
Since his BP remained uncontrolled despite five anti‐hypertensive medications, renal revascularization was pursued, with successful angioplasty of stenoses within the anterior and posterior divisions of the right renal artery; and successful angioplasty and stenting (7 × 18 mm) of the left renal artery (Figure 2). There was early branching of the right renal artery; hence, this vessel was not stented. Two weeks after the intervention, creatinine had improved to 102 µmol/L (1.1 mg/dL) and BP medications were reduced to bisoprolol 2.5 mg, amlodipine 10 mg, and candesartan 8 mg daily was added with optimal BP control (see Table 1). He was prescribed clopidogrel 75 mg and aspirin 81 mg daily for 3 months after the procedure. Due to concerns about adverse vascular effects, and the fact that his BCR‐ABL transcript levels had been undetectable for a period of 5 years, ponatinib was discontinued. Unfortunately, 5 months later he had recurrence with detectable transcript levels, initially at 0.1%, then a month later at 1.1%, and hence, ponatinib was restarted at 15 mg daily.
Figure 2.
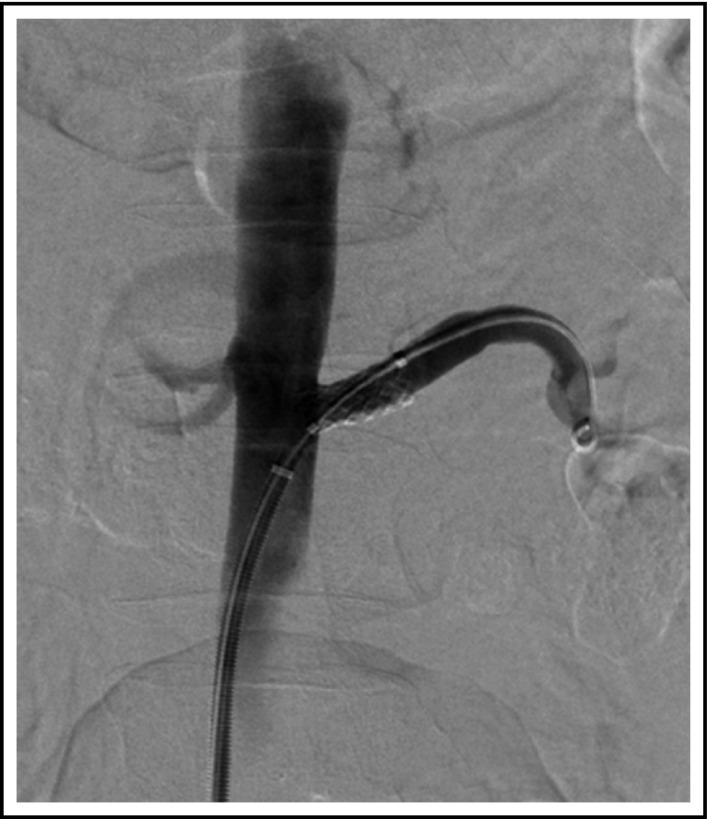
Successful revascularization. Balloon angioplasty of both renal artery branches on the right; angioplasty and stenting of the main renal artery on the left
Table 1.
Time course of blood pressure changes and kidney function
| Time | Clinical event | Blood pressure (mm Hg) | Blood pressure–lowering drugs | Creatinine (µmol/L) |
|---|---|---|---|---|
| July 2011 | Ponatinib started at 45 mg | 110/65 | None | 91 |
| September 2014 | Initial hypertension diagnosis | 157/96 | Candesartan 8 mg started | 87 |
| May 2015 | Candesartan 8 mg | 126 | ||
| Summer 2015 | Uncontrolled hypertension | Changed to Candesartan and hydrochlorothiazide (32/12.5 mg) | ||
| September 2015 | Initial Nephrology consultation |
Candesartan plus Hydrochlorothiazide (32/12.5 mg)—stopped Amlodipine 10 mg started |
230 | |
| October 2015 | Follow‐up laboratories | Amlodipine 10 mg | 116 | |
| January 2016 | Uncontrolled hypertension | 163/83 |
Amlodipine 10 mg Hydrochlorothiazide 25 mg Bisoprolol 5 mg Spironolactone 25 mg added |
117 |
| November 2016 | First renal revascularization | |||
| Late November 2016 | Follow‐up visit | 118/66 |
Amlodipine 10 mg Candesartan 8 mg Bisoprolol 2.5 mg |
112 |
| January 2017 | Ponatinib stopped |
Amlodipine 10 mg Candesartan 8 mg Bisoprolol 2.5 mg |
102 | |
| July 2017 | CML relapse and Ponatinib restarted at 15 mg | 140/72 |
Amlodipine 10 mg Candesartan 8 mg Bisoprolol 2.5 mg |
111 |
| October 2017 | Imaging reveals occlusion of one branch and recurrent stenosis of another branch of right renal artery | 128/68 |
Amlodipine 10 mg Candesartan 8 mg Bisoprolol 2.5 mg |
111 |
| November 2017 | Second renal revascularization | |||
| Feb 2018 | Follow‐up visit | 126/68 |
Amlodipine 10 mg Candesartan 8 mg Bisoprolol 2.5 mg |
118 |
| April 2019 | Last follow‐up visit | 130/80 |
Amlodipine 10 mg Candesartan 8 mg Bisoprolol 2.5 mg |
121 |
Abbreviation: CML, chronic myeloid leukemia.
Eight months following renal intervention, CT imaging to reassess vascular involvement was performed and revealed stable stenoses involving the origin of the celiac, superior, and inferior mesenteric arteries, but re‐occurrence of stenosis in the right renal artery. The superior divisional branch of the right renal artery was occluded, causing infarction of the posterior half of the kidney. The stenosis in the anterior divisional branch of the right renal artery had recurred post‐angioplasty (Figure 3A). Renal function and BP were stable. Given the recurrence, 4 months after repeat imaging (and 12 months after the initial angioplasty), he underwent successful angioplasty and stenting for the stenosis involving the anterior branch of the right renal artery (Figure 3B). Two weeks later, creatinine remained stable at 110 µmol/L (1.2 mg/dL) and BP at target (average of five readings on automated monitor of 130/67) on three anti‐hypertensive agents as previous.
Figure 3.
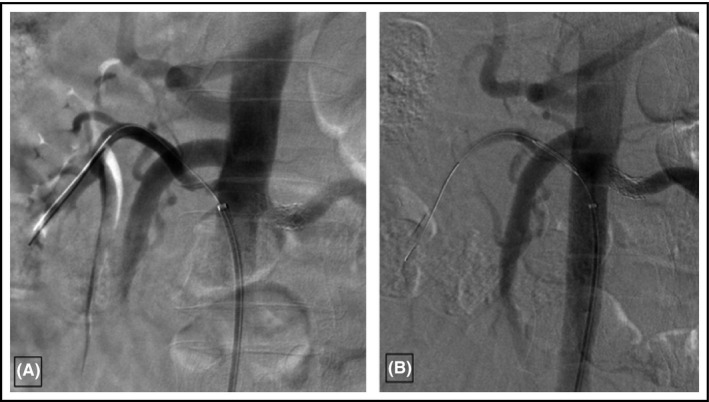
A, Re‐stenosis of the anterior divisional branch of the right renal artery. B, Successful repeat angioplasty of the right renal artery stenosis and stent placement
The patient was assessed by cardiology services for further vascular risk reduction and was found to have persistent hypertriglyceridemia, with total cholesterol 3.6 mmol/L, triglycerides 7.28 mmol/L, HDL 0.78 mmol/L, LDL 0.9 mmol/L, C‐reactive protein 4.3 mg/L, and lipoprotein(a) ˂9.7 mg/dL. CT angiography revealed no significant coronary artery stenosis, with mild atherosclerotic plaque burden in the coronaries. Further imaging revealed atherosclerotic disease at the bilateral carotid bifurcations and origin of both internal carotid arteries without significant stenosis (less than 50%). He was restarted on clopidogrel 75 mg and rosuvastatin 20 mg.
At follow‐up, 2 years after the initial intervention (and 1 year after the second intervention), renal function remained at baseline with excellent BP control on three agents. As of 2019, he remains on ponatinib 15 mg daily with undetectable transcript levels maintained.
3. DISCUSSION
The introduction of TKIs has improved the prognosis of CML, although they have been associated with VAEs (venous thrombosis, arterial hypertension, pulmonary hypertension) and PAOD. These adverse events seem to be a class effect with first‐generation TKIs (imatinib) often leading to peripheral edema and rarely even congestive heart failure. Newer agents such as dasatinib and bosutinib have been more commonly associated with pleural effusions, pulmonary hypertension, and diarrhea (bosutinib). Nilotinib and ponatinib have the highest reported cases of VAEs. These are summarized in a recent review on the adverse effects of different TKIs.2
In December 2012, the Federal Drug Administration (FDA) approved ponatinib 45 mg daily for “treatment of adult patients with chronic‐phase (CP), accelerated‐phase, or blast‐phase CML or Philadelphia chromosome‐positive acute lymphoblastic leukemia that is resistant or intolerant to prior TKI therapy,” following the results of the Ponatinib for Chronic Myeloid Leukemia Evaluation and Ph + Acute Lymphoblastic Leukemia (PACE) trial.6 The trial had initially reported ponatinib‐related serious arterial thrombotic events in 9% of patients, with only 3% felt to be treatment‐related. However, a prospective analysis of 19 patients who received ponatinib showed 42% of patients had arterial cardiovascular events after a median period of 8.5 months with a longer phase I trial showing overall vascular occlusive events in 37% of patients, with 23% serious VAEs.7 Cardiovascular, peripheral vascular, cerebrovascular, and venous thrombotic events were seen in 23%, 9%, 8%, and 5% of patients, respectively.8 Ponatinib‐related hypertension occurred in 67% of patients, prompting the FDA to suspend the drug in October 2013. It was reintroduced to the market in January 2014 with limited indications, a black box warning, and a maximum daily dose of 15 mg; in clinical practice, higher doses are still used for acute lymphoblastic leukemia and when initiating therapy for refractory CML not in remission. VAEs were previously thought to accumulate in ponatinib‐treated patients because of their prior exposure to nilotinib. More recent data suggest, however, that ponatinib can also induce VAEs in patients who had not received nilotinib.4, 5 Nilotinib, a second‐generation TKI, is thought to be very similar to ponatinib in pharmacokinetics and side effect profile. Risk factors for PAOD and VAEs with nilotinib include higher dose, known atherosclerosis, and pre‐existing risk factors such as advanced age, diabetes mellitus, cardiac history, arterial hypertension, cigarette smoking, and hyperlipidemia.2
Initial recognition of these vascular and adverse events was delayed since the majority of clinical trials were understandably underpowered for detection of VAEs. In addition, advanced patient age with CML and high incidence of atherosclerosis led to a delay in attribution of VAEs to the TKIs.5 There is also a latency period of months to years before patients on TKIs develop VAEs, and often these VAEs are either clinically silent or present with non‐specific symptoms including cramps or abdominal pain.2 The exact mechanism of vasculopathy remains unknown, and several hypotheses have been postulated with nilotinib including abnormal peripheral insulin resistance, and direct pro‐atherogenic and anti‐angiogenic effects.2
This is the first reported case of ponatinib‐induced VAEs and renal artery stenoses. Though we attribute renal artery stenosis to ponatinib, there is an unlikely possibility that vascular stenosis may have been pre‐existing prior to initiation of ponatinib. The chronology of the development of hypertension, the response to revascularization, as well as the other reports of vascular stenosis reported to the FDA suggest otherwise. In the present case, renal angioplasty was associated with adequate control of BP, and resumption of candesartan with preservation of kidney function. Similar results have been reported in another case report of bilateral renal artery stenoses with nilotinib.9
This case sheds light on the importance of having a high index of suspicion for VAEs and PAODs for patients on TKIs, especially nilotinib and ponatinib. Ponatinib was discontinued for a few months in our patient following diagnoses of these serious VAEs, which interestingly did not result in resolution or improvement of involved areas (right renal artery, abdominal aorta, and its major branches). Resumption of ponatinib was followed by recurrence of renal artery stenosis, and occlusion of a branch artery, suggesting a pro‐atherogenic mechanism. Additional risk factors in the patient were hypertriglyceridemia and a history of smoking.
Certain questions remain unanswered and require further research. This patient's renovascular hypertension remains stable despite continued ponatinib therapy, albeit at a lower dose. He has stopped smoking and is now on clopidogrel and rosuvastatin, which might be potential mitigating factors. Thus, the long‐term effects of ponatinib on stent patency, and the efficacy of anti‐platelet therapy and modification of risk factors in patients taking second‐ or third‐generation TKIs remain uncertain. The exact incidence of new‐onset hypertension and of hypertension attributable to renal artery stenosis in this setting remains to be ascertained. Until then, the clinician should remain cognizant of this potential development in patients receiving these agents and investigate patients who present with findings suggesting renovascular hypertension.
CONFLICT OF INTEREST
The authors have no conflict of interest or disclosures.
AUTHOR CONTRIBUTIONS
Syed O. Amin and Swapnil Hiremath drafted the initial manuscript. Syed O. Amin collected the data. All other authors contributed to critical review and revision of the manuscript.
ACKNOWLEDGMENTS
SH, MR, and KDB receive research salary support from the Department of Medicine, University of Ottawa
Amin SO, Ruzicka M, Burns KD, et al. Renovascular hypertension from the BCR‐ABL tyrosine kinase inhibitor ponatinib. J Clin Hypertens. 2020;22:678–682. 10.1111/jch.13843
REFERENCES
- 1. Touyz RM, Lang NN, Herrmann J, van den Meiracker AH, Danser AHJ. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension. 2017;70(2):220‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125(6):901‐906. [DOI] [PubMed] [Google Scholar]
- 3. Gafter‐Gvili A, Ram R, Gafter U, Shpilberg O, Raanani P. Renal failure associated with tyrosine kinase inhibitors–case report and review of the literature. Leuk Res. 2010;34(1):123‐127. [DOI] [PubMed] [Google Scholar]
- 4. Holstein SA, Stokes JB, Hohl RJ. Renal failure and recovery associated with second‐generation Bcr‐Abl kinase inhibitors in imatinib‐resistant chronic myelogenous leukemia. Leuk Res. 2009;33(2):344‐347. [DOI] [PubMed] [Google Scholar]
- 5. Pasvolsky O, Leader A, Iakobishvili Z, Wasserstrum Y, Kornowski R, Raanani P. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardio‐Oncology. 2015;1(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cortes JE, Kim DW, Pinilla‐Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome‐positive leukemias. N Engl J Med. 2013;369(19):1783‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gagnieu M‐C, Heiblig M, Blond E, et al. Cardio‐vascular events occurring on ponatinib in chronic phase chronic myeloid leukemia patients, preliminary analysis of a multicenter cohort. Blood. 2013;122(21):4020. [Google Scholar]
- 8. Talpaz M, Cortes JE, Kantarjian HM, et al. Longer‐term follow up of a phase 1 study of ponatinib in patients (pts) with Philadelphia chromosome‐positive (Ph+) leukemias. J Clin Oncol. 2014;32(15_suppl):7078‐7078. [Google Scholar]
- 9. Kristensen T, Randers E, Stentoft J. Bilateral renal artery stenosis in a patient with chronic myeloid leukemia treated with nilotinib. Leuk Res Rep. 2012;1(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]


