Abstract
High blood pressure (BP) is the single leading preventable cardiovascular disease (CVD) risk factor across the world. In order to decrease the global burden of CVD, broad hypertension screening programs that facilitate early hypertension diagnosis and treatment are essential. Accurate BP devices are a key element of hypertension control programs. With the overwhelming number of devices available now on the market, most of which have not been tested for accuracy, it can be challenging to select the optimal BP measurement device for clinical settings. This review details essential factors to consider when selecting a good‐quality BP device, particularly for use in low‐resource settings. Barriers to the procurement and use of good‐quality devices are reviewed and practical solutions proposed.
Keywords: accuracy, cardiovascular disease, cardiovascular health, high blood pressure, hypertension, prevention, validation
1. INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death globally. High blood pressure (BP) is the leading preventable CVD risk factor, responsible for 10.7 million CVD‐related deaths per year. 1 Approximately 40% of adults worldwide live with high BP, 2 which carries a 3.0 times increased risk of stroke, 3 a 1.9 times greater risk of myocardial infarction, 4 and a 1.5 times greater risk of renal failure. 5 Efforts to decrease the global burden of CVD must include broad hypertension screening programs that facilitate early diagnosis and treatment of hypertension. Proper BP measurement, which includes use of accurate devices, is essential. However, BP measurement errors are common in clinical practice. 6 Inaccurate BP values can lead to over‐ or underdiagnosis and over‐ or undertreatment of hypertension and contribute to poor patient outcomes. 7
Clinicians in low‐resource settings may find it challenging to obtain accurate BP measurements. Quality of hypertension screening and diagnosis in these settings may be hindered by (a) barriers to procurement and maintenance of high‐quality measurement devices; (b) provider training and skill maintenance; and (c) difficulties in obtaining multiple BP measurements when required for clinical diagnosis. Further, health care facilities in many low‐resource settings have limited or no access to biomedical engineers, new BP measurement technologies, or out‐of‐office BP monitoring capabilities.
2. BP MEASUREMENT DEVICE ACCURACY
Accurate devices are those that have been validated according to an established protocol, 8 , 9 , 10 , 11 , 12 provide multiple cuff sizes to ensure appropriate upper arm fit, and are maintained in good condition.
2.1. Device validation
Device validation refers to rigorous, objective clinical testing of a device against a gold standard to determine accuracy. Validation should be done by an independent organization, such as a testing laboratory or academic institution with publicly available protocols, rather than by the manufacturer itself. 13 Validation protocols require that devices be tested on a predetermined number of individuals meeting age, sex, arm circumference, and BP requirements. BP measured by the device under test is compared to BP measured either by simultaneous, blinded, two‐observer manual auscultation or by an intra‐arterial catheter. A device is considered “validated” if it passes protocol requirements. As an example, the International Organization for Standardization (ISO) validation protocol has two criteria, one of which requires that the mean BP difference between the device under test and the reference standard be no more than 5 ± 8 mm Hg.
There are key differences between the currently available protocols, with the most significant difference being the required sample size (Table 1). For example, the European Society of Hypertension International Protocol (ESH‐IP) requires a device to be tested in 52 fewer individuals than a device tested using the American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization (ANSI/AAMI/ISO) protocol. In 2018, the ESH joined forces with AAMI/ANSI/ISO to endorse a universal protocol that requires 85 subjects for each validation test. 14
TABLE 1.
Comparison of validation protocols for devices to be used in an adult population
| BHS 1993 | ESH‐IP 2002 | ESH‐IP 2010 | AAMI/ESH/ISO 81060‐2; 2019 | |
|---|---|---|---|---|
| Number of subjects | 85 |
33 Phase 1: 15 Phase 2: 18 |
33 | 85 |
| Age range of participants | Distribution by chance | ≥30 y | ≥25 y | >12 y |
| Sex of participants | Distribution by chance |
Phase 1: At least 5 male/5 female Phase 2: At least 10 male/10 female |
At least 30% male/30%female | At least 30% male/30%female |
| SBP ranges of included participants | At least 8 subjects should have an entry SBP < 90 mm Hg | At least 5 subjects in phase 1 and 11 subjects in phase 2 should have an entry SBP 90‐130 mm Hg | 10‐12 subjects with SBP < 130 mm Hg | At least 5% of the reference SBP readings ≤100 mm Hg |
| At least 8 subjects should have an entry SBP > 180 mm Hg | At least 5 subjects in Phase 1 and 11 subjects in Phase 2 should have an entry SBP 130‐160 mm Hg | 10‐12 subjects with SBP 130‐160 mm Hg | At least 5% of the reference SBP readings ≥ 160 mm Hg | |
| At least 20 subjects should have an entry SBP 90‐129 mm Hg | At least 5 subjects in Phase 1 and 11 subjects in Phase 2 should have an entry SBP 161‐180 mm Hg | 10‐12 subjects with SBP > 160 mm Hg | At least 20% of the reference SBP readings ≥ 140 mm Hg | |
| At least 20 subjects should have an entry SBP 130‐160 mm Hg | ||||
| At least 20 subjects should have an entry SBP 161‐180 mm Hg | ||||
| DBP ranges of included participants | At least 8 subjects should have an entry DBP < 60 mm Hg | At least 5 subjects in Phase 1 and 11 subjects in Phase 2 should have an entry DBP 40‐79 mm Hg | 10‐12 subjects and 22‐44 test readings with DBP < 80 mm Hg | At least 5% of the reference DBP readings ≤60 mm Hg |
| At least 8 subjects should have an entry DBP > 110 mm Hg | At least 5 subjects in Phase 1 and 11 subjects in Phase 2 should have an entry DBP 80‐100 mm Hg | 10‐12 subjects and 22‐44 test readings with DBP 80‐100 mm Hg | At least 5% of the reference DBP readings ≥ 100 mm Hg | |
| At least 20 subjects should have an entry DBP 60‐79 mm Hg | At least 5 subjects in Phase 1 and 11 subjects in Phase 2 should have an entry DBP 101‐130 mm Hg | 10‐12 subjects and 22‐44 test readings with DBP > 100 mm Hg | At least 20% of the reference DBP readings ≥ 85 mm Hg | |
| At least 20 subjects should have an entry DBP 80‐100 mm Hg | ||||
| At least 20 subjects should have an entry DBP 101‐110 mm Hg | ||||
| Reference cuff requirements | Bladder length: 80% of upper arm circumference | NS | Bladder length: 80%‐100% upper arm circumference |
Bladder length: 75%‐100% of the upper arm circumference Bladder width: 37%‐50% of the upper arm circumference |
| Reference device requirement | Mercury | NS | Mercury |
Mercury, aneroid, or invasive Aneroid devices used as reference must have a maximum error of ± 1 mm Hg |
| Number of participants tested per cuff sold with the device | NS | NS | NS | 1/2n of the total study population (n = number of cuffs, study population is typically 85) |
| Number of participants tested along the range of the cuff size | NS | Arm circumference ranges determined by chance | NS |
For multiple cuff systems: 40% with limb circumference in upper half of specified range of the use of the cuff, 40% with limb circumference below For single cuff systems (typically wide‐range cuffs): In addition to above, 20% with limb circumference in upper quarter of specified range, 20% below, 10% need to be in upper octile, and 10% need to be in lower octile |
| Minimal performance standards (ie, minimal degree of accuracy to “pass” validation) |
Overall: The percentages of device‐observer measurement differences that are <5, <10, and <15 mm Hg are calculated separately for each observer and separately for SBP and DBP The device is graded A, B, C, or D based on these percentages to determine whether device passes or fails |
Phase 1: must meet requirements for the number of device‐observer BP differences ≤5, 10, 15 mm Hg Phase 2: must meet requirements for (1) the number of device‐observer BP differences ≤5, 10, 15 mm Hg and (2) the number of subjects with 0, 2, or 3 absolute differences ≤5 mm Hg |
Overall: must meet requirements for (1) the number of device‐observer BP differences ≤5, 10, 15 mm Hg, and (2) the number of subjects with 0, 2, or 3 absolute differences ≤5 mm Hg |
Overall: average difference for all 255 measurements must be no more than ± 5 mm Hg with a standard deviations no more than 8 mm Hg Each participant: the averaged difference between each participant's measurements obtained from the reference sphygmomanometer and the measurements obtained by the device under test have additional requirements re: allowable difference ± standard deviation |
| Required observer agreement | 80% of the measurements by the observers should be within 5 mm Hg of each other and 95% within 10 mm Hg | Observer measurements must by ≤4 mm Hg to be included | Observer measurements must by ≤4 mm Hg to be included | Observer measurements must by ≤4 mm Hg to be included |
| Additional requirements |
In addition to the above, each device needs to pass the following testing:
|
|||
| Notes | BP measurements obtained during testing are either (1) assessed in real‐time using two observers or (2) recorded via a Sphygmocorder and assessed at a later date | Sphygmocorder not allowed in this protocol because “no validated model is currently available” | Intra‐arterial reference blood pressure measurements are also allowed under this protocol; there are different requirements for testing than those described above for an auscultatory reference BP measurement and the BP measurements obtained with this reference standard may be different than the BPs obtained by auscultation | |
| References | O’Brien E, et al (1993) 9 | O’Brien E, et al (2002) 30 | O’Brien E, et al (2010) 10 | Stergiou GS, et al (2019) 12 |
Devices need not undergo clinical validation testing in order to be marketed. In fact, most devices on the global market have not been validated. 15 For example, a study designed to assess accuracy of home BP devices reported that 26 of 85 participants were using a non‐validated device to manage their BP. 16 In 2005, only 66 of 124 internet sites selling home BP devices offered at least one validated BP device, 17 and only six sites clearly identified which devices were validated. Approximately half of the sites offered more than one cuff size for purchase, with up to 80% charging more for large adult cuff sizes (mean cost for large adult cuff in the United States is US$23.75).
It is also important to note that US manufacturers frequently describe devices as “FDA approved.” In truth, the US Food and Drug Administration does not “approve” BP devices, but merely allows legal marketing if they demonstrate substantial equivalence to another legally marketed device. 18
If not clearly labeled on the packaging or product Web site, one can determine whether a device has been validated by referencing Web sites endorsed by professional societies, such as STRIDE BP, 19 the British and Irish Hypertension Society, 20 and Hypertension Canada. 21 If a manufacturer claims that its device is validated, the specific protocol used for validation testing should be listed. Devices that have not been validated for accuracy should not be purchased.
This issue is particularly salient for resource‐constrained settings. Validation testing involves time and cost (approximately US$30‐40 000 per study). As a result, devices that have passed validation may be more expensive than those that have not undergone validation testing.
2.2. Cuff size
A frequent error in BP measurement is use of an inappropriate cuff size. 22 Too‐small and too‐large cuffs produce overestimated and underestimated BP readings, respectively. 23 The recommended bladder length is 75‐100 percent of the patient's mid‐upper arm circumference, and the ideal bladder width is 37‐50 percent of the mid‐upper arm circumference. 24 These dimensions are based on evidence gathered from the mid‐1970s (Figure 1), 25 with the length requirement being particularly important for manual blood pressure devices. Some oscillometric devices offer cuffs for a wide range of arm circumference ranges; as long as these devices are properly validated across the range of arm circumferences of intended use, the cuffs may not need to hold to the length guideline. Ideally, several different cuffs will be available to ensure proper fit to each patient with each cuff selected to fit the arm circumference it was intended for.
FIGURE 1.
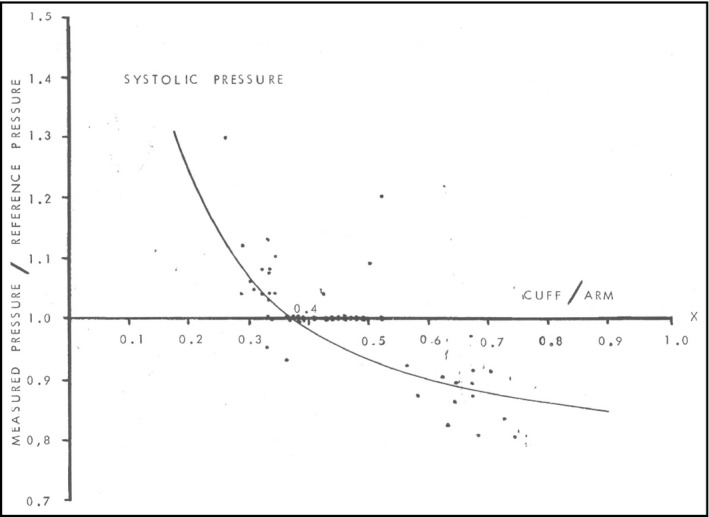
The association of the ratio of device measured:reference blood pressure vs the ratio of cuff width:mid‐arm circumference. Source: Geddes and Tivey (1976) 25
In many low‐resource settings, BP measurement is performed using a single cuff for all patients, regardless of arm circumference, due to either the lack of varying cuff sizes or the operational challenge of switching cuffs in an extremely busy clinical setting. Although best practice dictates individualized cuff selection, if needed, providers may consider using a medium‐size cuff for all patients in locations with low obesity rates where small and medium arm circumferences are more common, as long as the limitations to this strategy (in particular, underdiagnosis of elevated blood pressure among adults with thin arms) are acknowledged. Wrist or finger cuffs are not recommended as they are extremely sensitive to body position and will not provide readings as accurately as an upper arm cuff.
2.3. Cuff integrity
The physical integrity of the cuff is also critical to ensuring accurate BP measurements. The fabric of the cuff itself should not be worn or frayed, the Velcro attachments should adhere securely, and the bladder and tubing should be undamaged and intact. When these criteria are not met, the device may appear to be working properly, when in fact it may no longer be accurate. Maintaining cuff/tubing integrity can be difficult in low‐resource settings where there is insufficient funding to replace equipment as it wears out.
3. BP MEASUREMENT DEVICE SELECTION
3.1. Types of devices
There are a number of different types of BP measurement devices, each with advantages and disadvantages that are particularly pertinent to low‐resource settings (Table 2). Perhaps the most important difference between devices is the amount of operator skill each requires and the variable concern for human error impacting the measurement. Automated and semi‐automated devices are the easiest devices to operate and produce an objective estimation of BP independent of the individual performing the measurement. For these and other reasons, many professional societies recommend their use over the use of manual (mercury or aneroid) BP devices.
TABLE 2.
BP measurement devices and features
| Device | Required skill of operator, other operator considerations | Power requirements | Calibration needed? | Longevity * | Pros | Cons |
|---|---|---|---|---|---|---|
| Manual—mercury |
High Required good vision/hearing/manual dexterity to adjust and control proper deflation rate |
None | No | Indefinite |
|
|
| Manual—aneroid |
High Required good vision/hearing/manual dexterity to adjust and control proper deflation rate |
None | Yes, every 6 mo |
|
|
|
| Semi‐automated | Low (need to know how high to inflate the cuff) | Battery, solar (lower power needs due to manual cuff inflation with a hand bulb) | No, but to maintain accuracy parts need to be replaced when cuffs or tubing is worn or has lost integrity |
|
|
|
| Automated | Lowest | Battery, A/C, solar | No, but to maintain accuracy parts need to be replaced when cuffs or tubing is worn or has lost integrity |
|
|
|
Length of time before device or essential parts need to be replaced.
4. MANUAL MERCURY SPHYGMOMANOMETERS
The mercury sphygmomanometer, when used by trained observers, has long been considered the gold standard for non‐invasive BP measurement. 26 Because this method requires use of a stethoscope to listen for Korotkoff sounds (auscultation), it requires clinical expertise, manual dexterity, and excellent vision and hearing. Mercury sphygmomanometers do not require recalibration and remain accurate as long as the column at rest is at zero, the column is intact, and none of the parts have been damaged (ie, there is no air leak). Because of concerns about the toxicity of mercury, this type of device is being phased out in many areas. 27
5. MANUAL ANEROID SPHYGMOMANOMETERS
Instead of a column of mercury, aneroid devices use an analog gauge that usually features a circular dial and a needle to indicate BP. The method of measuring BP with an aneroid device is otherwise identical to that using a mercury sphygmomanometer and shares the requirements of clinical expertise, manual dexterity, and excellent vision and hearing. Periodic recalibration, usually every 6 months, is essential to maintain accuracy.
6. AUTOMATED DEVICES
Automated devices use a cuff that inflates automatically and estimate systolic and diastolic BP from oscillometric pulses via proprietary algorithms. This BP estimation is displayed on the device's digital display at the completion of the measurement. Because readings are automated, less operator skill is required to obtain BP measurements with these devices. While these devices do not require calibration, intact cuffs and tubing is essential to maintain accuracy.
7. SEMI‐AUTOMATED DEVICES
Semi‐automated devices are similar to automated devices in terms of the method of BP estimation, but cuff inflation is instead dependent on an operator manually inflating the cuff using a bulb to pump air. While these devices are advantageous in that they require less power to operate (and therefore are suitable to settings with irregular electrical supply), they do require operator skill (eg, not fully inflating the cuff prior to measurement could lead to inaccurate readings). As with automated devices, calibration is not required, but cuff and tubing integrity are essential for accuracy.
8. EMERGING TECHNOLOGY
Electronic devices such as smart phones and cuffless wearable devices use emerging technologies to detect heartbeats or pulses that are then used to estimate BPs. Studies suggest these “mHealth” applications are inaccurate and that more work needs to be done prior to recommending these devices for clinical care. 28 , 29
9. ACQUISITION AND PURCHASING ISSUES
Personnel, resources, clinical care needs, throughput, and patient population are all variables that factor into decision‐making for device procurement (Table 3). Once the ideal type of device for the clinical setting has been determined, the ideal model should be identified with validation, availability and affordability, and cuff sizes in mind.
TABLE 3.
Stepwise approach to device procurement
| Step 1: Determine the ideal device for the clinical setting | |||||
|---|---|---|---|---|---|
| Mercury device | Aneroid device | Semi‐automated device | Fully automated device | Notes | |
| Personnel | |||||
| Are there trained health care professionals who can operate, or who can be trained to operate the BP device? | |||||
| Yes | √ | √ | √ | √ | |
| No | √ | ||||
| Do you have resources to provide training/retraining every 6‐12 mo? | |||||
| Yes | √ | √ | √ | √ | |
| No | √ | ||||
| Do you have access to biomedical engineers for frequent (eg every 6 mo) calibration needs? | |||||
| Yes | √ | √ | √ | √ | |
| No | √ | √ | |||
| Power | |||||
| Is there adequate and reliable wall outlet or battery power available to support daily use of the device? | |||||
| Yes | √ | √ | √ | √ | |
| No | √ | √ | √ | ||
| Durability and sustainability | |||||
| Is the device meant for use in a single clinic or a mobile health center/community screening? | |||||
| Single Clinic | √ | √ | √ | √ | |
| Mobile Clinic | √ | √ | |||
| What is anticipated patient volume per year? | |||||
| <10 000 | √ | √ | √ | √ | |
| >10 000 | √ | √ | Automated devices intended for home use are not ideal; devices intended for professional use are preferred | ||
| Step 2: Identify potential devices for purchase | ||||
|---|---|---|---|---|
| Ideal | Acceptable | Not ideal | Do not purchase | |
| Validation | ||||
| Has the device been validated using a standard method? | Yes |
No Do not know |
||
| Which validation protocol was used to validate the device? | AAMI/ISO/ESH, BHS | ESH‐IP |
Do not know None |
|
| Availability/affordability | ||||
| Does the manufacturer have a local or regional office? | Yes | No | ||
| Does the manufacturer offer a warranty? | Yes | No | ||
| Does the manufacturer offer bulk pricing options? | Yes | No | ||
| Cuffs | ||||
| Does the device come with multiple cuff sizes? | Yes, provided with device | Yes, available for separate purchase | No | |
| Do the available cuff sizes match arm sizes in your population? | Yes | No | ||
9.1. Personnel
Ensuring that staff tasked with performing BP measurements have proper skill and training is critical. Automated devices require less training so may be more practical and accurate in settings where less‐skilled staff are responsible for BP measurement. Medically trained and skilled personnel may be able to perform manual measurements with acceptable accuracy; however, the need for regular training and ongoing competency assessments to ensure persistent accuracy cannot be understated. Evidence suggests that, even with sufficient training, some physicians do not accurately measure BP. 22 Access to biomedical engineers is essential to maintain calibration of aneroid devices and should factor heavily into decision‐making.
9.2. Resources and clinical care needs
The source of power required to operate the device also needs to be considered, whether electric power, battery power, solar power, or manual power (eg, hand pump). Electricity is often not sufficiently reliable in some parts of the world, necessitating generator or battery backup power sources. If batteries are the primary power source, whether by necessity or because portability is desired, the cost and frequency of battery replacement and the ability to safely dispose of used batteries will be factors.
9.3. Recording
Some devices are able to transfer blood pressure measurements automatically to electronic health records or to smartphones for later download. In some settings, this feature may be ideal because it can enhance workflow and reduce inaccurate or falsified data.
9.4. Patient population
Although BP readings will be most accurate when taken using a cuff wrapped directly over bare upper arm skin, cultural norms or societal expectations may necessitate placing the cuff over clothing. Accuracy does not appear to be compromised if the arm is covered by a thin layer of clothing. Wrist cuffs, while tempting in this scenario and with patients who have obesity, should not be used as the accuracy of these devices when used in typical clinical situations is suboptimal.
10. BEST PRACTICES
Once these characteristics have been considered, potential devices should be identified and evaluated for accuracy. Experience has established the following best practices to guide procurement of BP measurement devices that are of sufficiently good quality to provide accurate readings for all intended patients over the expected lifespan of the device (Table 4). Independent Web sites, such as that maintained by the British and Irish Hypertension Society, 20 provide useful information about device cost, features, and validation status to aid in purchasing decisions.
TABLE 4.
Minimal requirements for procuring good‐quality BP measurement devices
| Highly recommended | Recommended | Not recommended | |
|---|---|---|---|
| Validation | AAMI/ESH/ISO and/or BHS | ESH‐IP | None |
| Cuffs |
Multiple upper extremity cuffs that cover a mid‐arm circumference range of 22‐42 cm AND Cuff bladder length:width ratio approximately 2:1 |
Multiple upper extremity cuffs that cover a mid‐arm circumference range of 22‐42 cm AND Cuff bladder length:width ratio approximately 2:1 OR Single, validated cuff which covers majority of arm ranges of patient populations in regions or clinics |
Finger or wrist cuff OR Unvalidated wide‐range multiple cuff systems (designed to be used on arm circumferences ranging>=20 cm) |
10.1. Cost
Purchasing a BP measurement device from a company with a local or regional office can be a less expensive option with greater device support. Bulk purchasing can also reduce costs. Procuring devices from the internet may offer a less expensive option up‐front, but the lack of manufacturer device support when purchasing from third‐party sellers may present greater costs later on. Other cost considerations include the anticipated number of annual measurements; more durable professional‐grade equipment will be better able to withstand the demands of near constant use, resulting in decreased costs over time. In settings with lower clinical density resulting in more infrequent use, a higher‐quality home monitor device may be adequate, although consumer‐grade equipment will require more frequent replacement. The number of additional cuffs needed and whether batteries are required for use also need to be considered.
10.2. Cuffs
Obtaining multiple cuffs of different sizes, as recommended, will generally incur additional expense. Health care facilities with limited financial resources may be tempted to purchase devices offering a “one size fits all” wide‐range cuff; however, these single‐cuff devices may be inaccurate at the extreme lower and upper ends of cuff range even in validated systems. Additionally, it is important to note that automated devices are intended for use as a single system inclusive of the cuff, meaning that the accuracy and validation of the devices are linked to the cuffs designed for the device. For this reason, the cuff specifically made for the device should be used, as use of a less expensive off‐brand cuff may change the measurement result.
10.3. Maintenance
For automated devices, maintaining accuracy requires periodic inspection to ensure the integrity of cuffs, tubing, and connections, with parts replacement whenever there is wear and tear noted. There is no need for re‐calibration of the device. This is in contrast to maintenance needs for aneroid devices which require frequent calibration by a trained individual. In general, with usual use, one can expect automated devices designed for home use to provide accurate readings for approximately 30 000 measurements before significant device wear develops that impacts accuracy. Devices designed for clinical use can be expected to perform well for approximately 100 000 measurements.
10.4. Public kiosks
Use of public BP measurement kiosks is common and increasing. BP measurement kiosks are generally located in busy areas with high foot traffic, can receive heavy use, and are often not maintained regularly, making them more prone to produce inaccurate readings. 29 They typically only have a single cuff available, with many kiosks relying on an arm‐in approach to cuff placement. Unless the cuff adjusts for arm size, it may be too large or too small for a substantial proportion of the population presenting a risk that patients will obtain a forearm, not upper arm, BP measurement, particularly in those with obesity. While these types of devices can provide individual patients with at least a general idea of their BP, there is currently no data linking measurements obtained by these devices to CVD outcomes. 23 Long‐term outcome data for this BP measurement modality are needed.
11. CONCLUSION
The criteria for BP device quality and proper device use are well‐established. Inexpensive validated automatic or semiautomatic BP measurement devices are preferable for most clinical settings based on their relatively low cost, minimal operator training requirements, ease of use in clinical and nonclinical settings, and reduced need for maintenance. Barriers to uptake of these devices include a perception by health care providers and patients that they are not as accurate as traditional mercury sphygmomanometers. Advances in technology and implementation of a more rigorous universal device validation protocol should make this less of an issue than in the past, with future perceptions of automated devices becoming more favorable.
Ultimately, purchase cost will be a major driver of BP measurement device selection in most low‐resource settings. Decisions are likely to be based on the initial financial outlay; however, in addition to the initial expense of device acquisition, the costs of cuffs and ongoing maintenance must also be considered. Additionally, expected patient throughput and device durability should also be factors, as equipment that is unable to accommodate anticipated patient volume or that wears out and must be replaced frequently may lead to a higher cumulative cost over time, despite a lower initial cost.
Reliable and accurate BP measurement devices are an essential tool for efforts to improve hypertension detection and treatment globally. Assessment of clinical needs, along with regional and bulk purchasing options, should enable low‐resource settings to obtain validated BP measurement equipment of sufficiently high quality to provide front‐line support for a hypertension screening and control program of any scale.
CONFLICT OF INTEREST
TMB, DB, MF, TRF, PK, AEM, and MGJ have no conflict of interests. RP is co‐founder of mm Hg Inc, a University‐based start‐up in blood pressure measurement creating software and hardware innovations to improve measurement and monitoring.
AUTHOR CONTRIBUTIONS
Each of the listed authors (TMB, RP, MF, TRF, AEM, DEB, PK, and MGJ) meet the criteria for “Authorship” in accordance with the ICMJE recommendations as outlined below:
Substantial contributions to the conception or design of the work; and Drafting the work or revising it critically for important intellectual content; and Final approval of the version to be published; and Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Brady TM, Padwal R, Blakeman DE, et al. Blood pressure measurement device selection in low‐resource settings: Challenges, compromises, and routes to progress. J Clin Hypertens. 2020;22:792–801. 10.1111/jch.13867
Funding information
This review was conducted on behalf of Resolve to Save Lives, an initiative of Vital Strategies. Resolve to Save Lives is funded by grants from Bloomberg Philanthropies; the Bill and Melinda Gates Foundation; and Gates Philanthropy Partners, which is funded with support from the Chan Zuckerberg Foundation.
REFERENCES
- 1. GBD 2017 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10159):1923‐1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Health Observatory (GHO) data . Raised blood pressure [web page]. Geneva: World Health Organization; 2019. https://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en; Accessed 2 Mar 2020. [Google Scholar]
- 3. O’Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case‐control study. Lancet. 2016;388(10046):761‐775. [DOI] [PubMed] [Google Scholar]
- 4. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364(9438):937‐352. [DOI] [PubMed] [Google Scholar]
- 5. Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new‐onset kidney disease in a community‐based population. JAMA. 2004;291(7):844‐850. [DOI] [PubMed] [Google Scholar]
- 6. Badeli H, Assadi F. Strategies to reduce pitfalls in measuring blood pressure. Int J Prev Med. 2014;5(Suppl 1):S17‐S20. [PMC free article] [PubMed] [Google Scholar]
- 7. Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017;35(3):421‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stergiou GS, Alpert BS, Mieke S, Wang J, O’Brien E. Validation protocols for blood pressure measuring devices in the 21st century. J Clin Hypertens. 2018;20(7):1096‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Brien E, Petrie J, Littler W, et al. An outline of the revised British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(6):677‐679. [DOI] [PubMed] [Google Scholar]
- 10. O’Brien E, Atkins N, Stergiou G, et al. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15(1):23‐38. [DOI] [PubMed] [Google Scholar]
- 11. Non‐invasive sphygmomanometers – Part 2: Clinical investigation of automated measurement type. American National Standards Institute. ANSI/AAMI/ISO 81060–2:2019. Geneva: International Organization for Standardization; 2019. [Google Scholar]
- 12. Stergiou GS, Palatini P, Asmar R, et al. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens. 2019;37(3):459‐466. [DOI] [PubMed] [Google Scholar]
- 13. Sharman JE, O’Brien E, Alpert B, et al. Lancet Commission on Hypertension group position statement on the global improvement of accuracy standards for devices that measure blood pressure. J Hypertens. 2020;38(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO) Collaboration Statement. Hypertension. 2018;71(3):368‐374. [DOI] [PubMed] [Google Scholar]
- 15. Stergiou GS, Asmar R, Myers M, et al. Improving the accuracy of blood pressure measurement: the influence of the European Society of Hypertension International Protocol (ESH‐IP) for the validation of blood pressure measuring devices and future perspectives. J Hypertens. 2018;36(3):479‐487. [DOI] [PubMed] [Google Scholar]
- 16. Ringrose JS, Polley G, McLean D, Thompson A, Morales F, Padwal R. An assessment of the accuracy of home blood pressure monitors when used in device owners. Am J Hypertens. 2017;30(7):683‐689. [DOI] [PubMed] [Google Scholar]
- 17. Graves JW. A survey of validated automated home blood pressure monitors available for the internet shopper. Blood Pres Monit. 2005;10(2):103‐107. [DOI] [PubMed] [Google Scholar]
- 18. Consumers (medical devices [web page]. Silver Spring, MD: Food and Drug Administration; 2019. https://www.fda.gov/medical‐devices/resources‐you‐medical‐devices/consumers‐medical‐devices Accessed March 2, 2020. [Google Scholar]
- 19. STRIDE BP: validated blood pressure monitors [web page]. Athens: Athens University Department of Medicine; 2019. https://www.stridebp.org Accessed March 2, 2020. [Google Scholar]
- 20. BP monitors [web page]. Leicester, UK: British and Irish Hypertension Society; 2019. https://bihsoc.org/bp‐monitors Accessed March 2, 2020. [Google Scholar]
- 21. Blood pressure devices [web page]. Markham, ON: Hypertension Canada; 2018. https://hypertension.ca/hypertension‐and‐you/managing‐hypertension/measuring‐blood‐pressure/devices Accessed March 2, 2020. [Google Scholar]
- 22. Rakotz MK, Townsend RR, Yang J, et al. Medical students and measuring blood pressure: results from the American Medical Association Blood Pressure Check Challenge. J Clin Hypertens. 2017;19(6):614‐619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muntner P, Einhorn PT, Cushman WC, et al. Blood pressure assessment in adults in clinical practice and clinic‐based research, JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;73(3):317‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35‐e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geddes LA, Tivey R. The importance of cuff width in measurement of blood pressure indirectly. Cardiovasc Res Cent Bull. 1976;14(3):69‐79. [PubMed] [Google Scholar]
- 26. Murray A. In praise of mercury sphygmomanometers: appropriate sphygmomanometer should be selected. BMJ. 2001;322(7296):1248‐1249. [PMC free article] [PubMed] [Google Scholar]
- 27. Developing national strategies for phasing out mercury‐containing thermometers and sphygmomanometers in health care, including in the context of the Minamata Convention on Mercury: key considerations and step‐by‐step guidance. Geneva: World Health Organization; 2015. https://www.who.int/ipcs/assessment/public_health/WHOGuidanceReportonMercury2015.pdf Accessed March 2, 2020. [Google Scholar]
- 28. Goldberg EM, Levy PD. New approaches to evaluating and monitoring BP. Curr Hypertens Rep. 2016;18(6):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen DL, Townsend RR. Blood pressure readings using public kiosks or smart phone apps: caveat emptor (for now). J Clin Hypertens. 2017;19(10):946‐947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Brien E, Pickering T, Asmar R, et al. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7(1):3‐17. [DOI] [PubMed] [Google Scholar]


