Abstract
Pulse wave velocity (PWV) was a good marker of arterial stiffness and could predict cardiovascular (CV) outcomes. Recently, estimated PWV (ePWV) calculated by equations using age and mean blood pressure was reported to be an independent predictor of major CV events. However, there was no study comparing ePWV with brachial‐ankle PWV (baPWV) for CV and overall mortality prediction. We included 881 patients arranged for echocardiographic examination. BaPWV and blood pressures were measured by ankle‐brachial index‐form device. The median follow‐up period to mortality was 94 months. Mortality events were documented during the follow‐up period, including CV mortality (n = 66) and overall mortality (n = 184). Both of ePWV and baPWV were associated with increased CV and overall mortality after the multivariable analysis. ePWV had better predictive value than Framingham risk score (FRS) for CV and overall mortality prediction, but baPWV did not. In direct comparison of multivariable analysis using FRS as basic model, ePWV had a superior additive predictive value for CV mortality than baPWV (p = .030), but similar predictive valve for overall mortality as baPWV (p = .540). In conclusion, both ePWV and baPWV were independent predictors for long‐term CV and overall mortality in univariable and multivariable analysis. Besides, ePWV had a better additive predictive value for CV mortality than baPWV and similar predictive value for overall mortality as baPWV. Therefore, ePWV obtained without equipment deserved to be calculated for overall mortality prediction and better CV survival prediction.
Keywords: brachial‐ankle pulse wave velocity, estimated pulse wave velocity, mortality
1. INTRODUCTION
Arterial stiffness measured by pulse wave velocity (PWV) is associated with micro‐ and macro‐vascular complications, and it can also predict cardiovascular (CV) outcomes and mortality in the literature. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 Although there are several methods to measure PWV, brachial‐ankle PWV (baPWV) was considered as a good index of arterial stiffness and exhibited similar extents of associations with CV disease risk factors and clinical outcomes with carotid‐femoral PWV, which was the most recognized and established index of arterial stiffness. 10 Recently, estimated PWV (ePWV) calculated by equations using age and mean blood pressure (MBP) was also reported to be an independent predictor of major CV events. 11 , 12 , 13 , 14 However, there was no literature comparing ePWV with baPWV for long‐term CV and overall mortality prediction. Hence, the present study was designed to examine the ability of ePWV in prediction of long‐term CV and all‐cause mortality and compare the predictive value of long‐term CV and all‐cause mortality between ePWV and baPWV.
2. METHODS
2.1. Study population and design
Study subjects were randomly included from a group of patients who were arranged for echocardiographic examinations at Kaohsiung Municipal Siaogang Hospital from March 2010 to March 2012 due to ischemic heart disease, heart failure, hypertension, abnormal cardiac physical examination, survey for dyspnea, and the pre‐operative cardiac function survey. Patients with significant mitral or aortic valve diseases, atrial fibrillation, inadequate image visualization, or ankle‐brachial index (ABI < 0.9) were excluded. The reason why we excluded ABI < 0.9 was due to unreliable measurement of PWV under the situation of peripheral artery stenosis or occlusion. 15 , 16 Finally, 881 patients were enrolled in this study.
2.2. Ethics statement
The study protocol was approved by the institutional review board (IRB) committee of our Hospital. Informed consents have obtained in written form from patients and all clinical investigation was conducted according to the principles expressed in the Declaration of Helsinki.
2.3. Assessment of baPWV and ePWV
Around 10 min after the completion of echocardiographic examination, baPWV was evaluated using an ankle‐brachial index‐form device (VP1000; Colin, Aichi, Japan), which automatically and simultaneously measures blood pressures in bilateral arms and ankles by an oscillometric method. 17 , 18 For measuring baPWV, pulse waves that were acquired from the brachial and tibial arteries were recorded simultaneously, and the transmission time, which was defined as the time interval between the initial increase in brachial and tibial waveforms, was determined. The transmission distance from the arm to each ankle was calculated according to body height. The value of baPWV was automatically calculated as the transmission distance divided by the transmission time. After obtaining bilateral baPWV values, the higher value was used for later analysis.
For calculation of ePWV, we used the equation described in the study by Greve et al that was derived by the Reference Values for Arterial Stiffness' Collaboration. 11 , 14 The ePWV was calculated from age and MBP: ePWV = 9.587−0.402 × age + 4.560 × 10−3 × age2−2.621 × 10−5 × age2 × MBP + 3.176 × 10−3 × age × MBP−1.832 × 10−2 × MBP. MBP was calculated as diastolic blood pressure + 0.4(systolic blood pressure – diastolic blood pressure). After obtaining bilateral ePWV values, the higher value was used for later analysis.
2.4. Collection of demographic and medical data
Demographic and medical data including age, gender, smoking status, and comorbidities were obtained from medical records or interviews with patients. In addition, information about patient medications including aspirin, angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, β‐blockers, calcium channel blockers, and diuretics at enrollment was obtained from medical records.
2.5. Calculation of Framingham risk score (FRS)
Framingham risk score was used as the basic model to further compare the predictive value of ePWV and baPWV in multivariable analysis. FRS was calculated by a computer program and based on using a previously reported algorithm which includes the parameters of age, sex, total cholesterol, HDL cholesterol, systolic blood pressure, smoking, presence of diabetes, and being under treatment for hypertension. 19
2.6. Definition of CV and all‐cause mortality
All study patients were followed up till December 2018. Information of survival and causes of death were obtained from the official death certificate and final confirmation by the Ministry of Health and Welfare.
2.7. Statistical analysis
SPSS 22.0 software (SPSS, Chicago, IL, USA) was used for statistical analysis. Data were expressed as mean ± standard deviation, percentage, or median (25th–75th percentile) for follow‐up period. Continuous and categorical variables between groups were compared by independent samples t test and chi‐square test, respectively. The significant variables in the univariable analysis were selected for multivariable analysis. Time to the CV and overall mortality events and covariates of risk factors were modeled using the Cox proportional hazards model with forward selection. Receiver operating characteristic curves are used for comparing different models for prediction of CV and overall mortality. The test with the higher area under curve (AUC) is considered better. The incremental value of ePWV and baPWV over basic model to predict CV and overall mortality was studied by calculating the improvement in global chi‐square value. Discriminatory ability was evaluated by calculating the net reclassification improvement (NRI). All tests were 2‐sided and the level of significance was established as p < .05.
3. RESULTS
Among the 881 subjects, mean age was 61 ± 13 years. CV and overall mortality data were collected up to December 2018. Mortality data were obtained from the Collaboration Center of Health Information Application (CCHIA), Ministry of Health and Welfare, Executive Yuan, Taiwan. The follow‐up period to mortality events was 94 (25th–75th percentile: 87–101) months in all patients. Mortality events were documented during the follow‐up period, including CV mortality (n = 66) and overall mortality (n = 184).
Table 1 compares the clinical characteristics between patients with ePWV below and above the median (10.3 m/s). Compared to patients with ePWV below the median, patients with ePWV above the median had an older age, more female gender, higher prevalence of diabetes and hypertension, lower prevalence of smoking, higher systolic blood pressure, higher ePWV and baPWV, and higher percentage of aspirin, and calcium channel blocker use.
TABLE 1.
Comparison of clinical characteristics between patients with ePWV below and above the median (10.3 m/s)
| Baseline characteristics | ePWV below the median | ePWV above the median | p value |
|---|---|---|---|
| Number | 460 | 421 | |
| Age (years) | 52 ± 10 | 71 ± 9 | <.001 |
| Male gender (%) | 62.2% | 48.7% | <.001 |
| Smoking (%) | 20.2% | 9.0% | <.001 |
| Diabetes (%) | 21.3% | 32.5% | <.001 |
| Hypertension (%) | 62.1% | 80.0% | <.001 |
| Coronary artery disease (%) | 16.7% | 17.1% | .928 |
| Heart failure (%) | 6.1% | 7.1% | .587 |
| SBP (mmHg) | 126 ± 16 | 145 ± 20 | <.001 |
| Total cholesterol | 193 ± 43 | 188 ± 37 | .063 |
| Heart rate (min−1) | 70 ± 12 | 69 ± 12 | .657 |
| PWV | |||
| ePWV (m/s) | 8.5 ± 1.1 | 12.2 ± 1.5 | <.001 |
| baPWV (m/s) | 15.1 ± 2.5 | 20.2 ± 4.5 | <.001 |
| Medication | |||
| Aspirin | 27.9% | 34.8% | .029 |
| β‐blockers | 40.8% | 39.0% | .629 |
| CCBs | 32.5% | 43.8% | .001 |
| ACEIs/ARBs | 52.7% | 57.6% | .154 |
| Diuretics | 26.9% | 30.5% | .232 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; baPWV, brachial‐ankle pulse wave velocity; CCB, calcium channel blocker; ePWV, estimated pulse wave velocity; PWV, pulse wave velocity; SBP, systolic blood pressure.
The univariable analysis of Cox proportional hazards model found increased CV mortality was associated old age, the presence of diabetes, coronary artery disease, and heart failure, high systolic blood pressure, high heart rate, diuretic use, high ePWV, and high baPWV, and increased overall mortality was associated with old age, the presence of diabetes, coronary artery disease, and heart failure, high systolic blood pressure, low total cholesterol, high heart rate, diuretic use, high ePWV, and high baPWV. In direct comparison of this univariable analysis, ePWV had a better predictive value for CV mortality (chi‐square value: 47.00 versus 38.39, p = .003) but similar predictive value for overall mortality (chi‐square value: 134.18 versus 130.58, p = .058) as baPWV.
Table 2 shows the predictors of CV mortality using Cox proportional hazards model in the multivariable analysis. After adjusting significant variables in the univariable analysis, including age, diabetes, coronary artery disease, heart failure, systolic blood pressure, heart rate, diuretic use, both ePWV (hazard ratio [HR] = 2.321; 95% confidence interval [CI]: 1.800–2.994; p < .001) and baPWV (HR = 1.385; 95% CI: 1.102–1.742; p = .005) were significantly associated with CV mortality.
TABLE 2.
Predictors of CV mortality using Cox proportional hazards model (multivariable analysis with forward selection)
| Parameter | CV mortality (PWV: using ePWV) | CV mortality (PWV: using baPWV) | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (+13.71 year) | – | – | 2.186 (1.588–3.011) | <.001 |
| Diabetes (yes vs. no) | 2.070 (1.258–3.405) | .004 | 1.988 (1.211–3.262) | .007 |
| Coronary artery disease | 1.810 (1.012–3.239) | .046 | – | – |
| Heart failure | 7.343 (4.244–12.707) | <.001 | 7.526 (4.351–13.018) | <.001 |
| SBP (+20.80 mmHg) | – | – | – | – |
| Heart rate (+12.33 beat/min) | – | – | – | – |
| Diuretic use | – | – | – | – |
| PWV* | 2.321 (1.800–2.994) | <.001 | 1.385 (1.102–1.742) | .005 |
The HRs of continuous variables were calculated as a standard deviation change.
Age, diabetes, SBP, heart rate, diuretic use, and PWV were significant variables in the univariable analysis. Covariates in the multivariable model included the above significant variables in the univariable analysis. *Standard deviation for ePWV: +2.36 m/s; standard deviation for baPWV: +4.66 m/s.
Abbreviations: CI, confidence interval; HR, hazard ratio; other abbreviations as in Table 1.
Table 3 shows the predictors of overall mortality using Cox proportional hazards model in the multivariable analysis. After adjusting significant variables in the univariable analysis, including age, diabetes, coronary artery disease, heart failure, systolic blood pressure, total cholesterol, heart rate, diuretic use, both ePWV (HR = 1.640; 95% CI: 1.162–2.315; p = .005) and baPWV (HR = 1.570; 95% CI: 1.340–1.839; p < .001) were still significantly associated with overall mortality.
TABLE 3.
Predictors of overall mortality using Cox proportional hazards model (multivariable analysis with forward selection)
| Parameter | Overall mortality (using ePWV) | Overall mortality (using baPWV) | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (+13.71 year) | 1.672 (1.129–2.478) | .01 | 2.250 (1.791–2.826) | <.001 |
| Diabetes (yes vs. no) | 1.943 (1.382–2.733) | <.001 | 1.802 (1.279–2.539) | .001 |
| SBP (+20.80 mmHg) | – | – | – | – |
| Coronary artery disease | – | – | ||
| Heart failure | 3.660 (2.316–5.784) | <.001 | 3.802 (2.405–6.012) | <.001 |
| Total cholesterol (+40.77 mg/dl) | 0.742 (0.613–0.899) | .002 | 0.757 (0.628–0.912) | .03 |
| Heart rate (+12.33 beat/min) | – | – | – | – |
| Diuretic use | – | – | – | – |
| PWV* | 1.640 (1.162–2.315) | .005 | 1.570 (1.340–1.839) | <.001 |
The HRs of continuous variables were calculated as a standard deviation change.
Age, diabetes, SBP, total cholesterol, heart rate, diuretic use, and PWV were significant variables in the univariable analysis. Covariates in the multivariable model included the above significant variables in the univariable analysis. *Standard deviation for ePWV: +2.36 m/s; standard deviation for baPWV: +4.66 m/s.
Abbreviations: CI, confidence interval; HR, hazard ratio; other abbreviations as in Table 1.
Table 4 shows the comparison of AUC between FRS, ePWV, and baPWV for prediction of CV and overall mortality. The unadjusted AUC between FRS, ePWV, and baPWV for prediction of CV mortality was 0.681, 0.734, and 0.690, respectively. We found that there was a significant difference of AUC between ePWV and FRS (p = .044), but non‐significant difference between baPWV and FRS (p = .782). In addition, the unadjusted AUC between FRS, ePWV, and baPWV for prediction of overall mortality were 0.703, 0.766, and 0.722, respectively. We found that there was also a significant difference of AUC between ePWV versus FRS (p < .001), but non‐significant difference between baPWV and FRS (p = .367).
TABLE 4.
Comparison of unadjusted AUC between FRS, ePWV, and baPWV for prediction of CV and overall mortality
| Comparison of AUC | p value | |
|---|---|---|
| CV mortality | ||
| ePWV vs. FRS | 0.734 vs. 0.681 | .044 |
| baPWV vs FRS | 0.690 vs. 0.681 | .782 |
| Overall mortality | ||
| ePWV vs. FRS | 0.766 vs. 0.703 | <.001 |
| baPWV vs FRS | 0.722 vs. 0.703 | .367 |
Abbreviations: AUC, area under curve; baPWV, brachial‐ankle pulse wave velocity; ePWV, estimated pulse wave velocity; FRS, Framingham risk score.
Figure 1 shows the Nested Cox model for CV mortality prediction. We used FRS as the basic model. The basic model could significantly predict CV mortality (chi‐square value, 25.33, p < .001). We further added baPWV and ePWV into the basic model. Both basic model + ePWV and basic model + baPWV could provide an extra benefit in prediction of CV mortality than basic model (p < .001). In direct comparison between basic model + baPWV and basic model + ePWV, the basic model + ePWV had a better predictive value for CV mortality (p = .030).
FIGURE 1.
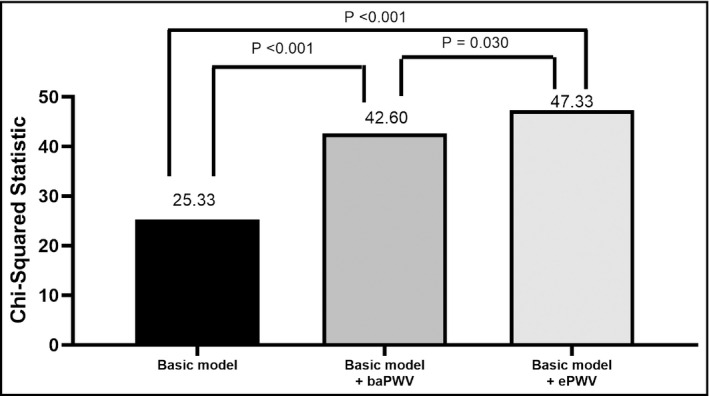
Direct comparison among basic model, basic model + brachial‐ankle pulse wave velocity (baPWV), and basic model + estimated pulse wave velocity (ePWV) for cardiovascular mortality prediction in multivariable analysis. Framingham risk score was used as the basic model
Figure 2 shows the Nested Cox model for overall mortality prediction. The basic model could significantly predict overall mortality (Chi‐square vale, 60.21, p < .001). We further added baPWV and ePWV into the basic model. Both basic model + baPWV and basic model + ePWV could provide an extra benefit in prediction of overall mortality than basic model (p < .001). In direct comparison between basic model + baPWV and basic model + ePWV, the basic model + ePWV had similar predictive value for overall mortality as basic model + baPWV (p = .543).
FIGURE 2.
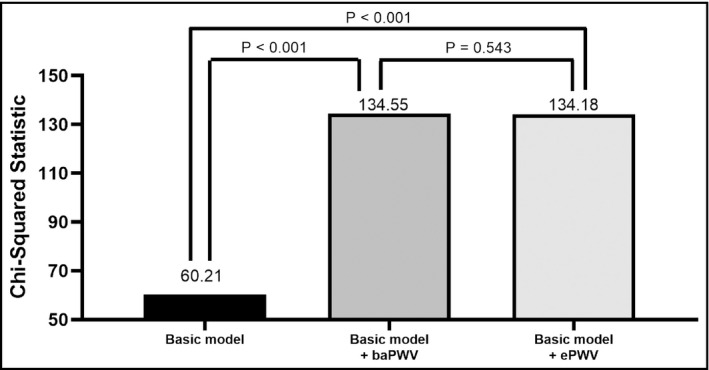
Direct comparison among basic model, basic model + brachial‐ankle pulse wave velocity (baPWV), and basic model + estimated pulse wave velocity (ePWV) for overall mortality prediction in multivariable analysis. Framingham risk score was used as the basic model
We also performed NRI to evaluate the discriminatory ability after adding ePWV and baPWV into basic model including FRS for prediction of CV and overall mortality. The results were shown in Table 5. We found that NRI improved significantly after adding ePWV and baPWV into FRS for prediction of CV (p ≤ .02) and overall mortality (P < .001).
TABLE 5.
Net reclassification improvement analysis for CV and overall mortality prediction after adding ePWV and baPWV into FRS model
| Model | Net reclassification improvement | p value |
|---|---|---|
| CV mortality | ||
| FRS + ePWV vs. FRS | 0.37 (0.15–0.59) | .001 |
| FRS + baPWV vs FRS | 0.48 (0.04–0.48) | .02 |
| Overall mortality | ||
| FRS + ePWV vs. FRS | 0.47 (0.33–0.62) | <.001 |
| FRS + baPWV vs FRS | 0.34 (0.19–0.48) | <.001 |
Abbreviations: AUC, area under curve; baPWV, brachial‐ankle pulse wave velocity; ePWV, estimated pulse wave velocity; FRS, Framingham risk score.
4. DISCUSSION
This study aimed to evaluate the ability of ePWV in predicting CV and overall mortality and compare the predictive value of CV and overall mortality between ePWV and baPWV. There are several major findings in the present study. First, both increased ePWV and baPWV were associated with increased CV and overall mortality in the univariable and multivariable analyses. Second, ePWV had better predictive value than FRS for prediction of CV and overall mortality. However, baPWV did not. Third, in direct comparison of univariable and multivariable analysis, ePWV had a better additive predictive value for CV mortality than baPWV but similar predictive value for overall mortality as baPWV.
The ePWV calculated by equations using age and MBP has shown to be a reliable parameter of arterial stiffness as measured carotid‐femoral PWV. 11 Greve et al reported that ePWV could predict composite CV endpoints of CV death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for ischemic heart disease independently of Systematic COronary Risk Evaluation (SCORE) or FRS as well as carotid‐femoral PWV. 11 In addition, in the secondary analysis of SPRINT study, Vlachopoulos et al also showed that ePWV could predict outcomes independent of the FRS and could be used to gauge the effect of treatment of hypertension. 14 In the present study, we consistently demonstrated that high ePWV was associated with increased CV and overall mortality.
Increased PWV, which reflects increased arterial stiffness, was reported to be an independent predictor of CV outcomes and prognosis. 1 , 2 , 3 , 4 , 5 , 6 , 26 PWV was also associated with atherosclerosis, 27 , 28 left ventricular diastolic dysfunction, 29 , 30 left ventricular mass index, and left ventricular hypertrophy. 31 , 32 , 33 , 34 , 35 Although several parameters can be used to measure arterial stiffness, the gold standard non‐invasive method was carotid‐femoral PWV, 18 which was reported to directly reflect aortic PWV. 36 , 37 In comparison, baPWV was a composite measure of several arterial segments, and some of these segments would be prone to arteriosclerosis (brachial and distal arteries). In Hatsuda's study, they found in patients with type 2 diabetes mellitus, central arterial stiffness played a more important role in the development of ischemic heart disease than peripheral arterial stiffness. 38 The ePWV was an estimate of central arterial stiffness, 11 but baPWV was a mixture of central and peripheral arterial stiffness. Central arterial stiffness might have a more important contribution in the development of CV disease. Therefore, our present study similarly showed ePWV had a superior predictive valve for CV mortality than baPWV both in the univariable and multivariable analyses.
Choo et al found in healthy subjects, carotid‐femoral PWV displayed a strong correlation with central heart‐femoral PWV, whereas baPWV displayed a moderate correlation with both central heart‐femoral PWV and peripheral femoral‐ankle PWV. 39 In the present study, both ePWV and baPWV were significant predictors of overall mortality in the univariable and multivariable analyses. In addition, baPWV also had similar predictive value for overall mortality as ePWV in the univariable (p = .058) and multivariable analysis (p = .541). The underlying mechanism of this finding was unknown. However, both central and peripheral arterial stiffness should have a certain role in survival predication. BaPWV, a mixture of central and peripheral arterial stiffness, might also exhibit a good predictive value for long‐term overall mortality as ePWV, an estimated measure of central arterial stiffness.
4.1. Study limitations
There were some limitations to this study. First, the sample size of our study was not very large, but the follow‐up period was relatively long, up to 105 months. Second, the majority of our patients were treated with CV drugs. For ethical reasons, we did not withdraw these medications. Hence, we could not exclude the influence of CV drugs on our study. However, we adjusted the associated usage of CV drugs in the multivariable analysis. Third, our study was aimed to evaluate the mortality events, so nonfatal events were not studied.
5. CONCLUSIONS
Our study was the first one to compare ePWV and baPWV for prediction of long‐term CV and overall mortality. We found both ePWV and baPWV were independent predictors for long‐term CV and overall mortality in univariable and multivariable analysis. ePWV had better predictive value than FRS for CV and overall mortality prediction but baPWV did not. In addition, ePWV had a better additive predictive value for CV mortality than baPWV and similar predictive value for overall mortality as baPWV. Therefore, ePWV obtained without equipment deserved to be calculated for overall mortality prediction and better CV survival prediction.
CONFLICT OF INTEREST
The authors have declared no competing interest exists.
AUTHOR CONTRIBUTIONS
Conceptualization, Po‐Chao Hsu, Wen‐Hsien Lee, Cheng‐An Chiu; Data curation, Wei‐Chung Tsai and Ying‐Chih Chen; Formal analysis, Hsueh‐Wei Yen and Wei‐Chung Tsai; Investigation, Po‐Chao Hsu, Chun‐Yuan Chu and Tsung‐Hsien Lin; Methodology, Wen‐Chol Voon, Wen‐Ter Lai and Sheng‐Hsiung Sheu; Supervision, Wen‐Chol Voon, Wen‐Ter Lai, Sheng‐Hsiung Sheu and Ho‐Ming Su; Validation, Wen‐Ter Lai, Sheng‐Hsiung Sheu and Ho‐Ming Su; Visualization, Wen‐Ter Lai, Sheng‐Hsiung Sheu, Ho‐Ming Su, and Cheng‐An Chiu; Writing – original draft, Po‐Chao Hsu and Wen‐Hsien Lee; Writing – review & editing, Ho‐Ming Su and Cheng‐An Chiu.
ACKNOWLEDGEMENTS
Mortality data were provided by the Collaboration Center of Health Information Application, Ministry of Health and Welfare, Executive Yuan. The authors thank the help from Ming‐Yen Lin (Division of Medical Statistics and Bioinformatics, Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung Medical University).
Contributor Information
Ho‐Ming Su, Email: cobeshm@seed.net.tw.
Cheng‐An Chiu, Email: 1020004@mail.kmuh.org.tw.
REFERENCES
- 1. Cardoso CR, Salles GF. Aortic stiffness as a surrogate endpoint to micro‐ and macrovascular complications in patients with type 2 diabetes. Int J Mol Sci. 2016;17(12):2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664‐670. [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236‐1241. [DOI] [PubMed] [Google Scholar]
- 4. Cardoso CR, Ferreira MT, Leite NC, et al. Prognostic impact of aortic stiffness in high‐risk type 2 diabetic patients: the Rio deJaneiro type 2 diabetes cohort study. Diabetes Care. 2013;36(11):3772‐3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wijkman M, Lanne T, Ostgren CJ, et al. Aortic pulse wave velocity predicts incident cardiovascular events in patients with type 2 diabetes treated in primary care. J Diabetes Complications. 2016;30(7):1223‐1228. [DOI] [PubMed] [Google Scholar]
- 6. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Jong MA, Van Roon AM, Bakker JT, et al. Digital arterial pressure pulse wave analysis and cardiovascular events in the general population: the prevention of renal and vascular end‐stage disease study. J hypertens. 2020;38(6):1064‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scandale G, Dimitrov G, Recchia M, et al. Arterial stiffness and 5‐year mortality in patients with peripheral arterial disease. J Hum Hypertens. 2020;34(7):505‐511. [DOI] [PubMed] [Google Scholar]
- 9. Matschkal J, Mayer CC, Sarafidis PA, et al. Comparison of 24‐hour and office pulse wave velocity for prediction of mortality in hemodialysis patients. Am J Nephrol. 2019;49(4):317‐327. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27(10):2022‐2027. [DOI] [PubMed] [Google Scholar]
- 11. Greve SV, Blicher MK, Kruger R, et al. Estimated carotid‐femoral pulse wave velocity has similar predictive value as measured carotid‐femoral pulse wave velocity. J Hypertens. 2016;34(7):1279‐1289. [DOI] [PubMed] [Google Scholar]
- 12. Greve SV, Blicher MK, Kruger R, et al. Elevated estimated arterial age is associated with metabolic syndrome and low‐grade inflammation. J Hypertens. 2016;34(12):2410‐2417. [DOI] [PubMed] [Google Scholar]
- 13. Greve SV, Laurent S, Olsen MH. Estimated pulse wave velocity calculated from age and mean arterial blood pressure. Pulse (Basel). 2017;4(4):175‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vlachopoulos C, Terentes‐Printzios D, Laurent S, et al. Association of estimated pulse wave velocity with survival: a secondary analysis of SPRINT. JAMA Netw Open. 2019;2(10):e1912831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka A, Tomiyama H, Maruhashi T, et al. Physiological diagnostic criteria for vascular failure. Hypertension. 2018;72(5):1060‐1071. [DOI] [PubMed] [Google Scholar]
- 16. Munakata M. Brachial‐ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10(1):49‐57. [DOI] [PubMed] [Google Scholar]
- 17. Tomiyama H, Yamashina A, Arai T, et al. Influences of age and gender on results of noninvasive brachial‐ankle pulse wave velocity measurement–a survey of 12517 subjects. Atherosclerosis. 2003;166(2):303‐309. [DOI] [PubMed] [Google Scholar]
- 18. Yokoyama H, Shoji T, Kimoto E, et al. Pulse wave velocity in lower‐limb arteries among diabetic patients with peripheral arterial disease. J Atheroscler Thromb. 2003;10(4):253‐258. [DOI] [PubMed] [Google Scholar]
- 19. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837‐1847. [DOI] [PubMed] [Google Scholar]
- 20. Asmar R, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26(3):485‐490. [DOI] [PubMed] [Google Scholar]
- 21. Miyano I, Nishinaga M, Takata J, et al. Association between brachial‐ankle pulse wave velocity and 3‐year mortality in community‐dwelling older adults. Hypertens Res. 2010;33(7):678‐682. [DOI] [PubMed] [Google Scholar]
- 22. Mattace‐Raso FU, van der Cammen TJ, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657‐663. [DOI] [PubMed] [Google Scholar]
- 23. Chen SC, Chang JM, Tsai YC, Su HM, Chen HC. Brachial‐ankle pulse wave velocity and brachial pre‐ejection period to ejection time ratio with renal outcomes in chronic kidney disease. Hypertens Res. 2012;35(12):1159‐1163. [DOI] [PubMed] [Google Scholar]
- 24. Su HM, Lin TH, Hsu PC, et al. Brachial‐ankle pulse wave velocity and systolic time intervals in risk stratification for progression of renal function decline. Am J Hypertens. 2012;25(9):1002‐1010. [DOI] [PubMed] [Google Scholar]
- 25. Chen SC, Lin TH, Hsu PC, et al. Impaired left ventricular systolic function and increased brachial‐ankle pulse‐wave velocity are independently associated with rapid renal function progression. Hypertens Res. 2011;34(9):1052‐1058. [DOI] [PubMed] [Google Scholar]
- 26. Chen SC, Chang JM, Liu WC, et al. Brachial‐ankle pulse wave velocity and rate of renal function decline and mortality in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(4):724‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wykretowicz A, Metzler L, Milewska A, et al. Noninvasively assessed pulsatility of ascending aortic pressure waveform is associated with the presence of coronary artery narrowing. Heart Vessels. 2008;23(1):16‐19. [DOI] [PubMed] [Google Scholar]
- 28. van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32(2):454‐460. [DOI] [PubMed] [Google Scholar]
- 29. Seeland U, Brecht A, Nauman AT, et al. Prevalence of arterial stiffness and the risk of myocardial diastolic dysfunction in women. Biosci Rep. 2016;36(5):e00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roos CJ, Auger D, Djaberi R, et al. Relationship between left ventricular diastolic function and arterial stiffness in asymptomatic patients with diabetes mellitus. Int J Cardiovasc Imaging. 2013;29(3):609‐616. [DOI] [PubMed] [Google Scholar]
- 31. Chung CM, Lin YS, Chu CM, et al. Arterial stiffness is the independent factor of left ventricular hypertrophy determined by electrocardiogram. Am J Med Sci. 2012;344(3):190‐193. [DOI] [PubMed] [Google Scholar]
- 32. Masugata H, Senda S, Hoshikawa J, et al. Elevated brachial‐ankle pulse wave velocity is associated with left ventricular hypertrophy in hypertensive patients after stroke. Tohoku J Exp Med. 2010;220(3):177‐182. [DOI] [PubMed] [Google Scholar]
- 33. Su HM, Lin TH, Hsu PC, et al. Impact of systolic time intervals on the relationship between arterial stiffness and left ventricular hypertrophy. Atherosclerosis. 2012;223(1):171‐176. [DOI] [PubMed] [Google Scholar]
- 34. Su HM, Lin TH, Hsu PC, et al. Association of brachial‐ankle pulse wave velocity, ankle‐brachial index and ratio of brachial pre‐ejection period to ejection time with left ventricular hypertrophy. Am J Med Sci. 2014;347(4):289‐294. [DOI] [PubMed] [Google Scholar]
- 35. Su HM, Lin TH, Hsu PC, et al. Association of bilateral brachial‐ankle pulse wave velocity difference with peripheral vascular disease and left ventricular mass index. PLoS ONE. 2014;9(2):e88331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blacher J, Guerin AP, Pannier B, et al. Arterial calcifications, arterial stiffness, and cardiovascular risk in end‐stage renal disease. Hypertension. 2001;38(4):938‐942. [DOI] [PubMed] [Google Scholar]
- 37. Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end‐stage renal disease. Circulation. 1999;99(18):2434‐2439. [DOI] [PubMed] [Google Scholar]
- 38. Hatsuda S, Shoji T, Shinohara K, et al. Regional arterial stiffness associated with ischemic heart disease in type 2 diabetes mellitus. J Atheroscler Thromb. 2006;13(2):114‐121. [DOI] [PubMed] [Google Scholar]
- 39. Choo J, Shin C, Barinas‐Mitchell E, et al. Regional pulse wave velocities and their cardiovascular risk factors among healthy middle‐aged men: a cross‐sectional population‐based study. BMC Cardiovasc Disord. 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]


