Abstract
The present meta‐analysis aims to compare renal arterial and venous Doppler parameters in women with preeclampsia and healthy pregnant controls. Medline, Scopus, Cochrane Central Register of Controlled Trials, Clinicaltrials.gov, and Google Scholar databases were systematically searched from inception to December 04, 2019. All observational studies reporting renal resistive index, pulsatility index, renal interlobar vein impedance, or pulse transit time among preeclamptic and healthy pregnant women were held eligible. Subgroup analysis was conducted on the basis of disease onset and side of measurement. Both pair‐wise and network meta‐analysis were performed using Review Manager 5.3 and R‐3.4.3 software. Fourteen studies were included, with a total of 1118 women. No difference of renal resistive (MD: 0.00, 95% CI: [−0.03, 0.04]) and pulsatility index (MD: −0.01, 95% CI: [−0.14, 0.12]) was evident between the two groups. Renal interlobar vein impedance was estimated to be significantly higher in preeclampsia (MD: 0.07, 95% CI: [0.06, 0.09]), while venous pulse transit time was significantly lower (MD: −0.10, 95% CI: [−0.14, −0.05]) in women with the disease. Subgroup analysis indicated that early‐onset preeclampsia was associated with significantly elevated renal interlobar vein impedance and lower venous pulse transit time than late‐onset disease. The outcomes of the present meta‐analysis suggest that preeclampsia is characterized by venous hemodynamic dysfunction as it is associated with significantly elevated renal interlobar vein impedance and shorter venous pulse transit time. Future large‐scale prospective studies should introduce cutoff values and determine the optimal timing of measurement in order to achieve optimal predictive accuracy.
Keywords: hemodynamics, meta‐analysis, preeclampsia, renal Doppler, venous Doppler
1. INTRODUCTION
Preeclampsia represents a hypertensive disorder of pregnancy with significant rates of maternal and fetal morbidity. It complicates 3%‐5% of gestations, leading to increased risk of fetal growth restriction, as well as to potentially life‐threatening maternal multiple organ dysfunction. 1 Since the only definitive treatment is fetal delivery, early‐onset preeclampsia constitutes a major cause of preterm birth. Effective prediction is essential in order to identify high‐risk women early in the course of pregnancy and subsequently provide preventive interventions, such as the prophylactic administration of low‐dose aspirin. 2 Current screening models are based on the evaluation of parameters of maternal medical and obstetrical history, combined with uterine artery Doppler ultrasonography and measurement of serum biomarkers, especially pregnancy‐associated plasma protein A (PAPP‐A) and placental growth factor (PlGF). 3 Specifically, a model using the combination of mean arterial pressure, uterine artery velocimetry, and serum PlGF values has recently gained interest as it is able to provide promising predictive accuracy (sensitivity: 75.8%, specificity: 80%). 4 Much research effort has been devoted to the investigation of several novel angiogenic and inflammatory markers 5 as potentially useful predictive markers, although the optimal algorithm to be widely used in clinical practice remains still under investigation.
The pathophysiology of preeclampsia is as complex multi‐step process based on the hypothesis that inadequate trophoblast invasion and poor spiral artery remodeling leads to placental oxidative stress and the release of various mediators that promote inflammation and generalized endothelial dysfunction. 6 Recent research has proposed that left renal vein compression by the gravid uterus in women with deficient ipsilateral collaterals may lead to increased intrarenal pressure, subsequently leading to renal ischemia and development of hypertension. 7 As a result, alterations of renal hemodynamics leading to renal compartment syndrome have been suggested as a potential contributor to the pathogenesis of preeclampsia. 8
Renal Doppler ultrasonography is a non‐invasive tool of evaluating both the arterial and venous blood flow, providing clinically useful information about renal microcirculation. More specifically, renal resistive index (RI) and pulsatility index (PI), measured at the level of interlobar arteries, represent markers of vascular impedance and are mainly affected by renal wedge capillary pressure along with various extra‐renal factors, such as pulse pressure and aortic stiffness. 9 Interestingly, RI has been suggested to present important prognostic value in patients with renovascular hypertension, as well as in those at risk of acute kidney injury. 10 Renal interlobar vein impedance index (RIVI) is the equivalent of arterial RI on the venous side, reflecting the compliance of kidney parenchyma and thus can serve as a helpful tool in the evaluation of intrarenal venous blood flow, especially in the context of renal obstruction. 11 Furthermore, pulse transit time (PTT) measured both in the arterial and venous side of circulation reflects the time interval between the corresponding characteristics of Doppler and electrocardiographic waves and is considered as a marker of vascular stiffness. 12
The value of renal Doppler parameters as tools for the prediction of preeclampsia has been assessed by recent observational studies; however, no firm consensus exists regarding their exact role in clinical practice. The present meta‐analysis aims to systematically gather all the available literature evidence in the field and evaluate the potential significance of renal hemodynamics in the disease by comparing RI, PI, RIVI, and PTT values among preeclamptic and healthy pregnant women.
2. MATERIALS AND METHODS
The present systematic review was prospectively registered at The International Prospective Register of Systematic Reviews—PROSPERO (CRD42019133455) and was designed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 13 Outcomes were reported according to the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. 14
2.1. Eligibility criteria
The meta‐analysis included all observational (prospective or retrospective cohort, case‐control, cross‐sectional) studies that reported any renal Doppler parameter (RI, PI, RIVI, PTT, systolic‐to‐diastolic ratio) among pregnant women, with and without preeclampsia. Small case series (<10 patients), case reports, conference abstracts or posters, review papers, and animal studies were not included. Uncontrolled studies and those examining non‐pregnant patients were also excluded. In addition, women with nonproteinuric gestational hypertension or preeclampsia complications, such as eclampsia and hemolysis, elevated liver enzymes, low platelet (HELLP) syndrome were excluded in order to enhance the homogeneity of the sample.
2.2. Literature search
Literature search was primarily conducted using the Medline, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and Clinicaltrials.gov databases. Google Scholar database and the reference list of all the included studies (“snowball” method) were also searched in order to recognize potential additional review papers. The date of the last search was set at December 04, 2019. The literature search was based on the following main algorithm using both Medical Subject Headings (MeSH) terms and keywords: “((renal OR kidney) AND ("Ultrasonography, Doppler, Color"[Mesh] OR resistive index OR resistance index OR resistivity index OR pulsatility index OR impedance OR pulse transit OR hemodynamic)) AND ("Pre‐Eclampsia"[Mesh] OR preeclampsia)”. No date or language restrictions were applied.
2.3. Study selection
Study selection was performed consecutively in 3 stages. Firstly, the titles and/or abstracts of all electronic papers were screened to assess their eligibility. Subsequently, all review papers that were presumed to meet the criteria were retrieved as full texts. Finally, all observational studies that reported renal Doppler parameters (RI, PI, RIVI, PTT, systolic‐to‐diastolic ratio) among preeclamptic women and healthy pregnant controls were held eligible. Any potential discrepancies concerning retrieval of review papers and statistical analyses were resolved by the consensus of all authors. The process of study selection was conducted by two researchers independently, and any potential disagreements were resolved by their consensus.
2.4. Data collection
The following data were extracted from each included study: name of first author, year of publication, country, study design, eligibility criteria, timing of measurement, ultrasound characteristics, number of patients, mean maternal age, gestational age at sampling, systolic blood pressure, body mass index, and birthweight. Outcomes of interest were the values of resistive index, pulsatility index, arterial and venous pulse transit time, systolic‐to‐diastolic ratio and renal interlobar vein impedance index among preeclamptic and healthy pregnant women.
2.5. Definitions
Renal RI was calculated with the formula: (peak systolic velocity ‐ end‐diastolic velocity)/peak systolic velocity, while PI was defined as (peak systolic velocity − end‐diastolic velocity)/time averaged velocity and systolic/diastolic ratio as peak systolic velocity − end‐diastolic velocity, measured at the level of interlobar arteries. 15 RIVI was calculated at the levels of interlobar veins by the formula: (peak systolic velocity − end‐diastolic velocity)/peak systolic velocity. 16 Arterial PTT was defined as the time interval between the electrocardiographic Q‐wave and the beginning of Doppler systole, corrected for the heart rate. Correspondingly, venous PTT referred to the time interval between the electrocardiographic P‐wave and the Doppler A‐wave, corrected for the duration of the heart cycle. 17 All indices were calculated by the authors of primary studies. Replication was not feasible due to lack of individual participant data. Preeclampsia was detected when new‐onset hypertension (systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg) combined with proteinuria occurred after the completion of the 20th week of gestation in a previously normotensive woman. Moreover, early‐onset preeclampsia was defined as preeclampsia diagnosed before the 34th week of pregnancy. 18
2.6. Quality assessment
The methodological quality of the included studies was evaluated with the Newcastle‐Ottawa Scale (NOS) score. 19 More specifically, case‐control studies were assessed concerning the risk of bias on the domains of selection of cases and controls, comparability of the two groups, ascertainment of exposure and non‐response rate. The risk of bias in cohort studies was judged by evaluating the selection and comparability of the exposed and non‐exposed cohorts, as well as the assessment of outcome and the adequacy of the follow‐up period. A modified form of the tool was implemented for cross‐sectional studies, taking into consideration sample representativeness, sample size, non‐response rate, ascertainment of exposure, comparability, assessment of outcomes and statistical test. 20 The methodological quality of studies was appraised with a star system; case‐control and cohort studies could be awarded up to 9 stars, while the maximum number of stars for cross‐sectional ones was 10. Studies were judged by two authors independently, while any discrepancies were resolved by their consensus.
2.7. Data analysis
All outcomes were initially evaluated qualitatively, while outcomes reported in 3 or more studies were also eligible for the quantitative meta‐analysis. The effect measure was defined to be mean difference (MD) and the confidence intervals (CI) were set at 95%. Pooled estimates were obtained by fitting a random effects (DerSimonian and Laird) statistical model. 21 The inter‐study heterogeneity was assessed with the inconsistency index (I 2) and the between‐study variance (τ 2). In case median and interquartile range were only provided, the formulas proposed by Wan et al 22 were used to estimate mean and standard deviation values. Publication bias was not assessed, since the small number of studies (<10 per outcome) rendered the interpretation of the statistical tests unreliable. 23
Subgroup analysis was performed on the basis of preeclampsia onset (early‐ vs late‐onset preeclampsia) and side of measurement (left vs right kidney). Quantitative analysis was implemented if the subgroups included 3 or more studies. Both pair‐wise and network meta‐analysis were performed in order to simultaneously evaluate all comparisons among the different subgroups. A Bayesian hierarchical model with Markov Chain Monte Carlo Convergence (MCMC) simulation was applied through JAGS in order to perform arm‐based analyses. The number of iterations concerning the adaptation process was set at 10 000, while the number of iterations in each chain was 200 000. Posterior density plots and league tables were constructed to comparatively depict the estimated outcomes of interest for all subgroups. In addition, subgroups were ranked by creating cumulative ranking curves and estimating their surface under the cumulative ranking curve (SUCRA). 24 To perform this type of analysis, the mean, standard deviation and patient number for each arm of the included studies were used. Statistical analyses were performed using the Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) and the R‐3.4.3 statistical environment (“metafor” and “pcnetmeta” packages).
2.8. Sensitivity analysis
New estimates were obtained after applying the Knapp‐Hartung correction in order to provide adjustments for the small‐sample size. 25 Moreover, the 95% prediction intervals were calculated as an estimation of the effects to be expected by future studies. Prediction intervals take into consideration the variation of the results across studies and present the existing heterogeneity at the same scale as the outcome of interest. Calculation of the prediction intervals was performed according to the formulas proposed by IntHout et al 26 Credibility ceilings were also calculated for each study under the assumption that an observational study is not able to provide more than a maximum certainty that a specific effect is in a particular direction, due to its inherent methodological limitations. The credibility ceiling test was conducted by following the methodology suggested by Salanti et al, 27 applying a 5% and 10% ceiling. Furthermore, leave‐one‐out analysis was performed in order to explore the influence of individual studies. To accomplish this, one study was sequentially omitted at a time and thus its effect on the overall outcome was evaluated. This analysis was conducted using the Open Meta‐Analyst software. 28 Sensitivity analyses were performed for the pair‐wise meta‐analyses.
3. RESULTS
3.1. Study selection
The process of study selection is schematically depicted in the PRISMA flowchart (Appendix 1—Figure S1). Overall, 1321 review papers were identified and 17 of them were retrieved as full texts. Subsequently, 3 studies were excluded after reading the full text. 29 , 30 , 31 Specifically, one study evaluated renal hemodynamics among pregnant and non‐pregnant women but did not include a preeclamptic group. 31 Moreover, one study was not included as it did not report the absolute RIVI values, 30 while another one represented a small case series of preeclamptic women without comparing them with healthy controls. 29 As a result, 14 studies 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 were included in the present review, with a total of 1118 women. Among them, 594 were diagnosed with preeclampsia, while 524 women were recruited as healthy pregnant controls.
3.2. Included studies
The methodological characteristics (country, study design, exclusion criteria, timing of measurement, ultrasound characteristics, and examined parameters) of the included studies are summarized in Table 1. The majority of the studies (64.3%) presented a case‐control design, while 4 of them (28.6%) adopted a cross‐sectional design and 1 study (7.1%) was a prospective cohort. The most common reasons for patient exclusions were chronic kidney disease, pre‐existing hypertension, and diabetes mellitus. All studies except for one 37 evaluated Doppler parameters during the 3rd trimester of pregnancy. Ultrasound indices were measured using 3.0‐7.5 MHz probes. The main patients' characteristics (maternal age, gestational age, systolic blood pressure, body mass index, birthweight) are described in Appendix 2 (Table S1).
TABLE 1.
Study characteristics
| Year; author | Country | Study design | Exclusion criteria | Timing of measurement | Ultrasound characteristics | Examined parameters | NOS score |
|---|---|---|---|---|---|---|---|
| 2016; Moura | Brazil | PC | Any major fetal malformation, SGA neonate, pregnancy loss before 24 wks | 11th‐13th week | 3.5‐5.0‐MHz convex probe, Voluson 730 Pro (GE Healthcare, Austria) | RIVI | 8/9 |
| 2015; Gyselaers | Belgium | C‐C | Pre‐existing hypertension, DM, thyroid disease, liver disease, any rheumatologic disease, history of organ transplant, multiple gestation | 3rd trimester | 3.5‐7‐MHz probe (Hitachi EUB 6500, Belgium) | RIVI, VPTT | 8/9 |
| 2014; Gyselaers | Belgium | Cross‐sectional | Pre‐existing hypertension, DM, thyroid disease, liver disease, history of organ transplant, multiple gestation | 3rd trimester | 3.5‐5‐MHz probe (Toshiba Aplio, Belgium) | RI, PI, APTT, RIVI, VPTT | 8/10 |
| 2014; Mesens | Belgium | Cross‐sectional | Pre‐existing DM, CKD, any rheumatologic/liver/cardiovascular disease, any clotting disorder, multiple pregnancy | 3rd trimester | 3.5‐7‐MHz probe (Hitachi EUB 6500, Belgium) | RIVI | 8/10 |
| 2013; Bahser | Germany | C‐C | Pre‐existing DM, CKD, vasculitis, any rheumatologic disease | 3rd trimester | Toshiba aplio 80 (Toshiba Medical Systems GmbH, Germany); Philips iU22 (Philips GmbH, Germany) | RI, PI | 7/9 |
| 2012; Tomsin | Belgium | C‐C | CKD, any liver disease, multiple pregnancy | 3rd trimester | 3.5‐7‐MHz probe (Hitachi EUB 6500, Belgium) | VPTT | 7/9 |
| 2010; Gyselaers | Belgium | C‐C | Pre‐existing hypertension, DM, CKD, thrombophilia, any rheumatologic disease, multiple gestation | 3rd trimester | 3.5‐7‐MHz probe (Hitachi EUB 6500, Belgium) | RIVI | 6/9 |
| 2010; Tomsin | Belgium | Cross‐sectional | CKD, any liver disease, multiple pregnancy | 3rd trimester | 3.5‐7‐MHz probe (Hitachi EUB 6500, Belgium) | VPTT | 7/10 |
| 2009; Gyselaers | Belgium | C‐C | CKD, multiple pregnancy | 3rd trimester | 3.5‐7‐MHz probe (Hitachi EUB 6500, Belgium) | RIVI | 7/9 |
| 2006; Lubomirova | Bulgaria | C‐C | Pre‐existing hypertension, CKD | 3rd trimester | 3.5‐MHz probe | RI, PI | 7/9 |
| 2004; Bateman | Australia | C‐C | CKD | 3rd trimester | NR | RI, RIVI | 6/9 |
| 1996; Kublickas | Sweden | C‐C | Pre‐existing hypertension, any drug intake | 3rd trimester | 3.0‐MHz probe (Vingmed CFM 700, Norway) | RI, PI, S/D ratio | 7/9 |
| 1992; Thaler | Israel | Cross‐sectional | NR | 3rd trimester | 3.5‐MHz probe (Elscint ESI 2000, Israel) | RI | 7/10 |
| 1991; Gudmundsson | Sweden | C‐C | CKD | 3rd trimester | 3.5‐MHz real‐time scanner and 2‐MHz pulsed Doppler unit | PI, S/D ratio | 7/9 |
Abbreviations: APTT, arterial pulse transit time; C‐C, case‐control; CKD, chronic kidney disease; DM, diabetes mellitus; NOS, Newcastle‐Ottawa Scale; NR, not reported; PC, prospective cohort; PI, pulsatility index; RI, resistive index; RIVI, renal interlobar vein impedance index; S/D, systolic/diastolic; SGA, small for gestational age; VPTT: venous pulse transit time.
3.3. Quality assessment
The outcomes of the Newcastle‐Ottawa Scale score are presented in Table 1. Overall, specifically, the overall quality was judged to be fair, as 2 studies (14.3%) were found to be at low risk of bias and 12 studies (85.7%) at moderate risk of bias (Appendix 3, Tables S2‐S4). Potential risk of bias may have arisen from the domain of patients' comparability in non‐age‐matched studies, as well as from the domain of cases representativeness in studies that did not report whether preeclamptic women were consecutively selected. No studies were found to be at high risk of bias.
3.4. Qualitative synthesis
The outcomes of arterial indices are summarized in Table S5 (Appendix 4). Renal resistive index was evaluated in 6 studies, with 3 of them finding no association with the disease. One study proposed that RRI was significantly higher in preeclamptic women, while another one observed this association only in patients with early‐onset disease. On the contrary, Kublickas et al 39 found increased RRI values in the healthy pregnant control group. Regarding pulsatility index, one study indicated significantly higher values in the disease, while another one proposed that PI was higher only in women with early‐onset preeclampsia. Nevertheless, the opposite effect was observed in 2 studies, while another one found no significant association of PI with the disease. Arterial pulse transit time was assessed in only 1 study, reporting significantly lower values in pregnancies complicated with preeclampsia, while systolic‐to‐diastolic ratio was evaluated in 2 studies, with one of them observing significantly lower values in preeclamptic women.
The results of venous circulation indices are presented in Table S6 (Appendix 4). Studies evaluating renal interlobar vein impedance during the 3rd trimester found significantly higher values in women with preeclampsia, indicating a more pronounced difference in early‐onset disease. However, one prospective study measuring RIVI during the 1st trimester of pregnancy proposed that its values were not different in women that subsequently developed preeclampsia. Concerning venous pulse transit time, preeclampsia was associated with significantly decreased values compared to healthy controls, while women with early‐onset disease tended to present lower values than those with late‐onset preeclampsia.
3.5. Quantitative synthesis
Pair‐wise meta‐analysis indicated that RRI values were not different among preeclamptic and healthy pregnant women (MD: 0.00, 95% CI: [−0.03, 0.04], 6 studies). PI also did not differ among the examined groups (MD: −0.01, 95% CI: [−0.14, 0.12], 5 studies) (Figure 1). RIVI was estimated to be significantly higher in preeclampsia (MD: 0.07, 95% CI: [0.06, 0.09], 7 studies), while VPTT was significantly lower (MD: −0.10, 95% CI: [−0.14, −0.05], 4 studies) in women with the disease (Figure 2).
FIGURE 1.
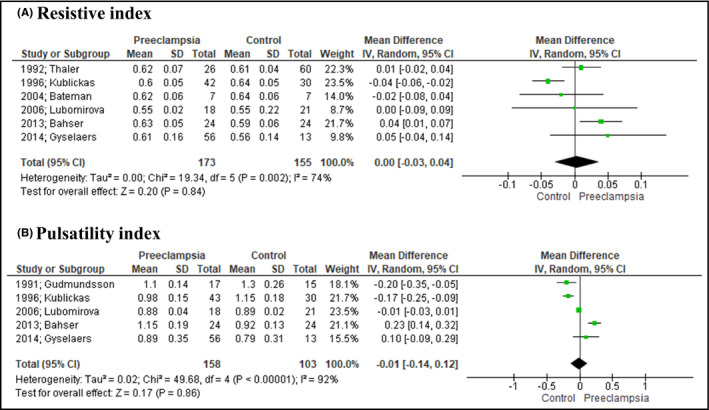
Forest plot depicting the outcomes of arterial indices. Renal resistive (A) and pulsatility (B) indices did not differ among preeclamptic and healthy pregnant women
FIGURE 2.
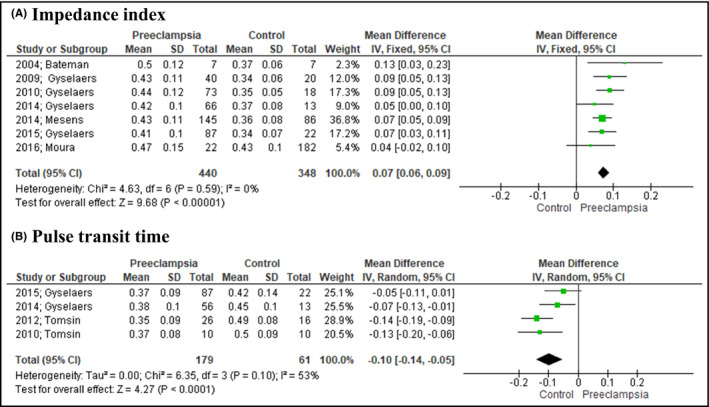
Forest plot illustrating the outcomes of venous indices. Preeclampsia was associated with significantly higher renal interlobar vein impedance (A) and lower venous pulse transit time (B)
Subgroup analysis demonstrated that RIVI was increased in both early (MD: 0.11, 95% CI: [0.09, 0.13], 4 studies) and late‐onset (MD: 0.05, 95% CI: [0.03, 0.07], 4 studies) preeclampsia compared to healthy pregnancies, with higher values to be observed in women with early‐onset disease (MD: 0.07, 95% CI: [0.05, 0.09], 4 studies). RIVI was estimated to be increased in preeclampsia when measured both in the right (MD: 0.09, 95% CI: [0.07, 0.10], 7 studies) and the left (MD: 0.06, 95% CI: [0.05, 0.07], 7 studies) kidney. Right kidney RIVI values were found to be significantly higher compared to left kidney ones in preeclamptic women (MD: 0.03 [0.01, 0.04], 7 studies).
VPTT was calculated to be significantly lower in both early (MD: −0.10, 95% CI: [−0.14, −0.06], 3 studies) and late‐onset (MD: −0.07, 95%: [−0.10, −0.03], 3 studies) disease that in the control group. Early‐onset preeclampsia was associated with decreased VPTT compared to late‐onset disease (MD: −0.04, 95% CI: [−0.07, −0.01], 3 studies). Low VPTT values were evident in the preeclampsia group when evaluated both in the right (MD: −0.08, 95% CI: [−0.10, −0.05], 4 studies) and the left (MD: −0.10, 95% CI: [−0.13, −0.07], 4 studies) kidney. No difference was found when the two sides of measurements were compared (MD: 0.00, 95% CI: [−0.01, 0.02], 4 studies) (Table 2).
TABLE 2.
Outcomes of the subgroup analysis
| Subgroup | Comparison | Studies no. | Mean difference (95% CI) | Heterogeneity | Test for overall effect | |
|---|---|---|---|---|---|---|
| P value | I 2 | P value | ||||
| Renal interlobar vein impedance index | ||||||
| Preeclampsia onset | EOP vs control | 4 | 0.11 (0.09, 0.13) | .70 | 0% | <.001 |
| LOP vs control | 0.05 (0.03, 0.07) | .81 | 0% | <.001 | ||
| EOP vs LOP | 0.07 (0.05, 0.09) | .77 | 0% | <.001 | ||
| Side of measurement | Left kidney PE vs left kidney control | 7 | 0.06 (0.05, 0.07) | .14 | 38% | <.001 |
| Right kidney PE vs right kidney control | 0.09 (0.07, 0.10) | .53 | 0% | <.001 | ||
| Left kidney PE vs right kidney PE | 0.03 (0.01, 0.04) | .61 | 0% | <.001 | ||
| Venous pulse transit time | ||||||
| Preeclampsia onset | EOP vs control | 3 | −0.10 (−0.14, −0.06) | .70 | 0% | <.001 |
| LOP vs control | −0.07 (−0.10, −0.03) | .38 | 0% | <.001 | ||
| EOP vs LOP | −0.04 (−0.07, −0.01) | .77 | 0% | .003 | ||
| Side of measurement | Left kidney PE vs left kidney control | 4 | −0.10 (−0.13, −0.07) | .51 | 0% | <.001 |
| Right kidney PE vs right kidney control | −0.08 (−0.10, −0.05) | .21 | 34% | <.001 | ||
| Left kidney PE vs right kidney PE | 0.00 (−0.01, 0.02) | .94 | 0% | .71 | ||
Abbreviations: CI, confidence intervals; EOP, early‐onset preeclampsia; I 2: inconsistency index; LOP, late‐onset preeclampsia.
The network of the comparisons used in the Bayesian meta‐analysis is illustrated in Appendix 5 (Figure S2). Density plots revealed that RIVI had the greatest probability to be highest in women with early‐onset preeclampsia when measured in the left kidney. In addition, VPTT was estimated to be lowest in early‐onset preeclamptic women, regardless the side of measurement (Figure 3). The outcomes of all comparisons among the different subgroups are summarized in league tables (Figure 4). Ranking of treatments indicated that RIVI was highest when measured in the left kidney of women with early‐onset preeclampsia (SUCRA: 93.9%) and lowest in the right kidney of healthy controls (SUCRA: 0.1%). Inversely, VPPT was estimated to be highest in the left kidney of healthy pregnant women (SUCRA: 98.9%) and lowest in the left kidney of those with early‐onset preeclampsia (8.6%) (Appendix 5, Figure S3; Table S7).
FIGURE 3.
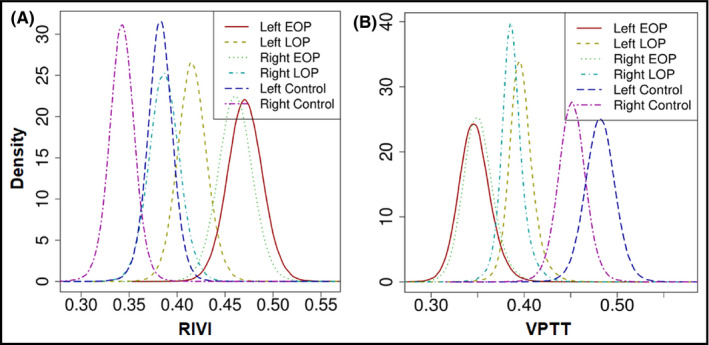
Density plots depicting the posterior densities for estimates of renal interlobar vein impedance and venous pulse transit time in the different subgroups. EOP, early‐onset preeclampsia; LOP, late‐onset preeclampsia; RIVI, renal interlobar vein impedance; VPTT, venous pulse transit time
FIGURE 4.
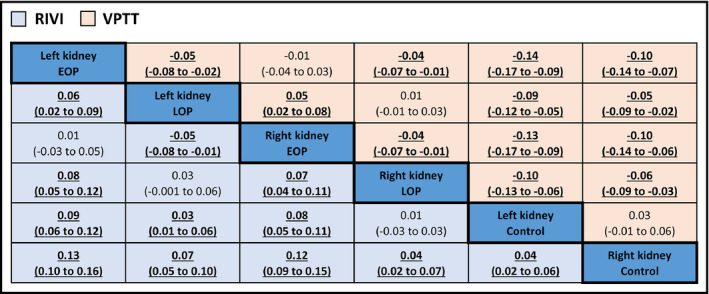
League table comparing renal interlobar vein impedance and venous pulse transit time among all subgroups. Significant differences (P value < 0.05) are highlighted in bold. EOP, early‐onset preeclampsia; LOP, late‐onset preeclampsia; RIVI, renal interlobar vein impedance; VPTT, venous pulse transit time
3.6. Sensitivity analysis
The Knapp‐Hartung adjustment resulted in similar outcomes with the primary analysis, with RIVI to be significantly higher (MD: 0.07, 95% CI: [0.06, 0.09]) and VPTT significantly lower (MD: −0.10, 95% CI: [−0.17, −0.03]) in preeclampsia. The estimated 95% prediction intervals were significant for both RIVI (−0.18 to −0.02) and VPTT (−0.18 to −0.02), indicating that significant effects should be expected by future studies. In addition, RIVI and VPTT passed both the 5% and 10% credibility ceiling tests, as their outcomes remained statistically significant. On the contrary, no significant effects were calculated for RI and PI (Table 3). The outcomes of the leave‐one‐out analysis demonstrated that the overall results regarding RI, PI, RIVI, and VPTT remained stable, since the omission of no study altered their statistical significance (Appendix 6, Figure S4).
TABLE 3.
Outcomes of the sensitivity analysis
| Outcome | Knapp‐Hartung adjustment | 95% prediction intervals | Credibility ceilings | |
|---|---|---|---|---|
| 5% | 10% | |||
| RI | 0.00 [−0.03, 0.04] | [−0.06, 0.07] | 0.00 [−0.02, 0.03] | 0.01 [−0.01, 0.03] |
| PI | −0.01 [−0.24, 0.21] | [−0.38, 0.36] | −0.02 [−0.14, 0.11] | −0.01 [−0.03, 0.01] |
| RIVI | 0.07 [0.06, 0.09]* | [0.06, 0.09]* | 0.06 [0.03, 0.09]* | 0.06 [0.02, 0.10]* |
| VPTT | −0.10 [−0.17, −0.03]* | [−0.18, −0.02]* | −0.07 [−0.12, −0.02]* | −0.07 [−0.13, −0.01]* |
Data expressed as mean difference [95% confidence intervals]
Abbreviations: PI, pulsatility index; RI, resistive index; RIVI, renal interlobar vein impedance index; VPTT, venous pulse transit time.
P value < .05.
4. DISCUSSION
The present meta‐analysis supports that preeclampsia is associated with altered renal venous hemodynamics, as it was linked to significantly elevated RIVI and lower VPTT values. These effects were estimated to be more pronounced in early than in late‐onset disease, while side of measurement was suggested to affect RIVI, as its values were higher when evaluated in the left kidney of preeclamptic women. On the contrary, no role was proposed for the arterial circulation indices, since renal RI and PI did not differ among preeclamptic and healthy pregnant women. Data regarding APTT was limited, and thus, no conclusions can be drawn about its potential utility in the disease.
Combining the evidence presenting above, it can be assumed that dysfunction of the venous compartment represents a significant part of the preeclamptic pathogenetic process, which may arise from intra‐abdominal hypertension combined with venous overfill and increased vascular tone. 46 High RIVI is associated with the presence of the venous pre‐acceleration nadir (VPAN) Doppler wave, which reflects a pronounced retrograde rebound of right atrial contraction, 47 while low VPTT implies a shorter time interval between atrial contraction and the A‐deflection. 48 These findings together indicate a fast and distant atrial contraction rebound through the venous compartment of the circulation, suggesting a state of increased vascular stiffness.
It is also interesting that preliminary data suggest abnormal RIVI and VPTT values as unique findings of preeclampsia, since nonproteinuric gestational hypertension was not associated with altered renal venous hemodynamics. 36 Moreover, it is important that increased RIVI has been identified in women who developed early‐onset preeclampsia several weeks before the onset of proteinuria, 30 while a significant correlation of RIVI with the degree of proteinuria (r = .218, P value = .009) was observed in patients with late‐onset disease. 34 From a pathophysiologic point of view, it can be thus hypothesized that renal venous congestion may represent an important step of preeclampsia, as it may lead to increased glomerular pressure and subsequently promote the development of proteinuria through endothelial and podocyte damage. 49
4.1. Strengths and limitations of the study
The present systematic review gathered, for the first time, all the available evidence regarding the role of renal Doppler parameters in women with preeclampsia. To accomplish this, 5 independent literature databases were searched, applying no date or language restrictions. A network meta‐analysis was implemented in order to simultaneously compare and rank all subgroups concerning the values of RIVI and VPTT. Furthermore, heterogeneity was further investigated with the calculation of prediction intervals, indicating that future studies are supposed to show a significant association of RIVI and VPTT with preeclampsia. Small‐sample adjustments were applied using the Knapp‐Hartung approach, obtaining stable results.
On the other hand, the majority of the included studies presented a case‐control design, and thus, potential risk of bias due to confounding or selection of participants cannot be safely excluded. To address the inherent limitations of observational studies, credibility ceilings were estimated, enhancing the robustness of the outcomes as their significance remained unchanged. Only one prospective study was included and thus separate analysis of longitudinal cohort studies was not feasible. Measurements were mainly conducted during the 3rd trimester of pregnancy; therefore, data about the potential predictive nature of RIVI and VPTT are currently lacking. Moreover, classification of cases into mild and severe ones was not performed in most studies, and thus, it remains unknown whether renal Doppler parameters differ between the two forms of the disease. Cases with chronic kidney disease were excluded from the majority of studies; however, patient eligibility criteria were unclear in the study of Gudmundsson et al, 33 although leave‐one‐analysis indicated no significant impact of this study on the overall outcome. It should be also stated that evaluation of ultrasound indices represents an objective method, while inter‐observer and intra‐observer were not widely reported in order to evaluate the effects of scanning technique on the observed outcomes.
4.2. Implications for current clinical practice and future research
The present meta‐analysis supports the role of RIVI and VPTT as promising preeclampsia markers, since their values significantly differed among women with the disease and healthy pregnant controls. These findings suggest that renal Doppler parameters may serve as candidate novel screening markers, while they provide new insights in the pathophysiology of preeclampsia by underlying the importance of venous compartment dysfunction. Specifically, RIVI was found to be highest when measured in women with early‐onset preeclampsia, indicating the severity of early‐onset disease which is commonly associated with hemodynamic dysfunction. In addition, the increased RIVI values in the left kidney may reflect the compression of the left renal vein by the gravid uterus, potentially leading to renal hypoperfusion. Nevertheless, several aspects should be elucidated in order to fully clarify the exact role of RIVI and VPTT in clinical practice. Future large‐scale should prospectively assess these parameters by conducting sequential measurements throughout pregnancy, in order to determine their exact pattern of increase or decrease and find out whether their alterations precede the clinical onset of preeclampsia. Cases need to be classified according to disease severity and onset, while the potential association of Doppler indices with both maternal and fetal outcomes remains to be examined. In addition, the efficacy of renal venous circulation markers should be investigated by introducing cutoff values and subsequently evaluating their accuracy in predicting the disease alone or in combination with other established biomarkers, such as PAPP‐A and PlGF. Finally, correlations with the degree of proteinuria, as well as with markers of podocyte dysfunction should be assessed in order to shed light on the exact effects of retrograde venous congestion in the course of preeclampsia.
5. CONCLUSIONS
The findings of the present meta‐analysis indicate that preeclampsia is associated with significantly elevated renal interlobar vein impedance and shorter venous pulse transit time, proposing the importance of venous compartment dysfunction in the pathogenetic process of the disease. No role was suggested for renal resistivity and pulsatility indices. Future studies should prospectively define the optimal timing of measurement, introduce cutoff values, and incorporate renal venous Doppler parameters in preeclampsia screening models to achieve optimal predictive accuracy.
STATEMENT OF ETHICS
No ethical approval was required. The present systematic review and meta‐analysis is based on aggregated data that were retrieved from studies already retrieved. We did not collect individual patient data and did not have direct contact with patients included.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Conception and design: I. Bellos, V. Pergialiotis. Analysis and interpretation of the data: I. Bellos. Drafting of the review paper: I. Bellos. Critical revision for important intellectual content: V. Pergialiotis. Final approval of the review paper: I. Bellos, V. Pergialiotis. Statistical expertise: I. Bellos. Collection and assembly of data: I. Bellos, V. Pergialiotis.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
None.
Bellos I, Pergialiotis V. Doppler parameters of renal hemodynamics in women with preeclampsia: A systematic review and meta‐analysis. J Clin Hypertens. 2020;22:1134–1144. 10.1111/jch.13940
REFERENCES
- 1. Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the United States, 1980–2010: age‐period‐cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre‐eclampsia. Lancet. 2016;387(10022):999‐1011. [DOI] [PubMed] [Google Scholar]
- 3. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613‐622. [DOI] [PubMed] [Google Scholar]
- 4. Chaemsaithong P, Pooh RK, Zheng M, et al. Prospective evaluation of screening performance of first‐trimester prediction models for preterm preeclampsia in an Asian population. Am J Obstet Gynecol. 2019;221(6):650.e1‐650.e16. [DOI] [PubMed] [Google Scholar]
- 5. Bellos I, Karageorgiou V, Kapnias D, Karamanli K‐E, Siristatidis C. The role of interleukins in preeclampsia: a comprehensive review. Am J Reprod Immunol. 2018;80(6):e13055. [DOI] [PubMed] [Google Scholar]
- 6. Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre‐eclampsia. Lancet. 2010;376(9741):631‐644. [DOI] [PubMed] [Google Scholar]
- 7. Tokunaga N, Kanayama N, Sugimura M, Kobayashi T, Terao T. Dilatation of the left renal vein in preeclampsia. J Matern Fetal Med. 2000;9(6):356‐359. [DOI] [PubMed] [Google Scholar]
- 8. Reuter DG, Law Y, Levy WC, et al. Can preeclampsia be considered a renal compartment syndrome? A hypothesis and analysis of the literature. J Am Soc Hypertens. 2016;10(11):891‐899. [DOI] [PubMed] [Google Scholar]
- 9. Di Nicolò P, Granata A. Renal resistive index: not only kidney. Clin Exp Nephrol. 2017;21(3):359‐366. [DOI] [PubMed] [Google Scholar]
- 10. Bellos I, Pergialiotis V, Kontzoglou K. Renal resistive index as predictor of acute kidney injury after major surgery: a systematic review and meta‐analysis. J Crit Care. 2019;50:36‐43. [DOI] [PubMed] [Google Scholar]
- 11. Vadana BMK, Pasumarthy A, Penumalli N, Bellapa NC. Renal venous Doppler study in obstructive uropathy. J Clin Diagn Res. 2015;9(11):TC13‐TC15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith RP, Argod J, Pépin JL, Lévy PA. Pulse transit time: an appraisal of potential clinical applications. Thorax. 1999;54(5):452‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1‐e34. [DOI] [PubMed] [Google Scholar]
- 14. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 15. McArthur C, Geddes CC, Baxter GM. Early measurement of pulsatility and resistive indexes: correlation with long‐term renal transplant function. Radiology. 2011;259(1):278‐285. [DOI] [PubMed] [Google Scholar]
- 16. Oktar SO, Yücel C, Ozdemir H, Karaosmanoglu D. Doppler sonography of renal obstruction: value of venous impedance index measurements. J Ultrasound Med. 2004;23(7):929‐936. [DOI] [PubMed] [Google Scholar]
- 17. Foo JYA, Lim CS. Pulse transit time as an indirect marker for variations in cardiovascular related reactivity. Technol Health Care. 2006;14(2):97‐108. [PubMed] [Google Scholar]
- 18. Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early‐onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013;3(1):44‐47. [DOI] [PubMed] [Google Scholar]
- 19. Wells G, Shea B, O'connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.medicine.mcgill.ca/rtamblyn/Readings%5CTheNewcastle‐Scaleforassessingthequalityofnonrandomisedstudiesinmeta‐analyses.pdf. Accessed December 17, 2017.
- 20. Modesti PA, Reboldi G, Cappuccio FP, et al. Panethnic differences in blood pressure in europe: a systematic review and meta‐analysis. PLoS One. 2016;11(1):e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 22. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta‐analyses: a large survey. CMAJ. 2007;176(8):1091‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salanti G, Ades AE, Ioannidis JPA. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163‐171. [DOI] [PubMed] [Google Scholar]
- 25. Knapp G, Hartung J. Improved tests for a random effects meta‐regression with a single covariate. Stat Med. 2003;22(17):2693‐2710. [DOI] [PubMed] [Google Scholar]
- 26. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salanti G, Ioannidis JPA. Synthesis of observational studies should consider credibility ceilings. J Clin Epidemiol. 2009;62(2):115‐122. [DOI] [PubMed] [Google Scholar]
- 28. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta‐Analyst: software for meta‐analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mesens T, Tomsin K, Oben J, Staelens A, Gyselaers W. Maternal venous hemodynamics assessment for prediction of preeclampsia should be longitudinal. J Matern Fetal Neonatal Med. 2015;28(3):311‐315. [DOI] [PubMed] [Google Scholar]
- 30. Gyselaers W, Mesens T. Renal interlobar vein impedance index: a potential new Doppler parameter in the prediction of preeclampsia? J Matern Fetal Neonatal Med. 2009;22(12):1219‐1221. [DOI] [PubMed] [Google Scholar]
- 31. Kublickas M, Randmaa I, Lunell NO, Westgren M. Effect of variations of heart rate within the normal range on renal artery Doppler indices in nonpregnant and pregnant women. J Clin Ultrasound. 1993;21(8):507‐510. [DOI] [PubMed] [Google Scholar]
- 32. Tomsin K, Mesens T, Molenberghs G, Gyselaers W. Venous pulse transit time in normal pregnancy and preeclampsia. Reprod Sci. 2012;19(4):431‐436. [DOI] [PubMed] [Google Scholar]
- 33. Gudmundsson S, Maršál K. Doppler ultrasound examination of the renal artery in healthy women, normotensive pregnant women and in pre‐eclampsia. Ultrasound Obstet Gynecol. 1991;1(4):258‐260. [DOI] [PubMed] [Google Scholar]
- 34. Mesens T, Tomsin K, Staelens AS, Oben J, Molenberghs G, Gyselaers W. Is there a correlation between maternal venous hemodynamic dysfunction and proteinuria of preeclampsia? Eur J Obstet Gynecol Reprod Biol. 2014;181:246‐250. [DOI] [PubMed] [Google Scholar]
- 35. Gyselaers W, Tomsin K, Staelens A, Mesens T, Oben J, Molenberghs G. Maternal venous hemodynamics in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2014;14(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gyselaers W, Staelens A, Mesens T, et al. Maternal venous Doppler characteristics are abnormal in pre‐eclampsia but not in gestational hypertension. Ultrasound Obstet Gynecol. 2015;45(4):421‐426. [DOI] [PubMed] [Google Scholar]
- 37. Bezerra Maia e Holanda Moura S, Praciano PC, Gurgel Alves JA, et al. Renal interlobar vein impedance index as a first‐trimester marker does not predict hypertensive disorders of pregnancy. J Ultrasound Med. 2016;35(12):2641‐2648. [DOI] [PubMed] [Google Scholar]
- 38. Thaler I, Weiner Z, Itskovitz J. Renal artery flow velocity waveforms in normal and hypertensive pregnant women. Am J Hypertens. 1992;5(6 Pt 1):402‐405. [DOI] [PubMed] [Google Scholar]
- 39. Kublickas M, Lunell NO, Nisell H, Westgren M. Maternal renal artery blood flow velocimetry in normal and hypertensive pregnancies. Acta Obstet Gynecol Scand. 1996;75(8):715‐719. [DOI] [PubMed] [Google Scholar]
- 40. Bateman GA, Giles W, England SL. Renal venous Doppler sonography in preeclampsia. J Ultrasound Med. 2004;23(12):1607‐1611. [DOI] [PubMed] [Google Scholar]
- 41. Lubomirova M, Andreev E, Bogov B, et al. Diagnostic value of the Conventional and Doppler ultrasound in pregnancy complicated with preeclampsia. Hippokratia. 2006;10(3):133‐137. [PMC free article] [PubMed] [Google Scholar]
- 42. Gyselaers W, Molenberghs G, Van Mieghem W, Ombelet W. Doppler measurement of renal interlobar vein impedance index in uncomplicated and preeclamptic pregnancies. Hypertens pregnancy. 2009;28(1):23‐33. [DOI] [PubMed] [Google Scholar]
- 43. Tomsin K, Mesens T, Molenberghs G, Peeters L, Gyselaers W. Time interval between maternal electrocardiogram and venous Doppler waves in normal pregnancy and preeclampsia: a pilot study. Ultraschall Med. 2010;33(07):E119‐E125. [DOI] [PubMed] [Google Scholar]
- 44. Gyselaers W, Mesens T, Tomsin K, Molenberghs G, Peeters L. Maternal renal interlobar vein impedance index is higher in early‐ than in late‐onset pre‐eclampsia. Ultrasound Obstet Gynecol. 2010;36(1):69‐75. [DOI] [PubMed] [Google Scholar]
- 45. Bahser N, Godehardt E, Hess AP, Blume C. Examination of intrarenal resistance indices indicate the involvement of renal pathology as a significant diagnostic classifier of preeclampsia. Am J Hypertens. 2014;27(5):742‐749. [DOI] [PubMed] [Google Scholar]
- 46. Gyselaers W. Maternal venous hemodynamic dysfunction in proteinuric gestational hypertension: evidence and implications. J Clin Med. 2019;8(3):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gyselaers W. Hemodynamics of the maternal venous compartment: a new area to explore in obstetric ultrasound imaging. Ultrasound Obstet Gynecol. 2008;32(5):716‐717. [DOI] [PubMed] [Google Scholar]
- 48. Naka KK, Tweddel AC, Doshi SN, Goodfellow J, Henderson AH. Flow‐mediated changes in pulse wave velocity: a new clinical measure of endothelial function. Eur Heart J. 2006;27(3):302‐309. [DOI] [PubMed] [Google Scholar]
- 49. Garovic VD, Wagner SJ, Petrovic LM, et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant. 2007;22(4):1136‐1143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


