Abstract
Repeated cuff‐based blood pressure (BP) measurements may cause discomfort resulting in stress and erroneous recording values. SOMNOtouch NIBP is an alternative cuff‐less BP measurement device that calculates changes in BP based on changes in pulse transit time (PTT) and a software algorithm. The device is calibrated with a single upper arm cuff‐based BP measurement. We tested the device against a validated 24‐h ambulatory BP monitoring (ABPM) device using both the previous (SomBP1) and the current software algorithm (SomBP2). In this study, 51 patients (mean age ± SD 61.5 ± 13.0 years) with essential hypertension underwent simultaneous 24‐h ABPM with the SOMNOtouch NIBP on the left arm and a standard cuff‐based oscillometric device on the right arm (OscBP). We found that mean daytime systolic BP (SBP) with OscBP was 140.8 ± 19.7 compared to 148.0 ± 25.2 (P = .008) and 146.9 ± 26.0 mmHg (P = .034) for SomBP1 and SomBP2, respectively. Nighttime SBP with OscBP was 129.5 ± 21.1 compared with 146.1 ± 25.8 (P < .0001) and 141.1 ± 27.4 mmHg (P = .001) for SomBP1 and SomBP2, respectively. Ninety‐five% limits of agreement between OscBP and SomBP1 were ± 36.6 mmHg for daytime and ± 42.6 mmHg for nighttime SBP, respectively. Agreements were not improved with SomBP2. For SBP, a nocturnal dipping pattern was found in 33% of the study patients when measured with OscBP but only in 2% and 20% with SomBP1 and ‐2, respectively. This study demonstrates that BP values obtained with the cuff‐less PTT‐based SOMNOtouch device should be interpreted with caution as these may differ substantially from what would be obtained from a validated cuff‐based BP device.
Keywords: ambulatory blood pressure/home blood pressure monitor, clinical management of high blood pressure (HBP), hypertension ‐ general, risk assessment, vasoreactivity
1. INTRODUCTION
Blood pressure (BP) measured in the doctor's office is closely associated with cardiovascular prognosis. 1 However, out‐of‐office BP (eg, 24‐h ambulatory BP monitoring [ABPM]) is an even better predictor of cardiovascular morbidity and mortality 2 , 3 as well as hypertension‐mediated organ damage. 4 For this reason, cuff‐based 24‐h ABPM is recommended in guidelines for hypertension stage classification as an 1A recommendation. 5 , 6 However, cuff‐based ABPM has several limitations as measurements are discontinuous, uncomfortable to many patients 7 , 8 , 9 and impacted by positional changes. 10 For decades, it has been known that pulse transit time (PTT), that is, the time it takes for the pulse wave to travel through the length of the arterial tree, covaries with BP. 11 , 12 Recently, cuff‐less devices designed to estimate BP based on changes in PTT have been released by a range of manufacturers with the aim of overcoming the limitations of cuff‐based BP devices. These include the Biobeat Watch (Biobeat Technologies, Petah Tikva, Israel), the Maisense Freescan (Maisense Inc Zhubei City, Taiwan) as well as the SOMNOtouch NIBP (Somnomedics GmbH, Randersacker, Germany). Most commonly, the devices measure PTT as the interval between the R‐wave of the electrocardiogram (ECG) and the arrival of the corresponding pulse wave at the index finger or at the wrist. Changes in PTT is then used to calculate changes in systolic and diastolic BP using an algorithm based on the non‐linear association between BP and PTT. As PTT can only be used for estimating changes in BP, PTT devices require an initial calibration BP with a cuff‐based BP device. The Biobeat Watch was recently approved by the United States Food and Drug Administration (FDA) for home BP measurement as the first cuff‐less device ever. A concern has been raised that this approval would make future FDA‐approval of other PTT‐based BP devices easier to attain, which could lead to a wave of approved BP devices, including smartwatches and smartphones, utilizing PTT. 13 PTT‐based estimation of BP changes may therefore in the near future be quite commonplace. Some PTT‐based devices, including the SOMNOtouch NIBP™, have undergone successful validation according to European Society of Hypertension (ESH) protocols. 14 , 15 However, this validation requires only 30 min of measurement after the initial calibration and, as stated in a recent position paper by the Lancet Commission on Hypertension Group, current ESH‐ and American Society of Hypertension validation protocols for BP devices may be insufficient to properly test the novel cuff‐less devices. 16 The commission therefore recommends the use of a recent draft ISO guideline when validating cuff‐less BP measurement devices. 17 We do not know if the SOMNOtouch device would pass the new ISO protocol. At present, to the best of our knowledge, the only published study comparing the PTT method with an oscillometric device for use in 24‐h ABPM in adults was conducted using the CE (Conformité Européenne) marked SOMNOtouch NIBP device. This study found both systolic and diastolic BP to be substantially higher using the PTT device compared with a standard upper arm cuff‐based oscillometric BP device. 18 Since then, a new software version (Domino Light v. 1.5.0) with an adjusted algorithm has been released for the SOMNOtouch device, with the aim of improving the accuracy of measurement during nighttime. No published studies have assessed the accuracy of measurements with the PTT device using the Domino Light software version 1.5.0 (SomBP2) compared to the previous and still widely used edition (Domino Light v. 1.4.0, SomBP1) and standard oscillometric BP measurement. We initiated this study to investigate the clinical usefulness of the SOMNOtouch device by comparing the BP measurements of the device with the measurements of a standard cuff‐based oscillometric device during 24‐h ABPM.
2. METHODS
2.1. Recruitment of study participants
A total of 60 adult patients with essential hypertension and an indication for 24‐h ABPM were recruited from The Hypertension Clinic at Aarhus University Hospital. Exclusion criteria were as follows: Known significant arterial abnormalities in the upper extremities, known cardiac arrhythmia, upper arm circumference below 15 cm or above 42 cm, or possible extreme uncontrolled hypertension (ie, resting office BP > 250/140 mmHg). To enable testing of the device at both normal and elevated BP levels, we aimed at including at least 15 patients in each of the following three categories: Normal BP, stage 1 hypertension, and stage ≥ 2 hypertension, based on the guidelines of the UK National Institute for Health and Care Excellence (NICE) for ABPM. 19 Informed consent was obtained from all patients. At inclusion, a simultaneous bilateral BP measurement was performed with three consecutive measurements using an automated oscillometric device (WatchBP Office®, Microlife, Corporation, Teipei, Taiwan) 20 after 5 min of rest. If the difference in systolic blood pressure (SBP) between the arms was ≥10 mmHg, the patient was not included in the study.
2.2. ABPM measurements
All staff members involved in the project were familiar with the SOMNOtouch NIBP device (SomBP), which has been used in our clinic since 2015, mainly for selected patients with high cuff‐based BP and at the same time symptoms of orthostatic hypotension. Mounting and calibration of the device were conducted according to the manufacturers’ instructions. With the patient sitting in an upright position with back support, a conventional oscillometric BP device (OscBP, Spacelabs 90 217, Spacelabs Healthcare, Hawthorne, USA) 21 was fitted on the right arm, while the SomBP was fitted on the left arm. The SomBP was calibrated with a single BP measurement with a cuff‐based sphygmomanometer (Big Ben® Square, Riester, Jungingen, Germany) as per manufacturer instructions. The Spacelabs devices as well as the Riester sphygmomanometer were maintained and regularly calibrated as recommended by the manufacturers. The OscBP recorded BP three times every hour during daytime and twice every hour during nighttime, while the SomBP measured BP continuously. The patients were instructed to engage in normal activities but refrain from strenuous exercise and, at the time of cuff inflation, to stop moving and talking, while keeping the arm still with the cuff at heart level.
As per ESH guidelines, a minimum of 20 completed daytime and 7 completed nighttime measurements during the ABPM were required for the 24‐h ABPM to be valid. 22 If this criterium was not met for one or both of the devices, data from both devices were excluded from the study. In these instances, patients were asked to repeat examination with both devices. SomBP1 data was extracted ad hoc using Domino Light 1.4.0 software. Reanalysis of SomBP‐data was performed post hoc with the Domino Light 1.5.0 software, and data were thereafter extracted locally by the primary investigator. This reanalysis was performed by a SOMNOmedics programmer blinded to the corresponding OscBP measurements. From the SomBP measurements, we used instantaneous BP measured four times every hour for our analysis as BP is reported at this interval in the blood pressure report of the device. A small questionnaire on device‐related pain and/or discomfort as well as sleeping habits was given to all participants.
2.3. Estimation of markers of arterial stiffness
To study the potential impact of aortic stiffness as well as peripheral wave reflection on the measurement of the PTT device, carotid‐femoral pulse wave velocity (cfPWV) as well as radially obtained pulse wave analysis (PWA) was performed in duplicate with the SphygmoCor system (version 8.2, Atcor Medical, Sydney, Australia). 23 A research nurse with more than a decade of experience using the Spygmocor device was responsible for these measurements. cfPWV and aortic augmentation index adjusted for heart rate (AIx@HR75) are presented as the mean of two measurements. Measurements were only included in the analysis if they were deemed to be of acceptable quality. cfPWV was chosen, as this is considered the gold standard of arterial stiffness estimation in clinical practice. 24
2.4. Statistical analysis
The study was conducted to test the null hypothesis that there is no difference between the BP measurements of the two ABPM devices. The study was designed to have at least 80% power to detect a 5‐mmHg systolic BP difference between the devices. Assuming a standard deviation of 10 mmHg at least 34 patients thus had to be included. We decided to include at least 50 patients to attain acceptable limits of agreement. 25 All statistical analyses were performed using STATA software (v. 16.0, Statacorp, Texas, USA). A P value of .05 was prespecified as statistically significant. Variables were checked for distribution through histograms and QQ‐plots. Data on continuous variables are presented as means ± SD unless stated otherwise. Means were compared using paired t test. Pearson's correlation was performed to test for association between different BP measurement modalities. Regression analysis was used to test association between BP measurements and potential covariates. Multivariate regression analysis was carried out to adjust associations for interaction. Linear regression models were checked by diagnostic plots of residuals. Categorical variables were compared using chi‐squared tests. For some dichotomous variables, Cohens kappa was used to test for agreement between devices.
3. RESULTS
3.1. General characteristics
A total of 60 patients were enrolled in the study. Nine (18%) of these were excluded after enrollment for the reasons outlined in Figure 1, leaving 51 patients in the current analysis. Patient baseline characteristics are shown in Table 1. Average age was 61.5 years and 59% were women. Average between‐arms difference was insignificant for both SBP and diastolic BP (DBP). Based on NICE guidelines for ABPM, 17 patients (33%) had a normal SBP, that is, BP below 135 mmHg, 18 patients (35%) had stage 1 systolic hypertension, that is, SBP ≥ 135 mmHg and <150 mmHg and 16 patients (31%) had stage ≥2 systolic hypertension, that is, SBP ≥ 150 mmHg assessed from the oscillometric awake mean BP. 19 Average heart rate (HR) over the full measurement was 68.3 ± 11.1 beats/min as measured with OscBP and 69.8 ± 10.4 beats/min with SomBP1 (P < .05). Curiously, 24‐h average HR was slightly lower with the SomBP2 (69.1 ± 10.3 beats/min) as compared with SomBP1 (P < .01). From the questionnaire, it was ascertained, that 3 patients (6%) found the PTT device to cause significant discomfort while 27 patients (53%) found the OscBP device to cause significant discomfort.
Figure 1.
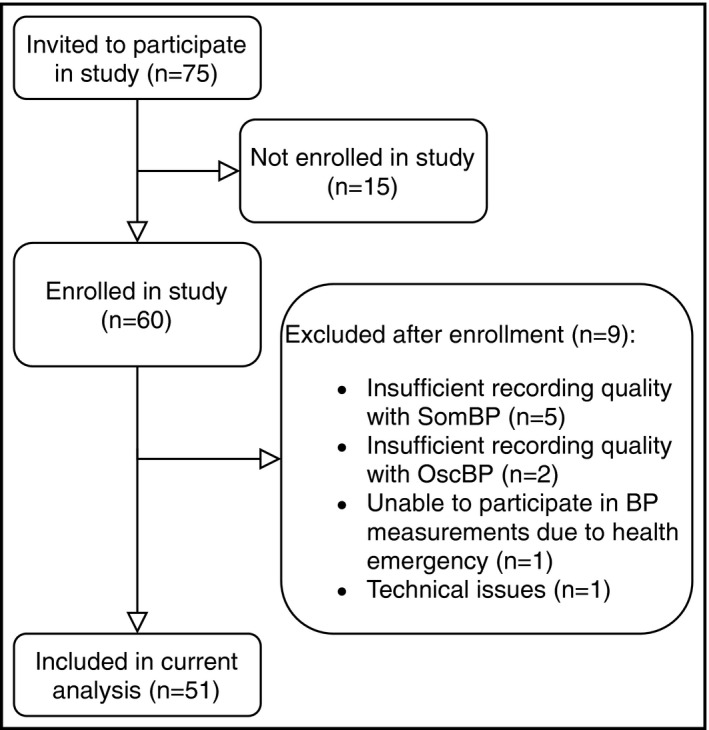
Study flow chart
Table 1.
Characteristics of included patients
| Age, years | 61.5 ± 13.0 |
| Sex, female | 30 (58.8) |
| BMI, kg/m2 | 28.7 ± 4.9 |
| Difference in systolic BP, left minus right arm, mmHg | 0.25 ± 3.6 |
| Difference in diastolic BP, left minus right arm, mmHg | −0.39 ± 3.3 |
| Mean valid 24‐h cuff‐based BP readings | 55.5 ± 9.2 |
| Percentage valid cuff‐based BP readings compared to expected number of readings during measurement, % | 89.7 ± 8.9 |
| Mean number of prescribed antihypertensive medications | 2.98 ± 1.4 |
Data are mean ± SD or number (%) as appropriate.
Abbreviation: BMI, Body mass index.
3.2. Comparison between OscBP and SomBP1
Mean daytime, nighttime, and 24‐h SBP were significantly higher for SomBP1 than for OscBP. The difference was most pronounced during nighttime, where SBP was 16.6 ± 21.8 mmHg lower with OscBP (Table 2). Comparing mean daytime SBP between OscBP and SomBP1, the agreements ≤2, ≤5, ≤10, ≤15, and ≤20 mmHg were 6 (12%), 12 (24%), 22 (43%), 31 (61%), and 35 (69%), respectively. For nighttime SBP, the agreements were 2 (4%), 5 (10%), 15 (29%), 22 (43%), and 29 (57%), respectively. Agreements tended to be poorer for nighttime averages than for daytime and 24‐h averages. A nocturnal BP dipping pattern with a drop from daytime SBP of ≥10% was seen in 17 patients (33%) with OscBP and 1 patient (2%) with SomBP1. The lone SomBP1 dipper was also found to be a dipper with OscBP.
Table 2.
Comparison of mean daytime, nighttime, and 24‐h blood pressure (BP) with the oscillometric Spacelabs device as well as the cuffless SOMNOtouch NIBP™ with the Domino Light v. 1.4.0 and v. 1.5.0 software
| BP, mmHg | P‐values | 95% limits of agreement, mmHg | ||||||
|---|---|---|---|---|---|---|---|---|
| OscBP | SomBP1 | SomBP2 | OscBP vs. SomBP1 | OscBP vs. SomBP2 | SomBP1 vs. SomBP2 | OscBP vs. SomBP1 | OscBP vs. SomBP2 | |
| Systolic | ||||||||
| Daytime | 140.8 ± 19.7 | 148.0 ± 25.2 | 146.9 ± 26.0 | .008 | 0.034 | 0.042 | ±36.6 | ±38.9 |
| Nighttime | 129.5 ± 21.1 | 146.1 ± 25.8 | 141.1 ± 27.4 | <.0001 | 0.001 | <0.0001 | ±42.6 | ±47.1 |
| Total | 136.9 ± 19.3 | 147.5 ± 25.2 | 145.3 ± 26.4 | .0002 | 0.005 | 0.0004 | ±36.7 | ±39.8 |
| Diastolic | ||||||||
| Daytime | 82.3 ± 10.2 | 85.1 ± 12.8 | 84.8 ± 13.4 | .020 | 0.045 | 0.195 | ±16.3 | ±16.6 |
| Nighttime | 72.2 ± 10.7 | 84.4 ± 12.9 | 81.2 ± 14.2 | <.0001 | <0.0001 | <0.0001 | ±20.6 | ±22.0 |
| Total | 78.9 ± 9.8 | 84.9 ± 12.9 | 83.9 ± 13.5 | <.0001 | 0.0002 | 0.001 | ±16.8 | ±17.3 |
DBP was significantly different between SomBP1 and OscBP during both daytime and nighttime. Overall, the agreement between the means of SomBP1 and OscBP was better for DBP than for SBP. For daytime DBP, the agreements ≤2, ≤5, ≤10, ≤15, and ≤20 mmHg were 13 (25%), 22 (43%), 37 (73%), 47 (92%), and 51 (100%), respectively. Again, agreements were poorer for nighttime averages at 5 (10%), 11 (22%), 24 (47%), 32 (63%), and 41 (80%), respectively. For DBP, a nocturnal dipping pattern was found in 34 patients (67%) with OscBP, while no patients were dippers with SomBP1.
Pearson correlation (r) between daytime, nighttime, and 24‐h averages for OscBP and SomBP1 are presented in Figure 2. Comparison of mean daytime, nighttime, and 24‐h SBP and DBP is presented in Bland–Altman plots (Figure 3). The plots reveal large differences in day and night BP averages with SomBP1 and OscBP in the individual patient. The 95% limits of agreement for mean daytime SBP with SomBP1 were ±36.6 mmHg compared to the corresponding OscBP. For nighttime SBP, the equivalent 95% limits of agreement were even wider at ±42.6 mmHg. The 95% limits of agreement for DBP can be seen in Table 2. The Bland–Altman plots revealed a slight tendency toward a gradual increase in the daytime and 24‐h SomBP1 compared to OscBP with increasing BP level. The Pearson coefficient for the association was small but significantly positive for daytime and 24‐h SBP and DBP (Figure 3). For nighttime SBP and DBP, the association was not significant (P = .08 and P = .10, respectively).
Figure 2.
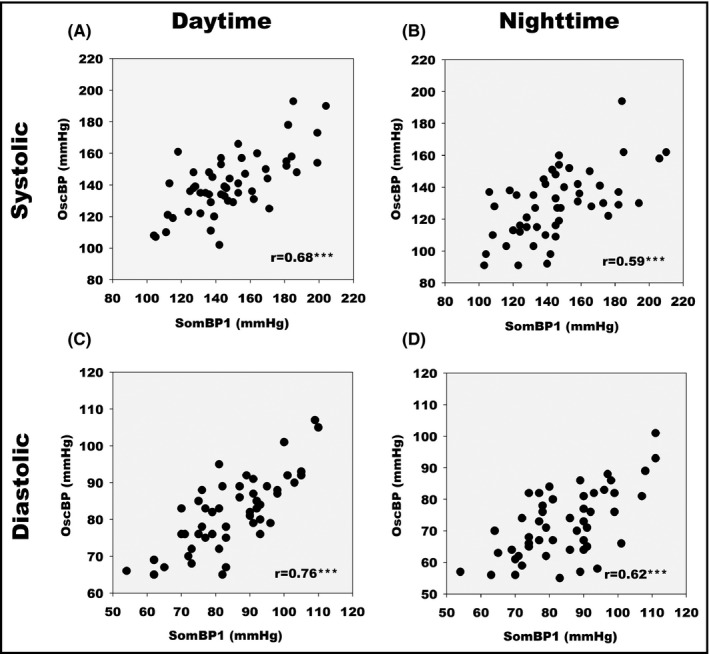
Scatter diagram of SomBP1 and OscBP mean blood pressure values. (A) Daytime systolic. (B) Nighttime systolic. (C) Daytime diastolic. (D) Nighttime diastolic. r indicates Pearson's correlation coefficients. *** indicates P < .0001
Figure 3.
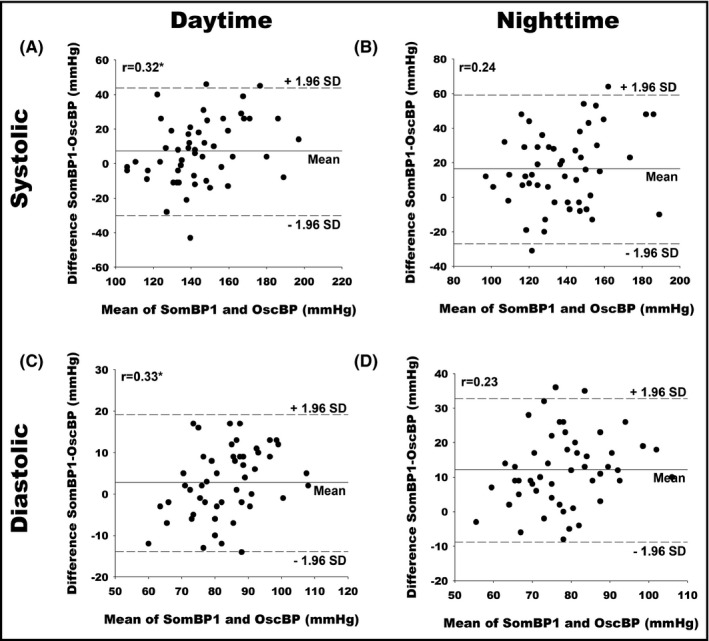
Bland–Altman plots comparing mean BP values of SomBP1 and OscBP. (A) Daytime systolic. (B) Nighttime systolic. (C) Daytime diastolic. (D) Nighttime diastolic. r indicates Pearson's correlation coefficients. SD, Standard deviation. * indicates P < .05
3.3. Comparison of OscBP and SomBP2
Overall, both SBP and DBP were lower for SomBP2 compared to SomBP1 and thereby closer to the mean SBP and DBP of OscBP. This difference was most pronounced for mean nighttime SBP. However, agreements in mean daytime, nighttime, and 24‐h SBP with OscBP were not improved with SomBP2 compared to SomBP1. Comparing mean daytime SBP between OscBP and SomBP2, the agreements ≤2, ≤5, ≤10, ≤15, and ≤20 mmHg were 5 (10%), 11 (21%), 21 (41%), 30 (59%), and 35 (69%), respectively. For nighttime SBP, the agreements were 4 (8%), 6 (12%), 16 (31%), 24 (47%), and 29 (57%), respectively. The number of patients displaying a nocturnal dipping pattern in SBP was 10 (20%) with SomBP2. Seven of these patients were non‐dippers with OscBP.
For daytime DBP, the agreements ≤2, ≤5, ≤10, ≤15, and ≤20 mmHg between OscBP and SomBP2 were 14 (27%), 21 (41%), 36 (71%), 48 (94%), and 51 (100%), respectively. For nighttime DBP, the agreements were 8 (16%), 15 (29%), 25 (49%), 36 (71%), and 45 (88%), respectively. In 7 patients (16%), DBP fell ≥10% during nighttime with SomBP2. Five of these patients were non‐dippers with OscBP.
Correlation coefficients for daytime, nighttime, and 24‐h SBP and DBP averages for OscBP and SomBP2 were largely the same as for SomBP1 (Figure 4). The 95% limits of agreement for daytime and nighttime SBP compared with OscBP were not improved for SomBP2 as compared to SomBP1 at ±38.9 and ±47.1 mmHg, respectively. For DBP, the 95% limits of agreement for both daytime and nighttime SomBP2 were similar to what was found for SomBP1 (Table 2). Once again, Bland–Altman plots of SomBP2 and OscBP revealed a slight association between the BP level and the difference in OscBP and SomBP2 for both mean SBP and DBP during daytime and 24‐h measurement while the association was also significant for both nighttime SBP and DBP (Figure 5).
Figure 4.
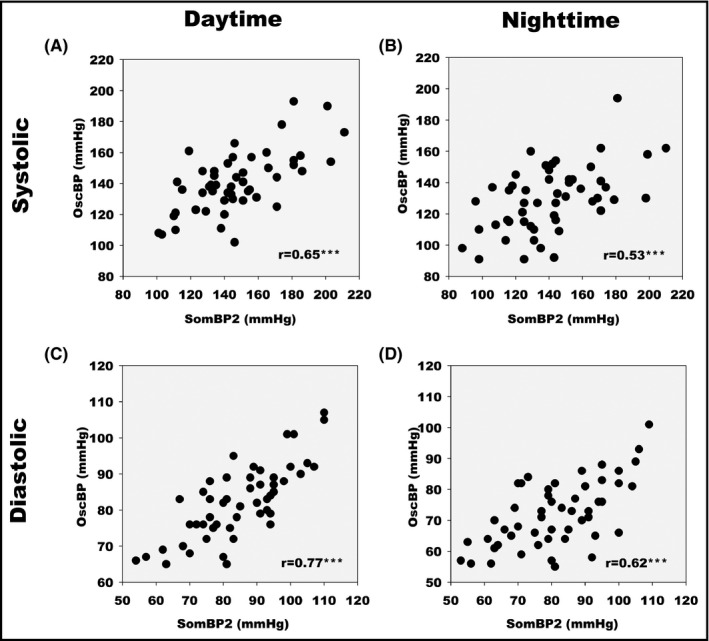
Scatter diagram of SomBP2 and OscBP mean blood pressure values. (A) Daytime systolic. (B) Nighttime systolic. (C) Daytime diastolic. (D) Nighttime diastolic. r indicates Pearson's correlation coefficients. *** indicates P < .0001
Figure 5.
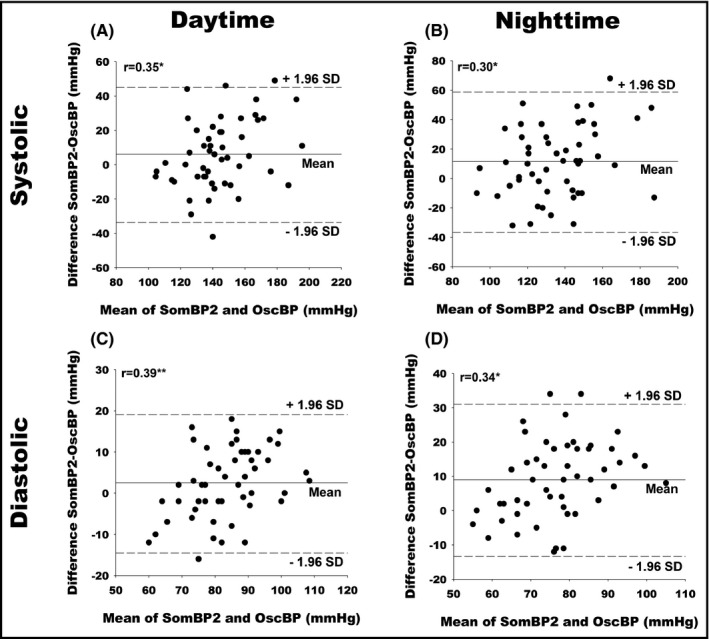
Bland–Altman plots comparing mean BP values of SomBP2 and OscBP. (A) Daytime systolic. (B) Nighttime systolic. (C) Daytime diastolic. (D) Nighttime diastolic. r indicates Pearson's correlation coefficients. SD, Standard deviation. * indicates P < .05. ** indicates P < .01
3.4. Diagnosing controlled/uncontrolled hypertension with the SOMNOtouch NIBP device
With daytime and nighttime hypertension defined as a BP > 135/85 mmHg and >120/70 mmHg, respectively, 5 and with OscBP as the reference, positive‐ and negative predictive values (PPV and NPV, respectively) as well as Cohens kappa (κ) for diagnosing hypertension with SomBP1 and ‐2 can be seen in Table 3. PPV and NPV for the diagnosis of daytime hypertension were similar for SomBP1 and SomBP2.
Table 3.
Positive and negative predictive values as well as Cohen kappa coefficient for diagnosing daytime and nighttime hypertension with SOMNOtouch NIBP™ using Domino Light software version 1.4.0 and version 1.5.0, using Spacelabs 90 217 measurements as the reference
| Positive predictive value (%; CI 95%) | Negative predictive value (%; CI 95%) | Cohens kappa coefficient (CI 95%) | |
|---|---|---|---|
| SomBP1 | |||
| Daytime hypertension (mean BP > 135/85) | 73.7 (56.9–86.6) | 46.2 (19.2–74.9) | 0.18 (−0.08–0.46) |
| Nighttime hypertension (mean BP > 120/70) | 71.4 (56.7–83.4) | 50.0 (1.3–98.7) | 0.05 (−0.10–0.21) |
| SomBP2 | |||
| Daytime hypertension (mean BP > 135/85) | 75.0 (57.8–87.9) | 46.7 (21.3–73.4) | 0.21 (−0.06–0.49) |
| Nighttime hypertension (mean BP > 120/70) | 75.6 (60.5–87.1) | 66.7 (22.3–95.7) | 0.26 (0.02–0.49) |
κ‐values for the diagnosis of daytime and nighttime hypertension with SomBP1 and ‐2 ranged from none to fair agreement. 26
3.5. Arterial stiffness and PTT‐based BP measurements
Carotid‐femoral PWV (cfPWV) was available in 41 patients (80%) and AIx@HR75 measurements were available in 39 patients (76%). AIx@HR75, cfPWV, age, gender, BMI, height, weight, and oscillometric BP level were all tested with simple‐ as well as multiple linear regression analysis for association with BP measurements with SomBP1 and SomBP2. Of the investigated variables, cfPWV, oscillometric BP level and AIx@HR75 showed a significant association with PTT‐based SBP measurements.
Simple linear regression coefficients for AIx@HR75 with daytime, nighttime, and 24‐h mean SBP for SomBP1 were 1.04, 0.93 and 1.01 (all P < .05), respectively, and for SomBP2 1.11, 1.07, and 1.09 (all P < .05), respectively. Simple linear regression coefficients for AIx@HR75 and the difference in mean SBP between SomBP1 and OscBP for daytime, nighttime, and 24‐h were 0.82, 0.78, and 0.80 (P = .053 for nighttime SBP, otherwise P < .05 ), respectively, and between SomBP2 and OscBP 0.89, 0.92, and 0.89 (all P < .05), respectively. All significant associations remained statistically significant in multiple regression analysis adjusting for the above‐mentioned covariates. Furthermore, in the multivariate analysis, the association between AIx@HR75 and the difference in nighttime SBP between SomBP1 and OscBP became statistically significant (P = .017).
For cfPWV, simple linear regression coefficients for the association with daytime, nighttime and 24‐h mean SBP for SomBP1 were 6.30, 6.58, and 6.37 (all P < .01), respectively, and for SomBP2 6.72, 6.95, and 6.75 (all P < .01), respectively. However, when adjusted for covariates in the multivariate analysis, all associations became insignificant. Furthermore, in both simple and multiple regression analysis, cfPWV was unassociated with the differences in mean SBP between OscBP and the PTT device.
All of the above‐described analyses were also carried out for DBP. In the multivariate analysis, DBPs measured with the PTT device were not associated with any of the covariates besides oscillometric BP level. Furthermore, differences in DBP between the PTT device and OscBP were not associated with any of the covariates in the multivariate analysis.
4. DISCUSSION
In this study, the cuffless SOMNOtouch NIBP device generally estimated daytime, nighttime, and 24‐h ambulatory BP to be considerably higher than measured with a thoroughly validated cuff‐based oscillometric ABPM‐device. This was the case for both the widely used Domino Light 1.4.0‐software and for the updated 1.5.0‐software. While the BP of patient group as a whole dropped considerably during ABPM with the OscBP device, this was not the case for SomBP1 where BP was largely the same during both daytime and nighttime. With SomBP2 nighttime and 24‐h means were lower and thereby closer to the means of OscBP. Despite the significantly lowered nighttime and 24‐h means with SomBP2, our data do not suggest that accordance with OscBP on the patient level was better for SomBP2 than SomBP1 as the 95% limits of agreement for SBP were more than 35 mm Hg using both the original and adjusted software. Furthermore, the weak association between BP level and the difference in OscBP minus SomBP revealed in the Bland–Altman plots suggests that the accuracy of the PTT device compared to OscBP may be lower at very high and very low values. The device might therefore be most accurate at intermediate BP levels. In this study, however, we did not have enough patients at either BP extreme to test this hypothesis properly.
Our findings with regards to SomBP1 are in accordance with the findings of the only previous study comparing the PTT device with an oscillometric device in adults for 24‐h ABPM, by Krisai and colleagues. 18 In their study, as in ours, daytime, nighttime, and 24‐h BP were all significantly higher with SomBP1 compared with OscBP. 18 This previous study did, however, not include data on SomBP2 and no prior paper has been published on the accuracy of the Domino Light 1.5.0‐software as compared to oscillometric measurement in 24‐h ABPM, despite the fact that this is currently the standard software to be used with the SOMNOtouch NIBP device.
It has been found, that a nocturnal drop in BP as compared with daytime BP, that is, “BP dipping,” significantly reduces the risk of cerebrovascular 27 and cardiovascular events. 28 , 29 , 30 In the manufacturer's clinical compendium for the SOMNOtouch NIBP, it is stated that nocturnal BP dipping can be determined more reliably with the PTT device compared with an oscillometric device. 31 The findings of our study do not support use of the PTT device for this purpose, as most dippers on the OscBP were non‐dippers on both the SomBP1 and SomBP2. The SomBP2 identified seven patients to be nocturnal dippers that were non‐dippers on the OscBP. However, as all major studies on the prognostic importance of nocturnal BP dipping have been based on oscillometric BP measurements, the clinical and prognostic importance of establishing a dipping pattern in patients who are non‐dippers with an oscillometric device is unclear and is most likely not outweighed by the apparent underdiagnosing of nocturnal dipping in the patient group as a whole.
PTT is dependent on the pulse wave velocity in arteries which in turn, is dependent on the elasticity and the diameter of the arterial vessel as described by the Moens‐Korteweg equation. The SomBP device uses a modified version of the Moens‐Korteweg equation to calculate BP indirectly by a non‐linear correlation between BP and PTT. 31 , 32 Arterial elasticity may, however, be significantly affected by changes in sympathetic tone. 33 , 34 , 35 Furthermore, changes in PTT may have a poorer correlation with BP in younger subjects than in older individuals because the arteries in young individuals are more flexible. 36 As a consequence, the intra‐ and interindividual relationship between PTT and BP may change over time depending on the activity level, sympathetic tone, and age of the individual. As the SomBP device is calibrated with a single resting BP, changes in physiological conditions after calibration, for example, physical activity and sleep, may change the relationship between PTT and BP and thereby cause the estimation of the BP based on PTT to become less precise. Interestingly, neither age nor cfPWV, as markers of arterial stiffness, were associated with SBP means gathered from the SomBP device in the multivariate analysis. The SOMNOtouch device measures PTT over only a small part of the aorta, that is, from the aortic valve to the origin of the subclavian artery, while the majority of the travel of the pulse wave from the heart to the finger is through muscular peripheral arteries. Any impact of aortic stiffness on the pulse wave may therefore be far outweighed by the PWV in the arteries of the upper extremities being different from cfPWV. This could explain why the SBP means on the SomBP was unassociated with cfPWV, in our adjusted model, but significantly associated with AIx@HR75 which is influenced by peripheral wave reflection.
Previous studies have found ABPM to cause significant discomfort 37 and cuff inflation itself has been found to cause a significant reactive increase in BP. 38 , 39 As the results of our questionnaire showed, 24‐h ABPM using the PTT device is significantly more comfortable for the patient than the oscillometric device. As measurements were carried out with both devices mounted at the same time, we cannot on the basis of our findings clearly state whether patient sleep and nocturnal dipping would be significantly better if measurements were carried out with just SomBP. It does, however, not seem unreasonable to assume, as more than 50% of all patients found the OscBP device to cause significant discomfort. Despite the apparent shortcoming in BP measurement accuracy, the potential of the PTT device to limit the impact of the discomfort associated with BP measurement on sleep is therefore supported by the findings of this study.
5. CONCLUSION
The cuff‐less PTT‐based SOMNOtouch NIBP overcomes many of the practical limitations that are typically associated with cuff‐based oscillometric 24‐h ABPM. Our data do, however, reveal several limitations of the PTT device when compared with a thoroughly validated oscillometric device. Firstly, agreement in measurement with the oscillometric device is low with wide 95% limits of agreement. Secondly, the PTT device consistently overestimates nighttime and 24‐h BP means. Finally, detection of nocturnal dipping is low. These limitations also apply to the newest software. However, with the newest software, detection of dipping was improved, and SBP and DBP means were lower.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Jakob Nyvad (JN), Kent Lodberg Christensen (KL), Niels Henrik Buus (NHB), and Mark Reinhard (MR) contributed to the design of the project. JN collected the data from the patients, supervised by KL, NHB, and MR. JN analyzed the data and wrote the manuscript in consultation with KL, NHB, and MR.
ACKNOWLEDGEMENTS
We would like to express our gratitude for the great help of our research nurses. Also, we would like to thank Somnomedics GmbH for being forthcoming with regards to this study.
Clinical trials registration: NCT04278001.
Funding information
This study was fully funded by the Department of Renal Medicine, Aarhus University Hospital.
REFERENCES
- 1. Myers MG. A short history of automated office blood pressure ‐ 15 years to SPRINT. J Clin Hypertens (Greenwich). 2016;18(8):721‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895‐905. [DOI] [PubMed] [Google Scholar]
- 3. Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348(24):2407‐2415. [DOI] [PubMed] [Google Scholar]
- 4. Gaborieau V, Delarche N, Gosse P. Ambulatory blood pressure monitoring versus self‐measurement of blood pressure at home: correlation with target organ damage. J Hypertens. 2008;26(10):1919‐1927. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021‐3104. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension. 2018;71(6):1269‐1324. [DOI] [PubMed] [Google Scholar]
- 7. Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G. Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens. 2007;20(2):154‐161. [DOI] [PubMed] [Google Scholar]
- 8. Heude E, Bourgin P, Feigel P, Escourrou P. Ambulatory monitoring of blood pressure disturbs sleep and raises systolic pressure at night in patients suspected of suffering from sleep‐disordered breathing. Clin Sci (Lond). 1996;91(1):45‐50. [DOI] [PubMed] [Google Scholar]
- 9. Mallion JM, de Gaudemaris R, Baguet JP, et al. Acceptability and tolerance of ambulatory blood pressure measurement in the hypertensive patient. Blood Press Monit. 1996;1(3):197‐203. [PubMed] [Google Scholar]
- 10. Schwan A, Pavek K. Change in posture during sleep causes errors in non‐invasive automatic blood pressure recordings. J Hypertens Suppl. 1989;7(6):S62‐63. [DOI] [PubMed] [Google Scholar]
- 11. Geddes LA, Voelz MH, Babbs CF, Bourland JD, Tacker WA. Pulse transit time as an indicator of arterial blood pressure. Psychophysiology. 1981;18(1):71‐74. [DOI] [PubMed] [Google Scholar]
- 12. Obrist PA, Light KC, McCubbin JA, Hutcheson JS, Hoffer JL. Pulse transit time: relationship to blood pressure and myocardial performance. Psychophysiology. 1979;16(3):292‐301. [DOI] [PubMed] [Google Scholar]
- 13. van Helmond N, Martin SS, Plante TB. Is cuffless blood pressure measurement already here? J Hypertens. 2020;38(4):774‐775. [DOI] [PubMed] [Google Scholar]
- 14. O'Brien E, Atkins N, Stergiou G, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(1):23‐38. [DOI] [PubMed] [Google Scholar]
- 15. Bilo G, Zorzi C, Ochoa Munera JE, Torlasco C, Giuli V, Parati G. Validation of the Somnotouch‐NIBP noninvasive continuous blood pressure monitor according to the European Society of Hypertension International Protocol revision 2010. Blood Press Monit. 2015;20(5):291‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharman JE, O'Brien E, Alpert B, et al. Lancet Commission on Hypertension group position statement on the global improvement of accuracy standards for devices that measure blood pressure. J Hypertens. 2020;38(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharman JE, O’Brien E, Alpert B, et al. Reply. J Hypertens. 2020;38(4):775. [DOI] [PubMed] [Google Scholar]
- 18. Krisai P, Vischer AS, Kilian L, Meienberg A, Mayr M, Burkard T. Accuracy of 24‐hour ambulatory blood pressure monitoring by a novel cuffless device in clinical practice. Heart. 2019;105(5):399‐405. [DOI] [PubMed] [Google Scholar]
- 19. National‐Guideline‐Centre (UK) . Hypertension in adults: diagnosis and management. NICE guidelines Web site. Published 2019. Accessed February 11, 2020.
- 20. Krogager C, Laugesen E, Rossen NB, Poulsen PL, Erlandsen M, Hansen KW. Evaluation of interarm blood pressure differences using the Microlife WatchBP Office in a clinical setting. Blood Press Monit. 2017;22(3):161‐165. [DOI] [PubMed] [Google Scholar]
- 21. Baumgart P, Kamp J. Accuracy of the SpaceLabs Medical 90217 ambulatory blood pressure monitor. Blood Press Monit. 1998;3(5):303‐307. [PubMed] [Google Scholar]
- 22. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31(9):1731‐1768. [DOI] [PubMed] [Google Scholar]
- 23. Carlsen RK, Peters CD, Khatir DS, et al. Estimated aortic blood pressure based on radial artery tonometry underestimates directly measured aortic blood pressure in patients with advancing chronic kidney disease staging and increasing arterial stiffness. Kidney Int. 2016;90(4):869‐877. [DOI] [PubMed] [Google Scholar]
- 24. Milan A, Zocaro G, Leone D, et al. Current assessment of pulse wave velocity: comprehensive review of validation studies. J Hypertens. 2019;37(8):1547‐1557. [DOI] [PubMed] [Google Scholar]
- 25. Altman DG. Practical Statistics for Medical Research. New York: Chapman and Hall/CRC; 1990. [Google Scholar]
- 26. McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276‐282. [PMC free article] [PubMed] [Google Scholar]
- 27. Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38(4):852‐857. [DOI] [PubMed] [Google Scholar]
- 28. O'Brien E, Sheridan J, O'Malley K. Dippers and non‐dippers. Lancet. 1988;2(8607):397. [DOI] [PubMed] [Google Scholar]
- 29. Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24(6):793‐801. [DOI] [PubMed] [Google Scholar]
- 30. Bankir L, Bochud M, Maillard M, Bovet P, Gabriel A, Burnier M. Nighttime blood pressure and nocturnal dipping are associated with daytime urinary sodium excretion in African subjects. Hypertension. 2008;51(4):891‐898. [DOI] [PubMed] [Google Scholar]
- 31. SOMNOmedics‐GmbH . Blood pressure measurement using PTT. SOMNOmedics GmbH. https://somnomedics.eu/solutions/blood‐pressure/Website. Published 2019. Accessed February 11, 2020
- 32. Gosling RG, Budge MM. Terminology for describing the elastic behavior of arteries. Hypertension. 2003;41(6):1180‐1182. [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Cao TS, Duan YY, Yang YL, Yuan LJ. Effects of cold pressor‐induced sympathetic stimulation on the mechanical properties of common carotid and femoral arteries in healthy males. Heart Vessels. 2011;26(2):214‐221. [DOI] [PubMed] [Google Scholar]
- 34. Joannides R, Richard V, Moore N, Godin M, Thuillez C. Influence of sympathetic tone on mechanical properties of muscular arteries in humans. Am J Physiol. 1995;268(2 Pt 2):H794‐801. [DOI] [PubMed] [Google Scholar]
- 35. Grassi G, Giannattasio C, Failla M, et al. Sympathetic modulation of radial artery compliance in congestive heart failure. Hypertension. 1995;26(2):348‐354. [DOI] [PubMed] [Google Scholar]
- 36. Proença J, Muehlsteff J, Aubert X, Carvalho P. Is pulse transit time a good indicator of blood pressure changes during short physical exercise in a young population? Conf Proc IEEE Eng Med Biol Soc. 2010;2010:598‐601. [DOI] [PubMed] [Google Scholar]
- 37. van der Steen MS, Lenders JW, Thien T. Side effects of ambulatory blood pressure monitoring. Blood Press Monit. 2005;10(3):151‐155. [DOI] [PubMed] [Google Scholar]
- 38. Charmoy A, Würzner G, Ruffieux C, et al. Reactive rise in blood pressure upon cuff inflation: cuff inflation at the arm causes a greater rise in pressure than at the wrist in hypertensive patients. Blood Press Monit. 2007;12(5):275‐280. [DOI] [PubMed] [Google Scholar]
- 39. Sheshadri V, Tiwari AK, Nagappa M, Venkatraghavan L. Accuracy in blood pressure monitoring: the effect of noninvasive blood pressure cuff inflation on intra‐arterial blood pressure values. Anesth Essays Res. 2017;11(1):169‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]


