Abstract
At present, the central role played by arginine in the modulation of the inflammatory cellular responses is well-recognized, and many pro-inflammatory stimuli are known to modulate the expression and activity of its transmembrane transporters. In this regard, we have addressed the effects of bacterial flagellin from Pseudomonas aeruginosa (FLA-PA) on the uptake of the amino acid in human epithelial respiratory cells. Among the arginine transporters, only ATB0,+, y+L, and y+ were operative in bronchial epithelial Calu-3 cells under control conditions; however, only the expression and activity of ATB0,+ were stimulated upon incubation with flagellin, whereas those of systems y+L and y+ were not stimulated. As a result, this induction, in turn, led to an increase in the intracellular content of arginine without making any change to its metabolic pathway. In addition, flagellin upregulated the amount of other amino acids substrates of ATB0,+, in particular, all the essential amino acids, such as valine, isoleucine, and leucine, along with the non-essential glutamine. At the molecular level, these effects were directly referable to the stimulation of a toll-like receptor-5 (TLR5) signaling pathway and to the induction of nuclear factor-κB (NF-κB) transcription factor. An induction of ATB0,+ expression has been observed also in EpiAirway™, a model of primary human normal tracheal-bronchial epithelial cells that mimics the in vitro pseudostratified columnar epithelium of the airways. In this tissue model, the incubation with flagellin is associated with the upregulation of messenger RNAs (mRNAs) for the chemokine IL-8 and for the cytokines IL-6 and interleukin-1β (IL-1β); as for the latter, a marked secretion in the extracellular medium was also observed due to the concomitant activation of caspase-1. The overall findings indicate that, in human respiratory epithelium, flagellin promotes cellular responses associating the increase of intracellular amino acids through ATB0,+ with the activation of the inflammasome. Given the role of the ATB0,+ transporter as a delivery system for bronchodilators in human airway epithelial cells, its induction under inflammatory conditions gains particular relevance in the field of respiratory pharmacology.
Keywords: SLC6A14 (ATB0,+); glutamine; inflammatory cytokines; inflammasome; NF-kappa B
Introduction
Arginine is a semi-essential amino acid acting as a key driver of metabolic processes associated with several pathological and physiological conditions (1). Specifically, an increasing number of evidence ascribe to a key role of arginine metabolism in the modulation of immune cell functions (2), and arginine is considered as the obligatory substrate of two metabolic pathways central to the inflammatory processes, i.e., the degradation by arginase to ornithine and urea and the conversion to nitric oxide (NO) by nitric oxide synthase (NOS) (3).
In mammalian cells, arginine crosses the plasma membrane through four distinct transport systems: y+, y+L, b0,+, and B0,+ [for review see Deves and Boyd (4), Closs et al. (5)]. The cationic amino acid-specific system y+ mediates a membrane potential-sensitive, Na+-independent arginine transport. The activity of system y+ is referable to the cationic amino acid transporter (CAT) family of monomeric transporters, which includes the ubiquitous SLC7A1-encoded CAT1 and the two transporters, CAT2A and CAT2B, derived from the alternative splicing of a SLC7A2 transcript. Conversely, system y+L mediates a Na+-independent transport of arginine and a Na+-dependent transport of neutral amino acids, particularly leucine. Under physiological conditions, it operates as an antiport by exchanging intracellular arginine with extracellular neutral amino acids and sodium. System y+L belongs to the group of the heterodimeric amino acid transporters (HAT) and is formed by the heavy subunit 4F2hc/CD98 and one of the two alternative light chains, y+LAT1 (encoded by SLC7A7) or y+LAT2 (encoded by SLC7A6) (6). Also, system b0,+ belongs to the group of the HAT and is formed by b0,+AT (encoded by SLC7A9) linked through a disulfide bridge to the heavy subunit rBAT. This system is expressed in the small intestine and the proximal tubule of the kidney, where it mediates the reabsorption of cystine by exchanging extracellular dibasic amino acids and cystine with intracellular neutral amino acids (7). Another transporter involved in the arginine absorption is ATB0,+, an influx system for neutral and cationic amino acids endowed with a high concentrative capacity, which is energized by the transmembrane gradients of Na+ and Cl− as well as by the membrane potential (8). This transporter, first described in human epithelial airway cells by Galietta et al. (9), is mainly expressed in the lung and the intestine (10, 11). In in vitro models, only some airway epithelial cells, such as Calu-3 and NCl-H441, actually express ATB0,+, while other cells, such as A549 and BEAS-2B cells, do not (12). Recently, we have demonstrated that SLC6A14/ATB0,+ is maximally expressed on the apical side of EpiAirway™, a model of primary human tracheal-bronchial epithelial cells where it mediates the uptake of carnitine (13).
Most of intracellular arginine is known to depend on the uptake from the extracellular milieu, since a de novo synthesis is not sufficient to meet the metabolic needs under particular conditions, such as infections or inflammation (14). Consistently, pro-inflammatory mediators, such as lypolisaccaride (LPS), tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), and interferon-γ (IFNγ), have been described over the past few years, to enhance the cellular uptake of arginine through an increased expression of CAT transporters in a variety of cell models, mainly immune and endothelial cells (15–20). More recently, also the ATB0,+ activity in bronchial epithelial cells has been reported to be modulated by inflammatory stimuli, such as LPS and TNFα (13), and flagellin from Pseudomonas aeruginosa (FLA-PA) (21). In this study, we further explored the effects of flagellin on human bronchial cells by extending the study to all arginine transporters as well as by addressing the molecular pathway involved in the stimulation of SLC6A14/ATB0,+. Moreover, we also evaluated the activation of inflammasome as a consequence of flagellin-mediated induction of ATB0,+ in normal tracheal-bronchial EpiAirway™ tissues.
Materials and Methods
Cell Culture and Experimental Treatments
Calu-3 cells (American Type Culture Collection, VA, USA), obtained from a human lung adenocarcinoma and derived from serous cells of proximal bronchial airways, were routinely grown in 10-cm diameter dishes in Eagle's Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate (1 mM), and 1% penicillin/streptomycin. Cells between passages 25 and 32 were routinely maintained under physiological conditions (37.5°C, 5% CO2, 95% humidity). EpiAirway™ tissues (AIR-100), an organotypic in vitro model of primary human tracheal-bronchial epithelial cells that form a fully differentiated, pseudostratified columnar epithelium, were provided by MatTek IVLSL (Bratislava, Slovakia) and were cultured by following the instructions of the manufacturer as described in a previous study (22).
The experimental treatment was performed by adding a purified FLA-PA (Invivogen, CA, USA) to complete growth medium; for EpiAirway™, FLA-PA was added to the apical compartment. Nuclear factor-κB (NF-κB) inhibitors were added to the cell culture 1 h before the addition of flagellin when employed. No significant cell loss was observed for up to 24 h under any of the experimental conditions.
Real-Time Quantitative PCR Analysis
Gene expression has been analyzed through real-time quantitative PCR (RT-qPCR) as described in a previous study (23). About 1 μg of complementary DNA (cDNA) was obtained upon a reverse transcription of total RNA with the RevertAid First Strand cDNA Synthesis Kit (ThermoFisher Scientific, MA, USA); the qPCR analysis was then performed on a StepOnePlus Real-Time PCR System (ThermoFisher Scientific, MA, USA) by employing specific forward/reverse primer pairs (Table 1) and SYBR™ Green or TaqMan Gene Expression Master Mix (ThermoFisher Scientific, MA, USA). The expression of the gene of interest under each experimental condition was calculated by using the standard curve method (24) after normalization of the housekeeping gene (Ribosomal like protein 15, RPL15, Gene ID:6138).
Table 1.
Sequences of the primer pairs employed for real-time quantitative PCR (RT-qPCR) analysis.
| Gene/protein name (Gene ID) | Forward primer | Reverse primer |
|---|---|---|
| ARG2/Arginase2 (ID: 384) | AAGCTGGCTTGATGAAAAGGC | GCGTGGATTCACTATCAGGTTGT |
| NOS3/eNOS (ID: 4846) | TGGTACATGAGCACTGAGATCG | CCACGTTGATTTCCACTGCTG |
| IL1B/IL-1β (ID: 3553) | Hs99999029_m1 (TaqMan® Assay, ThermoFisher Scientific) | |
| IL6/IL-6 (ID: 3569) | AACCTGAACCTTCCAAAGATGG | TCTGGCTTGTTCCTCACTACT |
| SLC1A5/IL-8 (ID: 3576) | ACTGAGAGTGATTGAGAGTGGAC | AACCCTCTGCACCCAGTTTTC |
| SLC6A14/ATB0,+ (ID: 11254) | GCTGCTTGGTTTTGTTTCTCCTTGGTC | GCAATTAAAATGCCCCATCCAGCAC |
| SLC7A1/CAT1 (ID: 6541) | CTTCATCACCGGCTGGAACT | GGGTCTGCCTATCAGCTCGT |
| SLC7A2/CAT2A (ID: 6542) | TTCTCTCTGCGCCTTGTCAA | TCTAAACAGTAAGCCATCCCGG |
| SLC7A2/CAT2B (ID: 6542) | TTCTCTCTGCGCCTTGTCAA | CCATCCTCCGCCATAGCATA |
| SLC7A6/y+LAT1 (ID: 9057) | Hs00187757_m1 (TaqMan® Assay, ThermoFisher Scientific) | |
| SLC7A7/y+LAT2 (ID: 9056) | Hs00909952_m1 (TaqMan® Assay, ThermoFisher Scientific) | |
| TLR5/TLR5 (ID: 7100) | TCCCTGAACTCACGAGTCTTT | GGTTGTCAAGTCCGTAAAATGC |
| RPL15/RPL15 (ID: 6138) | GCAGCCATCAGGTAAGCCAAG | AGCGGACCCTCAGAAGAAAGC |
SLC6A14 Promoter Sequence Analysis
The analysis of a SLC6A14 promoter sequence was performed by employing the tools available online1 The sequence upstream of the transcription start site (TSS) of the SLC6A14 gene was obtained with a Sequence Retrieval Tool available from The Eukaryotic Promoter Database (25). Then, the Search Motif Tool was sed to scan the promoter region with position weight matrices (PWM) of NF-κB transcription factors obtained from the open-source JASPAR database (26). The scan was performed on the fly using the FindM tool from the Signal Search Analysis toolkit (27) at the Swiss Institute of Bioinformatics.
Western Blot Analysis
For the determination of ATB0,+ expression, cells were lysed in a RIPA buffer added with a cocktail of protease inhibitors (Complete, Mini, EDTA-free, Roche). The Western Blot analysis was performed as described in a previous study (28). Briefly, 20 μg of proteins were separated on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (4–12% acrylamide) and were electrophoretically transferred to polyvinylidene fluoride (PVDF) membranes (Immobilione-P membrane, Merck, NJ, USA). Membranes were first incubated for 1 h at room temperature (RT) in a Tris-buffered saline solution (TBS; 50 mM Tris-HCl pH 7.5, 150 mM NaCl) containing 5% non-fat dried milk and then incubated overnight at 4°C in a Tris-buffered saline with Tween 20 (TBST) buffer added with 5% bovine serum albumin (BSA) and anti-ATB0,+ purified rabbit polyclonal antibody (1:5,000, Merck, NJ, USA), anti-p65 NF-κB, or anti-phospho-p65 (Ser536) NF-κB (1:2000, Cell Signaling TECHNOLOGY, MA, USA). Vinculin, detected with a monoclonal antibody (1:2,000; Merck, NJ, USA), was employed as internal standard. Immunoreactivity was visualized by using the Immobilon Western Chemiluminescent HRP Substrate (Merck, NJ, UA). Western Blot images were captured by using an iBright FL1500 Imaging System (ThermoFisher Scientific, MA, USA) and analyzed with the iBright Analysis Software.
Amino Acid Uptake
For transport studies, Calu-3 cells were cultured onto 96-well trays (Falcon). After two rapid washes in a pre-warmed transport buffer [Earle's Balanced Salt Solution (EBSS) containing (in mM) 117 NaCl, 1.8 CaCl2, 5.3 KCl, 0.9 NaH2PO4, 0.8 MgSO4, 5.5 glucose, 26 Tris/HCl, adjusted to pH 7.4], the cells were incubated for 30 s in the same solution containing [3H]arginine (50 μM, 5 μCi/ml) in the absence or presence of the indicated amino acids (2 mM each) employed as inhibitors. When sodium-independent transport was measured, a modified Na+-free EBSS (NMG-EBSS) was employed, with NaCl and NaH2PO4 replaced with N-methyl-D-glucamine and choline salts, respectively. The experiment was terminated by two rapid washes (<10 s) in an ice-cold 300 mM urea. Intracellular radioactivity from the cell monolayers was extracted in ethanol and measured by using the MicroBeta2® liquid scintillation spectrometer (PerkinElmer, MA, USA). The uptake was normalized for the protein content, determined directly in each well by using a modified Lowry procedure (29), and expressed as nmol/mg of protein/min.
Determination of the Amino Acid Intracellular Content
For the measurement of intracellular amino acids, Calu-3 cells, grown on 24-well trays were rapidly washed with an ice-cold PBS, and the intracellular pool was extracted with a 10-min incubation in 200 μl of ethanol at 4°C. After freeze-drying, samples were suspended in 150 μl Lithium Loading Buffer (Biochrom, Cambridge, UK), and the intracellular content of each amino acid species was determined through a high-pressure liquid chromatography (HPLC) analysis with a Biochrom 30 Amino Acid Analyzer (Biochrom, Cambridge, UK), employing a high-resolution lithium column and lithium buffers for elution (Biochrom, Cambridge, UK). The column effluent was mixed with an EZ Nin Reagent Kit (Biochrom, Cambridge, UK), passed through the high-temperature reaction coil, and read by the photometer unit at both 570 and 440 nm. Protein content in each condition was determined using a modified Lowry procedure (29), and the content of amino acids was expressed as nmol/mg of protein.
Interleukin-1β Measurement
Supernatants collected from the FLA-PA-treated EpiAirway™ tissues were assayed for secreted IL-1β using the Human IL-1 beta/IL-1F2 Quantikine ELISA Kit (R&D) according to the instructions of the manufacturer. Briefly, cell supernatants were added to the plate along with blank and standard samples. After 2 h incubation at RT, samples were first incubated for 1 h with Human IL-1β Conjugate and then with the substrate solution for further 20 min. In the end, the reaction was stopped with the stop solution, and the optical density of each well was read by using an EnSpire® Multimode Plate Reader (Perkin Elmer, MA, USA), at 450 nm. The amount of the cytokine in each sample was calculated from the standard curve and expressed as pg/ml of the incubation medium.
Caspase-1 Activity
The activity of caspase-1 was measured with a CaspaseGlo®-1 Inflammasome Assay (Promega, WI, USA) in the incubation medium of FLA-PA-treated EpiAirway™ tissues according to the instructions of the manufacturer. Briefly, 50 μl of cell supernatant or fresh growth medium (blank) was transferred into a 96-well plate and mixed with 50 μl of a CaspaseGlo®-1 Reagent, previously completed with a 6 μl/ml MG132 inhibitor. After the incubation at RT for 1 h, luminescence was read with the EnSpire® Multimode Plate Reader (Perkin Elmer, MA, USA); a blank value was subtracted from the signals measured in each well.
Statistical Analysis
GraphPad Prism 7 (GraphPad Software) was used for statistical analysis. The values of p were calculated with a two-tailed Student's t-test; p < 0.05 was considered significant.
Materials
Fetal bovine serum was purchased from EuroClone (Italy). L-[2,3,4-3H]-monohydrochloride Arginine (54.5 mCi/mmol) was obtained from PerkinElmer. Caffeic acid phenethyl ester (CAPE) was from Calbiochem (Italy) while all other chemicals were from Merck (Italy).
Results
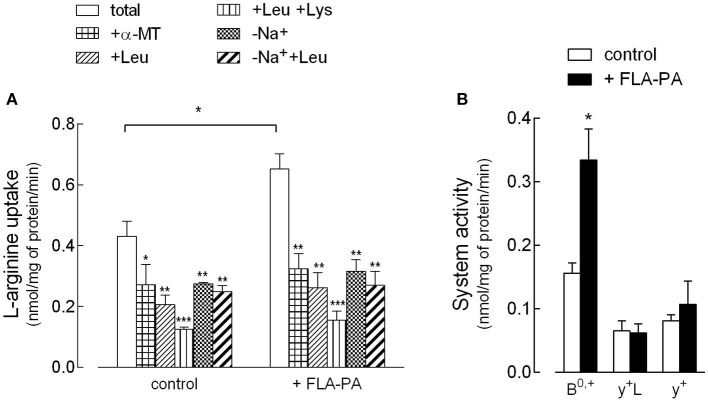
First, the effect of FLA-PA on the activity and expression of arginine transporters in Calu-3 bronchial epithelial cells has been investigated. In this regard, experimental conditions were adopted, which allowed the discrimination of the contribution of the different arginine transporters to the total uptake (Figure 1). Specifically, the presence/absence of Na+ was employed to calculate the activity of ATB0,+ as the sodium-dependent fraction of the transport, while further addition of leucine, α-methyltryptophan (α-MT), and lysine allowed the evaluation of the activity of systems y+L, y+, and b0,+. In the presence of Na+, leucine is known to inhibit both ATB0,+ and y+L transporters, while α-MT only inhibits ATB0,+ (30). As a result, the contribution of y+L can be obtained by subtracting the component inhibited by α-MT from the component inhibited by leucine. Second, since further addition of lysine also inhibits system y+, system y+ can be calculated as the difference between the uptake measured in the presence of leucine and the uptake measured in the presence of both leucine and lysine. Finally, the uptake mediated by b0,+ can be estimated as the quote inhibited by leucine in the absence of sodium. Figure 1A presents the results of the arginine uptake measured under these experimental conditions, both in control, untreated cells, and upon the incubation for 8 h with 2 μg/ml FLA-PA. These data have then been used to calculate the contribution of the different transport systems to the total uptake (Figure 1B). The results, besides excluding the presence of system b0,+ in Calu-3 cells, clearly demonstrated the activity of systems ATB0,+, y+L, and y+ under control conditions. Upon incubation with FLA-PA, a significant increase of total arginine uptake was observed and was referable to the induction of the activity of an ATB0,+ transporter, while neither system y+ nor system y+L was modified by the experimental treatment. Interestingly, the induction of ATB0,+ by flagellin was comparable when calculated as the sodium-dependent fraction of arginine uptake or, rather, as the α-MT-inhibited quote.
Figure 1.
Calu-3 cells were incubated for 8 h in the absence (control) or in the presence of 2 μg/ml flagellin from Psuudomonas aeruginosa (FLA-PA). (A) 30 s-[3H]arginine uptake (50 μM; 5 μCi/ml) was measured both in Na+-containing (total uptake) and Na+-free (-Na+) Earle's Balanced Salt Solution (EBSS), in the absence or in the presence of 2 mM methyltryptophan (α-MT), leucine, or leucine and lysine, as indicated. Bars represent the mean ± SEM of three independent determinations. *p < 0.05, **p < 0.01, ***p < 0.001 vs. total. (B) Data of (A) were employed to calculate the contribution of systems ATB0,+, y+L, and y+ to total uptake as follows: ATB0,+ = Na+-dependent uptake; y+L= “+Leu uptake” – “+α-MT uptake”; y+ = “+Leu uptake” – “+Leu+Lys uptake”. *p < 0.05 vs. control.
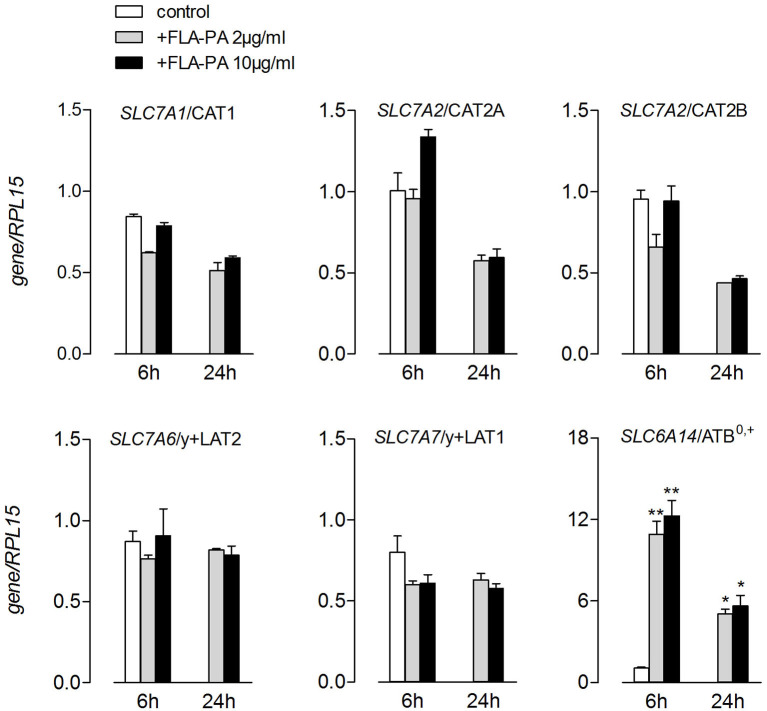
The transport data were in line with those of the gene expression (Figure 2). Indeed, the treatment with FLA-PA induced a marked increase of SLC6A14 mRNA, coding for a ATB0,+ transporter, without affecting the expression of neither system y+- (SLC7A1/CAT1, SLC7A2/CAT2A, and SLC7A2/CAT2B) nor system y+L- (SLC7A7/y+LAT1 and SLC7A6/y+LAT2) related genes. The same pattern of expression was observed when higher concentrations of flagellin (10 μg/ml) were employed or when the incubation time prolonged from 6 to 24 h. Altogether, these findings pointed to ATB0,+ as the sole arginine transporter targeted by flagellin in Calu-3 cells.
Figure 2.
Calu-3 were incubated for 6 and 24 h in the presence of the indicated concentrations of FLA-PA; the expression of the indicated genes was then measured by means of real-time quantitative PCR (RT-qPCR) and normalized for the housekeeping gene (RPL15). Data of three experiments, each performed in duplicate, are presented as mean ± SEM. *p < 0.05, **p < 0.01 vs. control.
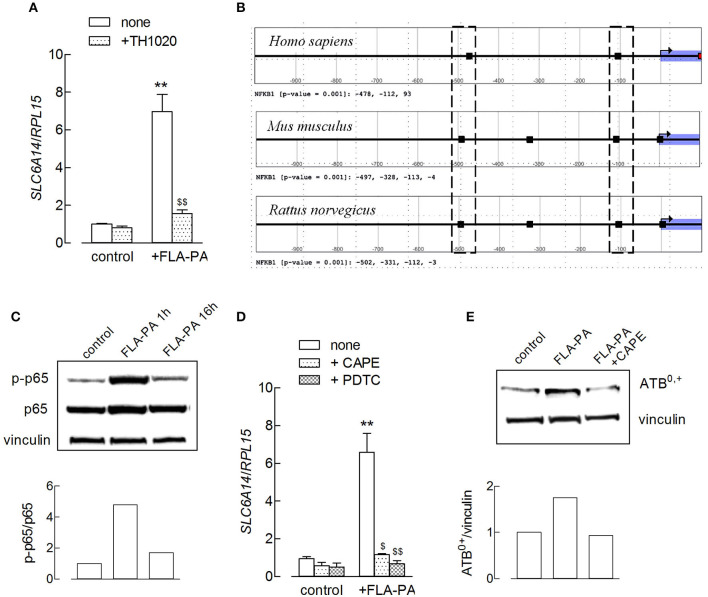
Flagellin is known to exert its pro-inflammatory effects through the activation of NF-κB via the stimulation of a Toll-like receptor-5 (TLR5) receptor (31, 32). Actually, the use of TLR5-agonist TH1020 completely prevented the flagellin-induced increase of SLC6A14/ATB0,+ expression (Figure 3A). To investigate the role of a NF-κB transcription factor, a bioinformatic analysis was first carried out on the promoter sequence of the SLC6A14 gene to identify the presence of putative NF-κB binding sites. NF-κB is a family of dimeric DNA binding transcription factors; five related protein subunits are known in mammals, NF-κB1/p50, NF-κB2/p52, RelA/p65, RelB, and c-Rel. Each family member shares a high amino acid sequence identity throughout a region of about 300 amino acids located near their NH2-termini and referred to as the Rel Homology Region (RHR). RHR is responsible for sequence-specific DNA binding, protein dimerization, and the association with a class of NF-κB inhibitor proteins known as IkB (33). The best-studied κB sites fit the consensus site pattern 5′-GGGRNWYYCC-3′ (where R, W, Y, and N denote purine, adenine or thymine, pyrimidine, and any nucleotide, respectively), although NF-κB can bind to degenerate κB sites (34). The analysis carried out in this study shows that the human SLC6A14 promoter region (from −1,000 to +100 relative to the transcriptional start site) contains three NF-κB1 motifs. A comparative promoter analysis revealed that the mouse and rat Slc6a14 promoter sequences present two NF-κB1 motifs in the positions equivalent to those of the human promoter, supporting an evolutionarily conserved regulatory mechanism (Figure 3B). These data suggest a direct involvement of NF-κB transcription factors in the modulation of SLC6A14 expression. In line with these findings, we show here that the incubation of Calu-3 cells with FLA-PA determined early, transient phosphorylation of NF-κB p65 subunit at Ser536 (Figure 3C), indicating the activation of the transcription factor under our experimental conditions. Moreover, the preincubation of cells with NF-κB inhibitors, such as CAPE or pyrrolidine dithiocarbamate (PDTC), completely prevented the effect of flagellin on SLC6A14 expression (Figure 3D); the addition of CAPE hampered the induction also at the protein level (Figure 3E). Hence, we can conclude that the induction of the SLC6A14 gene by flagellin directly depends upon the activity of the TLR5/NF-κB signaling pathway.
Figure 3.
(A) Calu-3 cells were pretreated for 1 h with TH1020 (20 μM), before the incubation with 2 μg/ml FLA-PA for 6 h. The expression of SLC6A14/ATB0,+ was then determined by means of RT-qPCR, as described in the section “Materials and methods”. Data of three experiments, each performed in duplicate, are presented as mean ± SEM. **p < 0.01 vs. control, $$p < 0.01 vs. none + FLA-PA. (B) Output of the Search Motif Tools scan of human, mouse, and rat SLC6A14 promoters for the nuclear factor-κB (NF-κB)1 position weight matrix profile MA0105.4 from Jaspar. The analysis has been performed in a region from −1,000 to +100 relative to transcription start site (TSS) and with the default cutoff (p-value) of 0.001. Hits are marked as black rectangles in the plot, and exact positions relative to the TSS are reported below each plot. The dotted boxes evidence NF-κB1 motifs located in equivalent positions in the promoter of the three mammalian species. (C) The amount of p-p65 and p65 proteins was determined by using the Western Blot analysis (see section “Material and methods”) in Calu-3 cells treated with 2 μg/ml FLA-PA; the densitometric analysis of protein expression is shown. A representative blot is reported that was repeated three times, showing comparable results. (D) Calu-3 cells were pretreated for 1 h with caffeic acid phenethyl ester (CAPE) (25 μg/ml) or pyrrolidine dithiocarbamate (PDTC) (100 μM) before the incubation with 2 μg/ml FLA-PA for 6 h. The expression of SLC6A14/ATB0,+ was then determined by means of RT-qPCR as described in section “Materials and methods”. Data of three experiments are presented as mean ± SEM, each performed in duplicate. **p < 0.01 vs. control, $p < 0.05, $$p < 0.01 vs. none + FLA-PA. (E) The amount of ATB0,+ protein was determined by using the Western Blot analysis (see section “Material and methods”) in Calu-3 cells treated for 8 h with 2 μg/ml FLA-PA, both in the absence and in the presence of CAPE; the densitometric analysis of protein expression normalized for that of vinculin is shown. A representative blot of three replicates that gave comparable results is shown.
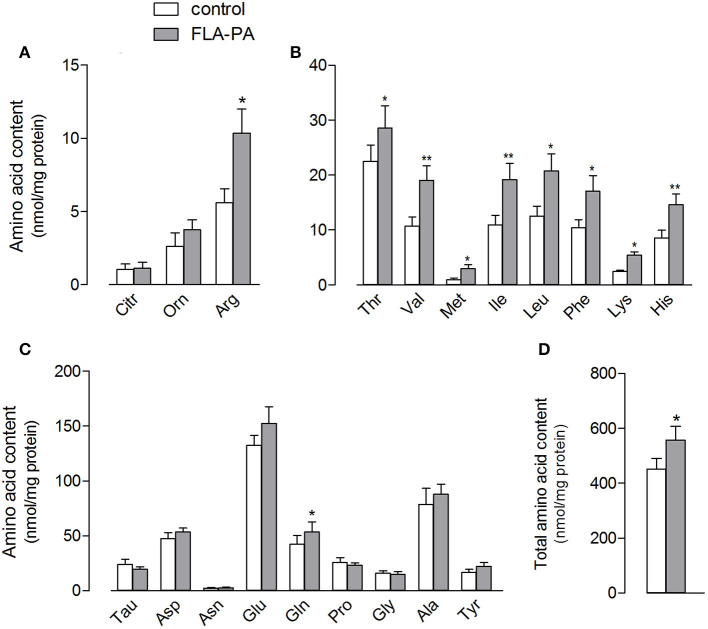
The stimulation of the ATB0,+ expression and activity by FLA-PA was also associated to changes in the intracellular amino acid content of Calu-3 cells (Figure 4), which increased from 451.9 ± 37.8 to 556.3 ± 50.8 nmol/mg of protein (Figure 4D). Specifically, in line with the changes observed in the arginine uptake, the intracellular amount of this amino acid nearly doubled in treated cells; however, this increase was not associated with any change in the content of its metabolites, citrulline and ornithine (Figure 4A). All essential amino acids, such as valine, isoleucine, and leucine, were significantly increased in the cells incubated with FLA-PA (Figure 4B), while almost all non-essential amino acids remained unaffected except glutamine (Figure 4C).
Figure 4.
Calu-3 cells were treated for 24 h with 2 μg/ml FLA-PA and the intracellular content of arginine and its metabolites (A), essential (B), and non-essential (C) amino acids was determined as described in section “Materials and methods”. Total amino acid content is also shown (D). Bars represent the mean ± SEM of four independent experiments. *p < 0.05, **p < 0.01 vs. control.
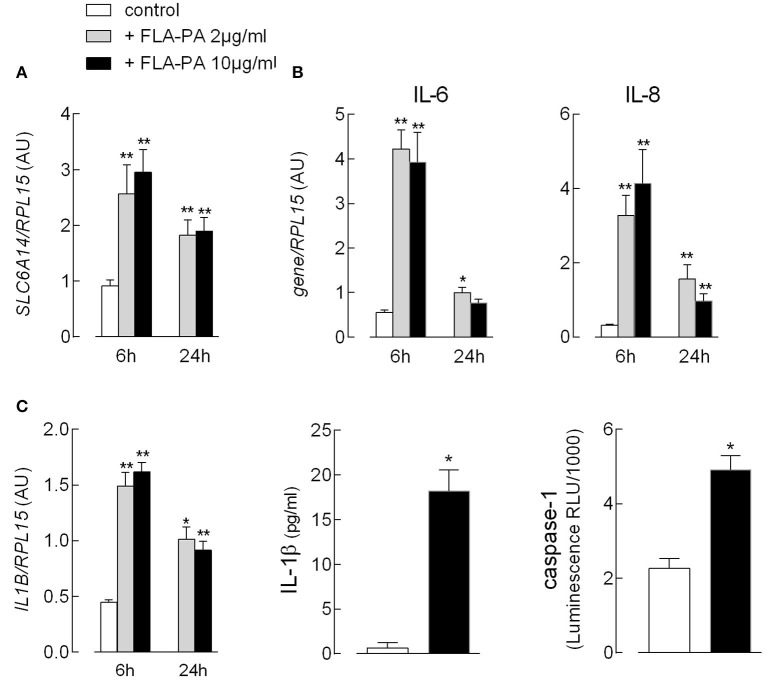
Since we recently demonstrated that EpiAirway™, a cellular model of normal human bronchial epithelium, maximally expresses SLC6A14 on the apical membrane (35), we next addressed the effect of FLA-PA in this model. First, we evaluated the expression of flagellin-targeted TLR5 and found it to be readily detectable and more abundant than that in Calu-3 cells and monocyte-derived macrophages (MDM) (Supplementary Figure 1). Similar to Calu-3 cells, the exposure of EpiAirway™ to bacterial flagellin caused a significant increase in the expression of ATB0,+ transporter (Figure 5A). Under the same condition, a marked induction of the pro-inflammatory phenotype was also observed, as indicated by the transient increase of mRNAs coding for the chemokine IL-8, as well as for the cytokines IL-6 (Figure 5B) and IL-1β (Figure 5C). For the cytokine IL-1β, it is important to notice that the incubation with flagellin was also associated with a markedly increased secretion of the cytokine in the extracellular medium, likely due to the concomitant activation of caspase-1 observed under the same experimental condition.
Figure 5.
EpiAirway™ tissues were incubated in the absence (control) or in the presence of the indicated concentrations of FLA-PA. After 6 h, the expression of the indicated genes (A,B) was measured by means of RT-qPCR analysis and shown after normalization for that of the housekeeping gene (RPL15). Data of three experiments, each performed in duplicate, are presented as mean ± SEM. (C) The activation of inflammasome was monitored after 16 h by addressing the expression of interleukin-1β (IL-1β) mRNA (left) and the cytokine secretion (middle) with RT-qPCR and ELISA assay, respectively; the activity of caspase-1 (right) was measured in parallel, as described in section “Materials and methods”. Bars represent the mean ± SEM of three experiments. *p < 0.05, **p < 0.01 vs. control.
Discussion
An increasing number of evidence in the past decade has highlighted the role of solute carrier (SLC) transporters in shaping the immune responses by modulating the bioavailability of many metabolites, including amino acids and their derivatives (36). In this context we demonstrate here that, in Calu-3 bronchial epithelial cells and EpiAirway™ tissues, the induction of a pro-inflammatory phenotype by bacterial FLA-PA is associated with an upregulation of the expression and activity of SLC6A14/ATB0,+ transporter for cationic and neutral amino acids.
ATB0,+ is a highly concentrative electrogenic transport system accepting all essential amino acids, along with arginine and glutamine (37). Recent studies identified SLC6A14 as a modifier gene in cystic fibrosis (CF), where its genetic variants modulate the severity of pulmonary disease (21, 38). In this context, the arginine transport through ATB0,+ has been shown to increase the residual function of F508del-CFTR chloride channel, suggesting a positive functional interaction between the CF modifier SLC6A14 and CFTR protein (39). The expression of SLC6A14/ATB0,+ is normally low under physiological conditions, while it is significantly induced in tumors like colon, cervical cancer, and breast cancer where its high activity is supposed to meet the increasing demand for amino acids essential for the rapid growth of the tumor (40). Similarly, ATB0,+ has been found upregulated also under intestinal inflammatory states, such as ulcerative colitis and Crohn's disease (41). As for the respiratory epithelium, we have recently demonstrated an induction of SLC6A14/ATB0,+ by LPS and TNF-α in Calu-3 and EpiAirway™ cells, in which the presence of the transporter was high and confined to the apical membrane (13).
In the same models, we demonstrate here that the expression of SLC6A14/ATB0,+ is also stimulated by FLA-PA and that this, in turn, causes a significant increase of the intracellular content of all essential amino acids, such as valine, phenylalanine, leucine, and isoleucine, along with the semi-essential arginine and non-essential glutamine, amino acids that are recognized as substrates of the transporter (40). The role of this amino acid upregulation has yet to be clarified. As for arginine, the immunomodulatory properties of the amino acid and its metabolites are well-recognized (42). Arginine is indeed the obligatory substrate for the synthesis of mediators crucial for the inflammatory response: NO, involved in processes associated with vasodilatation and cytotoxicity, which is produced by the NOS, and ornithine and urea, precursors for the production of polyamines and proline, generated by arginase (43). In our hands, however, the increased amount of intracellular arginine is not associated with a change of its metabolism, since neither citrulline, a byproduct of NO production, nor ornithine levels are modified in FLA-PA-treated cells, thus excluding the activation of either metabolic pathways. No change is consistently observed in the expression of NOS or arginase enzymes or in the NO synthesis as determined through the nitrite production in the cultured medium (data not shown). On the other hand, it is known that the intracellular concentration of the amino acid is not limiting for NOS (14), and the NO production in different cell models mostly depends upon the uptake of extracellular arginine by CAT transporters (18, 44, 45). Since we demonstrate in this study that the expression and activity of arginine transporters other than ATB0,+ do not change upon the incubation of Calu-3 cells with FLA-PA, we can conclude that the stimulation of ATB0,+ activity by flagellin only causes an increase of intracellular arginine availability without affecting its metabolism. Di Paola and colleagues have suggested that the induction of SLC6A14/ATB0,+ by FLA-PA in the airways aims to prevent the attachment of P. aeruginosa by depleting extracellular arginine from the airways surface liquid. According to their findings, SLC6A14 may thus have an important role in the modulation of lung disease in patients with CF, likely through its role in the host defense (21). Whether the relevance of SLC6A14 is actually restricted to the limitation of extracellular arginine or, rather, has a role in modulating the intracellular content of the amino acid for unrevealed functions remains to be investigated. Really, our results extend the role of ATB0,+ as a modulator of the intracellular and extracellular availability, not just of arginine but also of other amino acids, and support the hypothesis, of Broer and Fairweather (41), that the transporter expressed at places where the body interfaces with microbes, such as lung and intestine, may be involved in reducing the availability of nutrients essential to bacteria.
In respiratory and intestinal epithelia, as well as in monocytic cells, extracellular flagellin is known to target TLR5 and triggers a signaling cascade, leading to the activation of a NF-κB transcription factor for the induction of inflammatory genes (31, 46–49); the expression of numerous ATP-binding cassette (ABC) and SLC transporters in animal models has been shown to depend on the activity of the same transcription factor (50). In the present study, we have shown that a consensus domain for NF-κB is present also in the SLC6A14 gene; moreover, we confirmed that flagellin actually promotes NF-κB p65 phosphorylation and provided evidence that the flagellin-dependent induction of SLC6A14 requires NF-κB. Since also Signal Transducers and Activators of Transcription (STAT) proteins are described to be involved in the transcription of SLC6A14 (21), it is conceivable that both the transcription factors contribute to the FLA-PA-driven induction of the transporter. A similar hypothesis has been proposed in the model by Bao et al. for the intracellular signaling pathway primed by Salmonella typhimurium flagellin in macrophages (49).
In EpiAirway™, the exposure to FLA-PA not only induced the expression of SLC6A14 but also caused a massive increase in the expression of the chemokine IL-8, as well as of the cytokines IL-6 and IL-1β; under the same conditions, also caspase-1 was activated, leading to a large secretion of IL-1β in the extracellular medium, and, hence, showing for the first time the flagellin-dependent activation of inflammasome in EpiAirway™.
In conclusion, our data indicate that flagellin promotes the cellular responses that associate the activation of inflammasome with the upregulation of the intracellular content of amino acids through ATB0,+ that may be thus included in the list of genes upregulated by inflammatory stimuli. Since we have recently shown that ATB0,+ is a delivery system for bronchodilators in human airway epithelial cells (13), its induction under inflammatory conditions may gain a particular relevance in the field of respiratory pharmacokinetics.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
BR and VD designed the experimental plan. FF, AB, and RV performed in vitro experiments. GB performed the computational promoter analysis. AB and VD analyzed the results. BR and VD wrote the paper. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- EBSS
Earle's Balanced Salt Solution
- NF-κB
nuclear factor-κB transcription factor
- FLA-PA
flagellin from Pseudomonas aeuruginosa.
1The Eukaryotic Promoter Database: https://epd.epfl.ch/; JASPAR database: http://jaspar.genereg.net/; Signal Search Analysis toolkit: https://ccg.epfl.ch/ssa/.
Funding. This work was partially supported by Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (PRIN), by Ministero dell'Istruzione, dell'Università e della Ricerca, Italy (Prot. 2017R5ZE2C).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.641563/full#supplementary-material
The expression of toll-like receptor-5 (TLR5) was measured by means of RT-qPCR analysis and shown after normalization for that of the housekeeping gene (RPL15). Data of the three different cultures are presented as mean ± SEM. Monocyte-derived macrophages (MDM) were obtained from human monocytes after 5 day differentiation in the presence of 50 ng/ml GM-CSF.
References
- 1.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. (2009) 37:153–68. 10.1007/s00726-008-0210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asosingh K, Lauruschkat CD, Alemagno M, Frimel M, Wanner N, Weiss K, et al. Arginine metabolic control of airway inflammation. JCI Insight. (2020) 5:e127801. 10.1172/jci.insight.127801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris SM, Jr. Arginine metabolism: boundaries of our knowledge. J Nutr. (2007) 137:1602S−9S. 10.1093/jn/137.6.1602S [DOI] [PubMed] [Google Scholar]
- 4.Deves R, Boyd CA. Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev. (1998) 78:487–545. 10.1152/physrev.1998.78.2.487 [DOI] [PubMed] [Google Scholar]
- 5.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. (2004) 134:2752S−9S; discussion 65S−67S. 10.1093/jn/134.10.2752S [DOI] [PubMed] [Google Scholar]
- 6.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. (2004) 447:532–42. 10.1007/s00424-003-1086-z [DOI] [PubMed] [Google Scholar]
- 7.Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, et al. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. (2010) 6:424–34. 10.1038/nrneph.2010.69 [DOI] [PubMed] [Google Scholar]
- 8.Nakanishi T, Hatanaka T, Huang W, Prasad PD, Leibach FH, Ganapathy ME, et al. Na+- and Cl–coupled active transport of carnitine by the amino acid transporter ATB(0,+) from mouse colon expressed in HRPE cells and Xenopus oocytes. J Physiol. (2001) 532:297–304. 10.1111/j.1469-7793.2001.0297f.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galietta LJ, Musante L, Romio L, Caruso U, Fantasia A, Gazzolo A, et al. An electrogenic amino acid transporter in the apical membrane of cultured human bronchial epithelial cells. Am J Physiol. (1998) 275:L917–23. 10.1152/ajplung.1998.275.5.L917 [DOI] [PubMed] [Google Scholar]
- 10.Broer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev. (2008) 88:249–86. 10.1152/physrev.00018.2006 [DOI] [PubMed] [Google Scholar]
- 11.Sloan JL, Grubb BR, Mager S. Expression of the amino acid transporter ATB 0+ in lung: possible role in luminal protein removal. Am J Physiol Lung Cell Mol Physiol. (2003) 284:L39–49. 10.1152/ajplung.00164.2002 [DOI] [PubMed] [Google Scholar]
- 12.Ingoglia F, Visigalli R, Rotoli BM, Barilli A, Riccardi B, Puccini P, et al. Functional activity of L-carnitine transporters in human airway epithelial cells. Biochim Biophys Acta. (2016) 1858:210–9. 10.1016/j.bbamem.2015.11.013 [DOI] [PubMed] [Google Scholar]
- 13.Rotoli BM, Visigalli R, Barilli A, Ferrari F, Bianchi MG, Di Lascia M, et al. Functional analysis of OCTN2 and ATB0,+ in normal human airway epithelial cells. PLoS ONE. (2020) 15:e0228568. 10.1371/journal.pone.0228568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris SM, Jr. Arginine metabolism revisited. J Nutr. (2016) 146:2579S−86S. 10.3945/jn.115.226621 [DOI] [PubMed] [Google Scholar]
- 15.Durante W, Liao L, Cheng K, Schafer AI. Selective induction of a cationic amino acid transporter by tumor necrosis factor-alpha in vascular endothelium. Proc Assoc Am Physicians. (1996) 108:356–61. [PubMed] [Google Scholar]
- 16.Gill DJ, Low BC, Grigor MR. Interleukin-1 beta and tumor necrosis factor-alpha stimulate the cat-2 gene of the L-arginine transporter in cultured vascular smooth muscle cells. J Biol Chem. (1996) 271:11280–3. 10.1074/jbc.271.19.11280 [DOI] [PubMed] [Google Scholar]
- 17.Simmons WW, Closs EI, Cunningham JM, Smith TW, Kelly RA. Cytokines and insulin induce cationic amino acid transporter (CAT) expression in cardiac myocytes. Regulation of L-arginine transport no production by CAT-1, CAT-2A, CAT-2B. J Biol Chem. (1996) 271:11694–702. 10.1074/jbc.271.20.11694 [DOI] [PubMed] [Google Scholar]
- 18.Sala R, Rotoli BM, Colla E, Visigalli R, Parolari A, Bussolati O, et al. Two-way arginine transport in human endothelial cells: TNF-alpha stimulation is restricted to system y(+). Am J Physiol Cell Physiol. (2002) 282:C134–43. 10.1152/ajpcell.2002.282.1.C134 [DOI] [PubMed] [Google Scholar]
- 19.Visigalli R, Bussolati O, Sala R, Barilli A, Rotoli BM, Parolari A, et al. The stimulation of arginine transport by TNFalpha in human endothelial cells depends on NF-kappaB activation. Biochim Biophys Acta. (2004) 1664:45–52. 10.1016/j.bbamem.2004.04.001 [DOI] [PubMed] [Google Scholar]
- 20.Rath M, Muller I, Kropf P, Closs EI, Munder M. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front Immunol. (2014) 5:532. 10.3389/fimmu.2014.00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Paola M, Park AJ, Ahmadi S, Roach EJ, Wu YS, Struder-Kypke M, et al. SLC6A14 is a genetic modifier of cystic fibrosis that regulates pseudomonas aeruginosa attachment to human bronchial epithelial cells. MBio. (2017) 8:e02073–17. 10.1128/mBio.02073-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rotoli BM, Barilli A, Visigalli R, Ferrari F, Frati C, Lagrasta CA, et al. Characterization of ABC transporters in epiairway, a cellular model of normal human bronchial epithelium. Int J Mol Sci. (2020) 21:3190. 10.3390/ijms21093190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barilli A, Rotoli BM, Visigalli R, Dall'Asta V. Gliadin activates arginase pathway in RAW264.7 cells and in human monocytes. Biochim Biophys Acta. (2014) 1842:1364–71. 10.1016/j.bbadis.2014.04.021 [DOI] [PubMed] [Google Scholar]
- 24.Green MR, Sambrook J. Constructing a standard curve for real-time polymerase chain reaction (PCR) experiments. Cold Spring Harb Protoc. (2018). 2018. 10.1101/pdb.prot095026 [DOI] [PubMed] [Google Scholar]
- 25.Dreos R, Ambrosini G, Groux R, Cavin Perier R, Bucher P. The eukaryotic promoter database in its 30th year: focus on non-vertebrate organisms. Nucleic Acids Res. (2017) 45:D51–5. 10.1093/nar/gkw1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fornes O, Castro-Mondragon JA, Khan A, van der Lee R, Zhang X, Richmond PA, et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. (2020) 48:D87–92. 10.1093/nar/gkz1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosini G, Praz V, Jagannathan V, Bucher P. Signal search analysis server. Nucleic Acids Res. (2003) 31:3618–20. 10.1093/nar/gkg611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingoglia F, Visigalli R, Rotoli BM, Barilli A, Riccardi B, Puccini P, et al. Human macrophage differentiation induces OCTN2-mediated L-carnitine transport through stimulation of mTOR-STAT3 axis. J Leukoc Biol. (2017) 101:665–74. 10.1189/jlb.1A0616-254R [DOI] [PubMed] [Google Scholar]
- 29.Ingoglia F, Visigalli R, Rotoli BM, Barilli A, Riccardi B, Puccini P, et al. Functional characterization of the organic cation transporters (OCTs) in human airway pulmonary epithelial cells. Biochim Biophys Acta. (2015) 1848:1563–72. 10.1016/j.bbamem.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 30.Karunakaran S, Umapathy NS, Thangaraju M, Hatanaka T, Itagaki S, Munn DH, et al. Interaction of tryptophan derivatives with SLC6A14 (ATB0,+) reveals the potential of the transporter as a drug target for cancer chemotherapy. Biochem J. (2008) 414:343–55. 10.1042/BJ20080622 [DOI] [PubMed] [Google Scholar]
- 31.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. (2001) 410:1099–103. 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- 32.Smith KD, Ozinsky A. Toll-like receptor-5 and the innate immune response to bacterial flagellin. Curr Top Microbiol Immunol. (2002) 270:93–108. 10.1007/978-3-642-59430-4_6 [DOI] [PubMed] [Google Scholar]
- 33.Mulero MC, Wang VY, Huxford T, Ghosh G. Genome reading by the NF-kappaB transcription factors. Nucleic Acids Res. (2019) 47:9967–89. 10.1093/nar/gkz739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brignall R, Moody AT, Mathew S, Gaudet S. Considering abundance, affinity, and binding site availability in the NF-kappaB target selection puzzle. Front Immunol. (2019) 10:609. 10.3389/fimmu.2019.00609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barilli A, Visigalli R, Ferrari F, Di Lascia M, Riccardi B, Puccini P, et al. Organic cation transporters (OCTs) in EpiAirway, a cellular model of normal human bronchial epithelium. Biomedicines. (2020) 8:127. 10.3390/biomedicines8050127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss HJ, Angiari S. Metabolite transporters as regulators of immunity. Metabolites. (2020) 10:418. 10.3390/metabo10100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karunakaran S, Ramachandran S, Coothankandaswamy V, Elangovan S, Babu E, Periyasamy-Thandavan S, et al. SLC6A14 (ATB0,+) protein, a highly concentrative and broad specific amino acid transporter, is a novel and effective drug target for treatment of estrogen receptor-positive breast cancer. J Biol Chem. (2011) 286:31830–8. 10.1074/jbc.M111.229518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruffin M, Mercier J, Calmel C, Mesinele J, Bigot J, Sutanto EN, et al. Update on SLC6A14 in lung and gastrointestinal physiology and physiopathology: focus on cystic fibrosis. Cell Mol Life Sci. (2020) 77:3311–23. 10.1007/s00018-020-03487-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadi S, Wu YS, Li M, Ip W, Lloyd-Kuzik A, Di Paola M, et al. Augmentation of cystic fibrosis transmembrane conductance regulator function in human bronchial epithelial cells via SLC6A14-dependent amino acid uptake. Implications for treatment of cystic fibrosis. Am J Respir Cell Mol Biol. (2019) 61:755–64. 10.1165/rcmb.2019-0094OC [DOI] [PubMed] [Google Scholar]
- 40.Ganapathy ME, Ganapathy V. Amino Acid Transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. (2005) 5:357–64. 10.2174/156800805774912953 [DOI] [PubMed] [Google Scholar]
- 41.Broer S, Fairweather SJ. Amino acid transport across the mammalian intestine. Compr Physiol. (2018) 9:343–73. 10.1002/cphy.c170041 [DOI] [PubMed] [Google Scholar]
- 42.Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell. (2016) 167:829–42 e13. 10.1016/j.cell.2016.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. (2005) 5:641–54. 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- 44.Nicholson B, Manner CK, Kleeman J, MacLeod CL. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem. (2001) 276:15881–5. 10.1074/jbc.M010030200 [DOI] [PubMed] [Google Scholar]
- 45.Closs EI. Expression, regulation and function of carrier proteins for cationic amino acids. Curr Opin Nephrol Hypertens. (2002) 11:99–107. 10.1097/00041552-200201000-00015 [DOI] [PubMed] [Google Scholar]
- 46.Benedikz EK, Bailey D, Cook CNL, Goncalves-Carneiro D, Buckner MMC, Blair JMA, et al. Bacterial flagellin promotes viral entry via an NF-kB and Toll Like Receptor 5 dependent pathway. Sci Rep. (2019) 9:7903. 10.1038/s41598-019-44263-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. (2006) 291:C218–30. 10.1152/ajpcell.00605.2005 [DOI] [PubMed] [Google Scholar]
- 48.Gewirtz AT, Rao AS, Simon PO, Jr, Merlin D, Carnes D, et al. Salmonella typhimurium induces epithelial IL-8 expression via Ca(2+)-mediated activation of the NF-kappaB pathway. J Clin Invest. (2000) 105:79–92. 10.1172/JCI8066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bao W, Wang Y, Fu Y, Jia X, Li J, Vangan N, et al. mTORC1 regulates flagellin-induced inflammatory response in macrophages. PLoS ONE. (2015) 10:e0125910. 10.1371/journal.pone.0125910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu KC, Lin CJ. The regulation of drug-metabolizing enzymes and membrane transporters by inflammation: Evidences in inflammatory diseases and age-related disorders. J Food Drug Anal. (2019) 27:48–59. 10.1016/j.jfda.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The expression of toll-like receptor-5 (TLR5) was measured by means of RT-qPCR analysis and shown after normalization for that of the housekeeping gene (RPL15). Data of the three different cultures are presented as mean ± SEM. Monocyte-derived macrophages (MDM) were obtained from human monocytes after 5 day differentiation in the presence of 50 ng/ml GM-CSF.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.