Abstract
The aldosterone‐to‐renin ratio (ARR) is a common screening test for primary aldosteronism in hypertensives. However, there are many factors which could confound the ARR test result and reduce the accuracy of this test. The present review's objective is to identify these factors and to describe to what extent they affect the ARR. Our analysis revealed that sex, age, posture, and sodium‐intake influence the ARR, whereas assay techniques do not. Race and body mass index have an uncertain effect on the ARR. We conclude that several factors can affect the ARR. Not taking these factors into account could lead to misinterpretation of the ARR.
Keywords: aldosterone, hyperaldosteronism, hypertension, renin, screening
The aldosterone‐to‐renin ratio (ARR) is a common screening test for primary aldosteronism in hypertensives. Our analysis revealed that several factors can affect the ARR. Not taking these factors into account could lead to misinterpretation of the ARR.
1. INTRODUCTION
Current guidelines recommend the use of the aldosterone‐to‐renin ratio (ARR) for the screening of patients suspected of having primary aldosteronism (PA). 1 Aldosterone can be measured using either plasma or serum concentrations (PAC and SAC respectively), while renin can be determined using either plasma renin activity or direct renin concentration (PRA and DRC, respectively).
The ARR is not without its pitfalls as both aldosterone and renin are influenced by a variety of endogenous and exogenous stimuli such as age, diet, medications, and several others. 1 The choice in assay technique for the detection of either aldosterone or renin could additionally have an impact.
Although guidelines and position papers increasingly acknowledge the desirability to standardize test conditions, 2 a systematic analysis of confounding factors has scarcely been performed. This is unfortunate because in clinical practice, hypertensive patients are often subjected to unnecessary diagnostic investigations due to a moderately elevated ARR despite the cause potentially being the result of a confounder. Therefore, we aimed to identify and gauge the impact of a number of confounding factors that could significantly influence the validity and interpretation of the ARR.
2. METHODS
In accordance with the PRISM recommendations, 3 we performed a literature search on PubMed and the Cochrane database based on a combination of MeSH and free terms to find articles discussing the ARR and its confounders. To this end, we used the search string: ((((Hyperaldosteronism [MeSH Terms] OR Conn Syndrome OR Conn's Syndrome OR Aldosteronism OR Hyperaldosteronism) AND (("Renin"[Mesh] OR"Aldosterone"[Mesh]) AND (ARR OR Aldosterone renin ratio OR Aldosterone‐to‐renin ratio))))). Our search encompassed all articles published between January 1981 and October 2019. Finally, cross‐references of relevant literature were included.
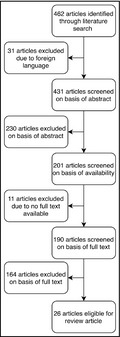
Our literature search retrieved 462 potential articles. Papers not published in English (n = 31) were excluded. After reading the abstracts, another 230 articles were rejected because they did not contain information needed to perform the present analysis. The remaining articles were read in full and considered eligible if they (1) had a clearly described and reproducible method for the determination of renin and aldosterone and (2) clearly described the type of participants that were investigated, for example, patients referred for evaluation of treatment‐resistant hypertension, and (3) applied confirmatory testing such as salt supplementation, or saline infusion in participants with an elevated ARR. When multiple articles were derived from the same research group on the same topic, only the most recent one was analyzed. Finally, articles on PA treatment studies or on cardiovascular‐related usage of the ARR as well as papers which focused on PA confirmatory tests or detection of genetic variations were excluded (Figure 1).
FIGURE 1.

Flowchart depicting the selection of papers
Studies accepted for this review were independently screened by two of the authors (GPV, RMA), who applied the selection criteria to the articles identified and reviewed the full‐text versions thereof. In the event of disagreement, said articles were discussed among the aforementioned authors in order to obtain consensus. In the event that consensus was not obtained, a third author (P.L) was decisive. In the end, a total of 26 articles qualified for inclusion in our analysis.
For the purpose of simplicity, dimensionless units for the ARR are used throughout this article. Unless otherwise stated, the PRA is measured in mL/dL·h, the DRC in mU/L, and the PAC in ng/dL.
3. RESULTS
3.1. Patient‐related factors
3.1.1. Age
Four of the studies which we selected assessed the effect of age on the ARR in adults. In the one by Yin et al, 4 SAC and PRA were inversely correlated with age, both in 153 healthy normotensive individuals and in 274 patients with essential hypertension (EH) seen at an outpatient clinic. However, the logarithmically transformed ARR correlated positively with age in both groups. The ARR reached a peak after the age of 60 in patients with EH, and between the ages of 40 and 49 in normotensive individuals. Per contra, the ARR was age‐independent in the PA group (n = 39). This study also addressed the accuracy and cutoff value of the ARR in two age groups, <40 and ≥40 years old. Two cutoff values were established, which were similar in the two age groups. The screening accuracy and the cutoff values of the ARR were not influenced by advancing age. Importantly, in this study antihypertensive medication had been discontinued as much as possible but if this was not possible patients were maintained on treatment with a calcium channel blocker or an alpha‐adrenoceptor blocker.
Based on measurements of PAC and PRA in 216 patients with PA and 657 patients with EH, Luo et al 5 compared the ARR in four age categories: ≤39, 40–49, 50–59 and ≥60 years. Antihypertensive agents had been stopped in all patients but wherever necessary slow‐release verapamil and/or doxazosin or terazosin were administered instead to control blood pressure. Moreover, premenopausal women on oral contraceptive agents and postmenopausal women taking hormonal replacement therapy were not included in the study.
In patients with EH, PRA decreased significantly with age though this change was not significant when only patients aged 50–59 and ≥60 years old were compared. Similar changes were seen in PAC, but this was only significant when the age categories ≥50 years and <50 years were contrasted. The ARR had a weak positive association with age, barring a comparison between patients aged 50–59 years and those ≥60 years. In the PA group, there was a significant decline in PRA between patients aged <39 and ≥60 years. However, PAC and ARR did not significantly change with age. Altogether, the diagnostic accuracy of the ARR fell at higher ages, particularly in patients 60 years and older.
Although not primarily intended to study the effect of age on the ARR, Rossi et al 6 also found a positive correlation between these two variables in a large series of newly diagnosed hypertensive patients who were either untreated or treated with a calcium channel blocker and/or doxazosin. Finally, Nakama et al retrospectively investigated 110 patients with EH and 45 with PA and divided them in a group under 65 years of age and a group 65 years and older. They found that PAC and PRA were lower in both groups of elderly patients with the net effect of increasing the ARR value. 7 Again, patients were either untreated or received a calcium channel and/or alpha‐adrenoceptor blocker.
3.1.2. Sex
Our search retrieved two articles looking at how female sex, and in particular female sex hormones, could affect the ARR. In a small study comparing the ARR in 19 healthy women, not on contraceptive pills, with that in 21 healthy men (all untreated), Ahmed et al 8 found that compared to men the ARR was consistently and significantly higher in women during all phases of the menstrual cycle, with the least difference during menses. Using PRA as the renin assay, the median ARR amounted to 3.85 (range 1.13–8.02) during menses, 3.91 (range 0.87–8.15) during the follicular phase and 4.78 (range 1.07–10.7) during the luteal phase. In comparison, the ARR of healthy men was 2.18 (range 0.75–5.74). Thus, healthy women during the luteal phase can be expected to have an ARR about two times higher than their male counterparts. A similar trend was observed when DRC was applied as the renin assay with an almost threefold lower ARR in males than in females in their luteal phase. There was no significant difference in the ARR, using either PRA or DRC, between the follicular and the menstrual phase. However, the ARR was higher in the luteal phase compared to the follicular phase but only when DRC was used for the calculation. The latter is in contrast with a comparable study by Pizzolo et al 9 in 33 healthy premenopausal women without oral contraceptive pills. Using DRC, these investigators failed to find a difference in the ARR between the follicular and the luteal phase. However, when the normotensive female population was started on oral contraceptives, the average ARR shifted from 18.69 ± 87 to 35.52 ± 22.7, a statistically significant increase. This upregulation was mainly driven by a roughly 50% rise in measured aldosterone.
In both male and female hypertensive patients with high ARR values, no significant difference in ARR values was found. However, it should be noted that some patients were subsequently found to have PA. In contrast, healthy normotensive women were more likely to have an elevated ARR (≥32) compared with men (13.6% vs. 2.3%; p < .05). 9
3.1.3. Race
Only one paper that fulfilled our selection criteria provides information on the potential influence of race on the ARR. 10 In a prospective study of 265 consecutive patients with resistant hypertension (115 African‐American, 150 Caucasian), Nishizaka and associates found an overall prevalence of 22% of PA. The prevalence was 24% in the African‐American population and 20% in the Caucasian patients, a difference that was not statistically significant. Although this study was not primarily designed to evaluate whether race could have an effect on the ARR, the average test result was comparable in both ethnic groups. It is of note that no medications were discontinued with the exception of some diuretics.
3.1.4. Body mass index
Two articles addressed the influence of BMI on the ARR. In a large cohort of newly diagnosed hypertensive patients, in whom treatment with a calcium channel blocker, an alpha‐adrenoceptor blocker or their combination was permitted, Rossi et al 6 found that despite a positive association between BMI and plasma aldosterone levels, in particular in overweight and obese individuals, the ARR did not correlate with BMI. The latter was also true in a smaller, prospective study (n = 59) in patients suspected of having PA. 11 In that study, the ARR accuracy was higher in patients with BMI <30 compared with obese patients, when using values >20 as the ARR cutoff point. It should be noted that when antihypertensive treatment could not be safely withheld, patients could not only be treated with verapamil, doxazosin, and terazosin but also with hydralazine, a drug with a well‐known stimulating effect on the renin‐angiotensin‐aldosterone system. 12
3.1.5. Sodium intake
As sodium balance is a major modulator of the renin‐angiotensin system, changes in dietary salt intake are likely to modify the results of ARR testing. Three of the papers that we retrieved have addressed this issue. Baudrand et al 13 explored among 79 patients, allegedly without antihypertensive medication, and with an ARR above 20 on a high salt diet whether the results of the ARR would be different when measured under conditions of low salt intake. Participants who had a PRA ≤ 1.0 and a serum aldosterone ≥6.0 (required for a “positive screen”) were placed on a low salt diet for one week. Adherence to the diet was checked by measuring sodium output in 24‐h urine collections. At the end of this dietary period, only 35 of the 79 patients still tested positive. In the other 44 patients with a discordant screen on high and low sodium intake, PA could still be confirmed in 25 of them. Put differently, the data showed that among the patients with confirmed PA, only half had a positive screen on the low salt diet. Another study in mild‐to‐moderate hypertensives in whom treatment was withdrawn and who had been put on a high salt diet for 4 days suggested that acute variations in sodium balance do not adversely affect the test results but in that study a low salt state was not achieved by dietary measures but through furosemide natriuresis. 14 Finally, Williams et al 15 analyzed the data from 118 normotensive and 347 hypertensive volunteers who were either untreated or used a dihydropyridine calcium channel blocker, a thiazide diuretic or both. Although the primary aim of this study was to establish the prevalence of PA in general, the hypertensive participants also completed a crossover study consisting of 7 days of high sodium intake (>200 mmol/day) and 7 days of low sodium intake (<10 mmol/day). The ARR was determined in the upright position at the end of the low salt period to assess its responsiveness to sodium restriction. Both in the patients with PA and in those with EH the ARR fell significantly after sodium restriction but the ARR remained significantly elevated in PA as compared to EH patients.
3.1.6. Posture
The effect of posture on the ARR was addressed in three studies. For instance, Barigou et al 16 studied 53 patients who were hospitalized for ARR testing and in whom non‐dihydropyridine calcium channel blockers, alpha‐adrenoceptor blockers, and central antihypertensive drugs (not further specified) were allowed to control blood pressure. DRC and aldosterone levels were determined in the supine position after sleep and after 1 h in the upright position and 2 h later after 15 min of sitting, respectively. The mean seated ARR (16 ± 22) was lower than mean supine ARR (19 ± 29) and mean upright ARR (22 ± 31). Although no significance levels were presented by the authors, they claimed that the seated ARR was significantly lower than supine ARR.
These results are at variance with those of Pilz et al 17 who found the opposite in 160 outpatients who had been referred to screen for endocrine hypertension. The great majority of these patients (n = 151) turned out to have essential hypertension and in these the ARR fell from a median of 1.54 (interquartile range 0.66–2.98) in sitting position to a median of 1.28 (range 0.55–2.60) after 1 h of recumbency, a statistically significant difference. In the 61 patients with PA, on the other hand, recumbency had no significant effect on the ARR. While patients on drugs that interfere with the renin‐angiotensin‐aldosterone system are said to have been excluded from the study, it seems that this only involved certain diuretics and aliskiren and that other drugs were still allowed. In females below the age of 50 years, oral contraceptives were allowed as well.
The impact of posture on the diagnostic potential of the ARR was also addressed by G. Giacchetti et al 18 who retrospectively scrutinized the data from 157 patients who had been referred for suspicion of PA and in whom only the use of calcium channel blockers and alpha‐adrenoceptor blockers was permitted. In all these patients, PRA and SAC had been measured after at least 2 h in the supine position and again after 2 h of being upright. From the data presented, it seems that the ARR did not change when going from the lying to the upright position in the 96 patients with essential hypertension while it tended to rise in the 61 patients with PA. Significance levels were, however, not provided by the authors. At any rate, they claimed that the upright ARR is superior as a screening test for PA.
3.1.7. Circadian rhythm
Although several studies have addressed the circadian rhythms of renin, aldosterone, and the ARR, none of these fulfilled our selection criteria.
3.2. Assay‐related factors
Many studies have examined whether the ARR based on chemiluminescent or other automated assays for measuring the plasma concentrations of renin (DRC) and aldosterone would yield results as reliable as the ARR based on the classical but rather cumbersome methods by which plasma renin activity (PRA) and aldosterone are measured by radioimmunoassay. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 By and large, these studies showed that these newer, automated methods are a good alternative for the old ones.
As far as reproducibility of the ARR is concerned, the available data suggest that the test is highly reproducible. 28 , 29
4. DISCUSSION
Our analysis has shown that the results of ARR measurements, while being generally reproducible, are confounded by many factors (Table 1). There has been a fairly large body of research conducted on this topic, but only a small subset thereof met our strict selection criteria. The reasons for our stringency are twofold. Firstly, when dealing with hypertensive patients in clinical practice, a high likelihood of PA is rarely evident upon first observation. As such, it is only with a full understanding of what variations, with regard to fluctuations in the ARR, can be expected in EH patients that we are best able to identify a truly abnormal ARR value indicating PA. Secondly, the purpose of our review was to draw attention to what we truly do (and do not) know about ARR confounders. Therefore, we wished to put emphasis only on studies in which highly standardized conditions were maintained, rather than to prematurely draw weakly supported conclusions. While this did have the effect of limiting our scope loonily 26 articles, the lack of comprehensive standardised research on the topic is in and of itself an important finding with regard to grasping how well understood the ARR is.
TABLE 1.
Summary of the effects of various confounders on levels of aldosterone and renin and on the aldosterone‐to‐renin ratio (ARR)
| Confounder | Aldosterone | Renin | ARR |
|---|---|---|---|
| Age | ↓ | ↓ | ↑ |
| Female sex/estrogen | ↑↑ | ↑ | ↑↑ |
| Black race | ↑↑ | – | ↑↑† |
| BMI | ↑† | – | ↑† |
| Circadian rhythm | 08:00 ↓, 12:00 ↑ | 00:00 ↓, 12:00↑ | 20:00 ↓, 08:00 ↑ |
| Supine position (vs. standing and seated) | ↓ | ↓↓ | ↓↓ |
| High sodium intake | ↓ | ↓↓ | ↑↑ |
| Assay technique | – | – | – |
The ARR shows a fairly consistent positive relationship with age in normotensives and EH patients. Concerningly, though, this relationship was less evident in PA populations. As such, there is a realistic possibility of reduced sensitivity and specificity of the ARR in older populations. Outside of the scope of our review due to a lack of confirmatory testing, two studies found no relationship between age and the ARR in pediatric populations, except for a positive relationship in female pediatric populations in one of the two. 30 , 31 The potential amplification of the ARR in girls is likely hormone‐mediated and in accordance with the observation of the ARR in general being higher in female populations. The latter may be cycle‐dependent, though, as several studies with the exception of the one by Pizzolo et al 9 have shown that the luteal phase of the menstrual cycle is associated with high ARR. This effect may be mediated by the rise in progesterone during the luteal phase. Although the lack of confirmatory testing prevented its inclusion in our review, a clear trend toward higher ARR values in the luteal phase was also shown in the study by Fommei et al 32 who took four serial measurements of aldosterone and renin during the course of a single menstrual cycle. Similar trends were noted in the Ohasama and Framingham Heart studies. 33 , 34 These hormonally mediated effects also have implications with regard to hormone replacement therapy and oral contraceptives.
We were disappointed to find that very few studies addressing race and the ARR met our criteria for inclusion. In the only eligible article comparing African‐American and Caucasian populations, no clear difference was found. However, this finding was tangential to the primary objective of the study, which was to estimate the prevalence of PA. 10 In one study, African‐Americans had more than twice as many positive ARR screenings compared to their Caucasian counterparts, 35 while a similar trend of elevated ARR values in African‐American treatment‐resistant EH participants was noted in another. 36 The most likely explanation for these findings is either that the PA prevalence of PA is elevated in African‐Americans compared to Caucasians, or that false‐positive ARR results are more common African‐Americans. These two explanations have contradictory clinical implications, and thus, rigorous investigation into this topic will be a clear boon for clinicians who frequently treat patients of African ancestry.
BMI is another topic in which the lack of significant highly standardized research was surprising. For now, the current research that meets our criteria seems to indicate a rise of aldosterone concentrations with BMI but not enough to tangibly affect the ARR. In the Framingham Heart Study, ARR did not correlate with BMI when controlling for other variables. 37 However, in a large (n = 2086) study by Dudenbostel and associates, with BMI divided into quartiles, a trend of increasing ARR was noted from quartile 1 through to 3, with a plateau reached between quartiles 3 and 4. 36 While this study did not meet our inclusion criteria, its large scale does warrant some discussion. It is, indeed, possible that the absence of a significant relationship as found by the studies that were included in our analysis was due to a disproportionate number of participants at the higher end of the BMI spectrum, where the relationship appears to flatten out. Another possibility is that a state of treatment resistance somehow alters the relationship between the BMI and ARR.
In view of the immense role that sodium intake has on the RAAS, this is undoubtedly an important aspect with regard to the ARR. In the three publications retrieved for our review, low versus high sodium intake consistently resulted in lower and higher ARR values, respectively. It is, however, important to note that these studies had some shortcomings, and thus, the results should be interpreted with caution. For example, fixed dietary regimens were used in the study by Baudrand et al, 13 while a crossover design would have given us clearer insight into the topic. In the case of Williams et al 15 a crossover trial was performed, but diuretics, a major factor in sodium balance, were not discontinued. In studies not eligible for our review, similar trends were noted, such as in the publication by Kerstens et al. 38 While the impact of sodium intake on the ARR is quite clear, no research proposed what an optimal sodium intake for screening purposes would be. As such, while controlling for this variable has significant importance, how best to standardize sodium intake is still beyond our present knowledge.
Potassium is another dietary mineral whose intake is likely to have an effect on the RAAS. However, to the best of our knowledge, no research has been done investigating the effect potassium has on the ARR. So far, studies have focused only on renin and aldosterone values individually, with mixed results. 39 , 40 , 41 This is a fairly large and concerning oversight in the body of research available. The interplay between potassium and the RAAS is quite significant and thus warrants further investigation.
It seems that posture in the form of being seated, upright or supine, while not having a particularly large effect on renin levels, does have a pronounced but unpredictable effect on aldosterone levels and thus the ARR overall. Therefore, standardization of posture is a cornerstone in improving ARR reproducibility. Recently, the group of Stowasser showed that the seated saline suppression test to confirm or exclude PA is superior to the recumbent suppression test. 42 However, since that study comprised only patients with a high ARR, it remains uncertain to what extent posture has an influence on the ratio in patients with EH at large.
In the articles, we reviewed aldosterone was measured using antibody‐based assays, namely a radioimmunoassay and a chemiluminescent assay. Usage of neither assay technique resulted in a significant difference in ARR measurements. A new measurement technique, being the liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) technique, was tested and compared with the conventional assay techniques. Median serum PACLC‐MS/MS levels were 27.8% lower than plasma PACRIA levels. This did not, however, yield a significant change in the sensitivity or the specificity of the ARR when a different cutoff value was used. 43
Finally, although ARR testing is by convention done in the early morning, there is uncertainty about the truly optimal time of the day for performing the test. Unfortunately, no articles on this topic survived our selection process. One notable study from those that were excluded is that by Lamarre‐Cliché et al. 44 These authors measured circadian patterns of renin, aldosterone, and the ARR. They found that the ARR crested in the morning with the lowest trough in the evening. Thus, in their study, the time of day at which the measurements were performed had a significant impact on how many participants had positive ARR screenings. Although no confirmatory tests were done in this study, the results emphasize the importance of standardizing the time of ARR measurement.
The aldosterone‐to‐renin ratio has become a widely used screening test for PA. As a result of newer assay techniques and declining costs, the test will likely be implemented on a larger scale in the future. However, in order to avoid unnecessary diagnostic procedures, the practicing clinician needs to be well aware of the shortcomings of the test and the many potential confounding factors which influence the ratio between aldosterone and renin. Standardizing the measurement procedure seems to be of great importance as recently shown by Vorselaars et al. 45
Unfortunately, there are still many unknowns regarding optimal standardization. Our review shows that investigations focusing on potential confounders have virtually never used an unbiased population. Of particular concern was that in almost all studies, at least some of the participants were still actively being treated with antihypertensive agents. In a separate review, we have examined what is known about the effect of various antihypertensive drugs on the ARR. There is a pressing need for better standardized studies regarding the ARR in order to gain the much needed knowledge about the optimal circumstances for this screening procedure and interpretation of its results.
Our findings have implications for clinical practice. Physicians ordering this test for screening purposes should be well aware of the factors which could influence the result. Knowledge of these factors is required to ensure that test conditions are standardized insofar as it is possible. Random test results should be interpreted with great caution. While our results may be less relevant for those with a low or markedly elevated ARR, the decision to proceed with additional investigations could well be of importance in those patients with an ARR close to the upper normal limit of the test.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
All authors were involved in the conception and design of this study as well as data acquisition and analysis. GPV and RMA drafted the manuscript. AAK and PWdL were involved in data acquisition, interpretation of data, and revision of the manuscript. All authors approved the final version of the manuscript and are accountable for all aspects of the work.
Funding information
None.
REFERENCES
- 1. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889‐1916. [DOI] [PubMed] [Google Scholar]
- 2. Mulatero P, Monticone S, Deinum J, et al. Genetics, prevalence, screening and confirmation of primary aldosteronism: a position statement and consensus of the Working Group on Endocrine Hypertension of The European Society of Hypertension. J Hypertens. 2020;38(10):1919‐1928. [DOI] [PubMed] [Google Scholar]
- 3. McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta‐analysis of diagnostic test accuracy studies: the PRISMA‐DTA statement. JAMA. 2018;319(4):388‐396. [DOI] [PubMed] [Google Scholar]
- 4. Yin G, Zhang S, Yan L, et al. Effect of age on aldosterone/renin ratio (ARR) and comparison of screening accuracy of ARR plus elevated serum aldosterone concentration for primary aldosteronism screening in different age groups. Endocrine. 2012;42(1):182‐189. [DOI] [PubMed] [Google Scholar]
- 5. Luo Q, Li NF, Yao XG, et al. Potential effects of age on screening for primary aldosteronism. J Hum Hypertens. 2016;30(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 6. Rossi GP, Belfiore A, Bernini G, et al. Body mass index predicts plasma aldosterone concentrations in overweight‐obese primary hypertensive patients. J Clin Endocrinol Metab. 2008;93(7):2566‐2571. [DOI] [PubMed] [Google Scholar]
- 7. Nakama C, Kamide K, Kawai T, et al. The influence of aging on the diagnosis of primary aldosteronism. Hypertens Res. 2014;37(12):1062‐1067. [DOI] [PubMed] [Google Scholar]
- 8. Ahmed AH, Gordon RD, Taylor PJ, Ward G, Pimenta E, Stowasser M. Are women more at risk of false‐positive primary aldosteronism screening and unnecessary suppression testing than men? J Clin Endocrinol Metab. 2011;96(2):E340‐E346. [DOI] [PubMed] [Google Scholar]
- 9. Pizzolo F, Raffaelli R, Memmo A, et al. Effects of female sex hormones and contraceptive pill on the diagnostic work‐up for primary aldosteronism. J Hypertens. 2010;28(1):135‐142. [DOI] [PubMed] [Google Scholar]
- 10. Nishizaka MK, Pratt‐Ubunama M, Zaman MA, Cofield S, Calhoun DA. Validity of plasma aldosterone‐to‐renin activity ratio in African American and white subjects with resistant hypertension. Am J Hypertens. 2005;18(6):805‐812. [DOI] [PubMed] [Google Scholar]
- 11. Tirosh A, Hannah‐Shmouni F, Lyssikatos C, et al. Obesity and the diagnostic accuracy for primary aldosteronism. J Clin Hypertens (Greenwich). 2017;19(8):790‐797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomes AS, Sinaiko AR, Tobian L, et al. Renal vein renin sampling in essential hypertension using hydralazine and the tourniquet test. Radiology. 1984;153(3):619‐623. [DOI] [PubMed] [Google Scholar]
- 13. Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab. 2016;101(11):3989‐3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwartz GL, Turner ST. Screening for primary aldosteronism in essential hypertension: diagnostic accuracy of the ratio of plasma aldosterone concentration to plasma renin activity. Clin Chem. 2005;51(2):386‐394. [DOI] [PubMed] [Google Scholar]
- 15. Williams JS, Williams GH, Raji A, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006;20(2):129‐136. [DOI] [PubMed] [Google Scholar]
- 16. Barigou M, Ah‐Kang F, Orloff E, Amar J, Chamontin B, Bouhanick B. Effect of postural changes on aldosterone to plasma renin ratio in patients with suspected secondary hypertension. Ann Cardiol Angeiol (Paris). 2015;64(3):169‐174. [DOI] [PubMed] [Google Scholar]
- 17. Pilz S, Kienreich K, Gaksch M, et al. Aldosterone to active renin ratio as screening test for primary aldosteronism: reproducibility and influence of orthostasis and salt loading. Horm Metab Res. 2014;46(6):427‐432. [DOI] [PubMed] [Google Scholar]
- 18. Giacchetti G, Ronconi V, Lucarelli G, Boscaro M, Mantero F. Analysis of screening and confirmatory tests in the diagnosis of primary aldosteronism: need for a standardized protocol. J Hypertens. 2006;24(4):737‐745. [DOI] [PubMed] [Google Scholar]
- 19. Burrello J, Monticone S, Buffolo F, et al. Diagnostic accuracy of aldosterone and renin measurement by chemiluminescent immunoassay and radioimmunoassay in primary aldosteronism. J Hypertens. 2016;34(5):920‐927. [DOI] [PubMed] [Google Scholar]
- 20. Dorrian CA, Toole BJ, Alvarez‐Madrazo S, Kelly A, Connell JM, Wallace AM. A screening procedure for primary aldosteronism based on the Diasorin Liaison automated chemiluminescent immunoassay for direct renin. Ann Clin Biochem. 2010;47(Pt 3):195‐199. [DOI] [PubMed] [Google Scholar]
- 21. Ferrari P, Shaw SG, Nicod J, Saner E, Nussberger J. Active renin versus plasma renin activity to define aldosterone‐to‐renin ratio for primary aldosteronism. J Hypertens. 2004;22(2):377‐381. [DOI] [PubMed] [Google Scholar]
- 22. Glinicki P, Jeske W, Bednarek‐Papierska L, et al. The ratios of aldosterone / plasma renin activity (ARR) versus aldosterone / direct renin concentration (ADRR). J Renin Angiotensin Aldosterone Syst. 2015;16(4):1298‐1305. [DOI] [PubMed] [Google Scholar]
- 23. Juutilainen A, Savolainen K, Romppanen J, et al. Combination of LC‐MS/MS aldosterone and automated direct renin in screening for primary aldosteronism. Clin Chim Acta. 2014;433:209‐215. [DOI] [PubMed] [Google Scholar]
- 24. Lonati C, Bassani N, Gritti A, Biganzoli E, Morganti A. Measurement of plasma renin concentration instead of plasma renin activity decreases the positive aldosterone‐to‐renin ratio tests in treated patients with essential hypertension. J Hypertens. 2014;32(3):627‐634. [DOI] [PubMed] [Google Scholar]
- 25. Manolopoulou J, Fischer E, Dietz A, et al. Clinical validation for the aldosterone‐to‐renin ratio and aldosterone suppression testing using simultaneous fully automated chemiluminescence immunoassays. J Hypertens. 2015;33(12):2500‐2511. [DOI] [PubMed] [Google Scholar]
- 26. Rossi GP, Ceolotto G, Rossitto G, et al. Prospective validation of an automated chemiluminescence‐based assay of renin and aldosterone for the work‐up of arterial hypertension. Clin Chem Lab Med. 2016;54(9):1441‐1450. [DOI] [PubMed] [Google Scholar]
- 27. Deng L, Xiong Z, Li H, Lei X, Cheng L. Analytical validation and investigation on reference intervals of aldosterone and renin in Chinese Han population by using fully automated chemiluminescence immunoassays. Clin Biochem. 2018;56:89‐94. [DOI] [PubMed] [Google Scholar]
- 28. Rossi GP, Seccia TM, Palumbo G, et al. Within‐patient reproducibility of the aldosterone: renin ratio in primary aldosteronism. Hypertension. 2010;55(1):83‐89. [DOI] [PubMed] [Google Scholar]
- 29. Grubler MR, Kienreich K, Gaksch M, et al. Aldosterone to active renin ratio is associated with nocturnal blood pressure in obese and treated hypertensive patients: the Styrian hypertension study. J Clin Hypertens (Greenwich). 2014;16(4):289‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez‐Aguayo A, Aglony M, Campino C, et al. Aldosterone, plasma renin activity, and aldosterone/renin ratio in a normotensive healthy pediatric population. Hypertension. 2010;56(3):391‐396. [DOI] [PubMed] [Google Scholar]
- 31. Genovesi S, Antolini L, Orlando A, et al. Aldosterone‐to‐renin ratio depends on age and sex in children attending a clinic for cardiovascular risk assessment. J Hypertens. 2018;36(2):344‐352. [DOI] [PubMed] [Google Scholar]
- 32. Fommei E, Ghione S, Ripoli A, et al. The ovarian cycle as a factor of variability in the laboratory screening for primary aldosteronism in women. J Hum Hypertens. 2009;23(2):130‐135. [DOI] [PubMed] [Google Scholar]
- 33. Newton‐Cheh C, Guo CY, Gona P, et al. Clinical and genetic correlates of aldosterone‐to‐renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49(4):846‐856. [DOI] [PubMed] [Google Scholar]
- 34. Satoh M, Kikuya M, Ohkubo T, et al. Aldosterone‐to‐renin ratio and nocturnal blood pressure decline in a general population: the Ohasama study. J Hypertens. 2011;29(10):1940‐1947. [DOI] [PubMed] [Google Scholar]
- 35. Schwartz GL, Chapman AB, Boerwinkle E, Kisabeth RM, Turner ST. Screening for primary aldosteronism: implications of an increased plasma aldosterone/renin ratio. Clin Chem. 2002;48(11):1919‐1923. [PubMed] [Google Scholar]
- 36. Dudenbostel T, Ghazi L, Liu M, Li P, Oparil S, Calhoun DA. Body mass index predicts 24‐hour urinary aldosterone levels in patients with resistant hypertension. Hypertension. 2016;68(4):995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Seaghdha CM, Hwang SJ, Vasan RS, et al. Correlation of renin angiotensin and aldosterone system activity with subcutaneous and visceral adiposity: the framingham heart study. BMC Endocr Disord. 2012;12:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kerstens MN, Kobold AC, Volmer M, Koerts J, Sluiter WJ, Dullaart RP. Reference values for aldosterone‐renin ratios in normotensive individuals and effect of changes in dietary sodium consumption. Clin Chem. 2011;57(11):1607‐1611. [DOI] [PubMed] [Google Scholar]
- 39. Cannon PJ, Ames RP, Laragh JH. Relation between potassium balance and aldosterone secretion in normal subjects and in patients with hypertensive or renal tubular disease. J Clin Invest. 1966;45(6):865‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weidmann P, de Chatel R, Schiffmann A, et al. Interrelations between age and plasma renin, aldosterone and cortisol, urinary catecholamines, and the body sodium/volume state in normal man. Klin Wochenschr. 1977;55(15):725‐733. [DOI] [PubMed] [Google Scholar]
- 41. Brunner HR, Baer L, Sealey JE, Ledingham JG, Laragh JH. The influence of potassium administration and of potassium deprivation on plasma renin in normal and hypertensive subjects. J Clin Invest. 1970;49(11):2128‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stowasser M, Ahmed AH, Cowley D, et al. Comparison of seated with recumbent saline suppression testing for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):4113‐4124. [DOI] [PubMed] [Google Scholar]
- 43. Guo Z, Poglitsch M, McWhinney BC, et al. Aldosterone LC‐MS/MS assay‐specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):3965‐3973. [DOI] [PubMed] [Google Scholar]
- 44. Lamarre‐Cliche M, de Champlain J, Lacourciere Y, Poirier L, Karas M, Larochelle P. Effects of circadian rhythms, posture, and medication on renin‐aldosterone interrelations in essential hypertensives. Am J Hypertens. 2005;18(1):56‐64. [DOI] [PubMed] [Google Scholar]
- 45. Vorselaars W, Valk GD, Vriens MR, Westerink J, Spiering W. Case detection in primary aldosteronism: high‐diagnostic value of the aldosterone‐to‐renin ratio when performed under standardized conditions. J Hypertens. 2018;36(7):1585‐1591. [DOI] [PubMed] [Google Scholar]


