Abstract
This meta‐analysis aims to compare serum uric acid levels among preeclamptic and healthy pregnant women across the various trimesters and provide a summary of the effect size of this biomarker in predicting adverse pregnancy outcomes. MEDLINE, Scopus, CENTRAL, Clinicaltrials.gov, and Google Scholar databases were systematically searched from inception. Observational studies were held eligible if they reported serum uric acid among preeclamptic and healthy pregnant women. Meta‐analysis was conducted regarding uric acid concentration, diagnostic accuracy, and association with perinatal outcomes. The credibility of evidence was appraised using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. The analysis included 196 studies, comprising 39 540 women. Preeclampsia was associated with significantly elevated uric acid levels during the 1st (mean difference [MD]: 0.21 mg/dL, 95% confidence intervals [CI]: 0.06‐0.35) trimester, 2nd (MD: 1.41 mg/dL, 95% CI: 0.78‐2.05) trimester, and 3rd (MD: 2.26 mg/dL, 95% CI: 2.12‐2.40) trimester. Higher uric acid was estimated for severe preeclampsia, eclampsia, and hemolysis, elevated liver enzymes, low platelet syndrome. The sensitivity for adverse perinatal outcome prediction ranged from 67.3% to 82.7% and the specificity from 47.7% to 70.7%. In conclusion, it is suggested that serum uric acid levels are increased in preeclampsia and can be used to predict disease severity and pregnancy complications. Future prospective studies should verify these outcomes, assess the optimal cutoffs, and incorporate uric acid to combined predicting models.
Keywords: meta‐analysis, predict, preeclampsia, urate, uric acid
1. INTRODUCTION
Preeclampsia represents a major pregnancy complication, as it is linked to high maternal and fetal morbidity rates. 1 It is estimated to affect approximately 5%‐8% of gestations, although its incidence presents wide variation worldwide. 2 Early‐onset disease is defined as preeclampsia diagnosed before the 34th week of pregnancy and is associated with worse prognosis, as it is often complicated by fetal growth restriction and preterm birth. 3 The pathophysiology of preeclampsia is complex and remains still under investigation. It is hypothesized that poor trophoblast invasion and deficient spiral artery remodeling lead to placental ischemia‐reperfusion injury 4 and the release of various angiogenic, 5 oxidative, 6 and inflammatory 7 mediators into maternal circulation, promoting generalized endothelial dysfunction, increased vascular reactivity, and activation of the coagulation cascade. 8 Risk stratification is essential in order to identify the subpopulation of pregnant women that would benefit from preventive measures early in the course of gestation, especially the administration of aspirin. 9 Recent research has proposed a variety of novel biomarkers as potential predictive tools, such as soluble fms‐like tyrosine kinase‐1 (sFlt‐1) and placental growth factor (PlGF) 10 ; nevertheless, the optimal screening model to be widely used in clinical practice remains a matter of debate. 11
Uric acid is the end product of purine metabolism, produced via the action of xanthine oxidase and plays a central role in free radical scavenging, presenting both pro‐oxidant and antioxidant properties. 12 During normal pregnancy, serum urate concentration is significantly decreased due to plasma volume expansion, while its clearance is amplified by the increased glomerular filtration rate and the uricosuric effects of estrogen. 13 On the other hand, preeclampsia has been considered as a state of hyperuricemia, mainly due to increased urate tubular reabsorption, stimulated by the presence of relative hypovolemia and the action of angiotensin II. 14 Uric acid excretion is also impaired as a result of lactate competition in the proximal tubule, while its production is magnified by the increased trophoblast turnover. 15 Moreover, it has been assumed that uric acid may have a role in the progression of the disease, since its elevated concentration may inhibit nitric oxide production, leading to inadequate trophoblast invasion and impaired endothelial repair. 16 Uric acid, both in its crystallized and soluble forms, has been also suggested to be capable of activating the NLRP3 (nucleotide‐binding oligomerization domain‐, leucine‐rich repeat‐, and pyrin domain‐containing protein 3) inflammasome, resulting in upregulated expression of pro‐inflammatory cytokines, especially interleukin‐1β. 17
Serum uric acid concentration has been proposed as a preeclampsia biomarker by several observational studies, although no firm consensus exists about whether its alterations precede the onset of disease, as well as regarding its utility to predict adverse pregnancy and neonatal outcomes. Previous meta‐analyses 18 , 19 , 20 in the field have indicated its potential predictive efficacy, although the small number of the included studies and the existing heterogeneity of the presented outcomes preclude the draw of safe conclusions. Evidence in the field of pregnancy complications seems to be conflicting, and the accuracy of the method seems to be debated.
In this context, the present meta‐analysis aims to accumulate all the available literature in the field and elucidate the possible prognostic role of serum uric acid in the disease, as well as to provide a summary effect estimate concerning the effectiveness of this protein in predicting preeclampsia complications and adverse perinatal outcomes. To achieve this, serum uric acid levels are compared among pregnant women with and without preeclampsia and the sensitivity and specificity of the marker for the prediction of maternal and neonatal complications are estimated.
2. MATERIALS AND METHODS
The present meta‐analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 21 The protocol of the study was prospectively registered (dx.doi.org/10.17504/protocols.io.bcndiva6).
2.1. Eligibility criteria
The main outcome of interest was predefined to the comparison of serum uric acid levels among preeclamptic and healthy pregnant women in all gestational trimesters. In addition, the prognostic role of uric acid was planned to be evaluated by comparing its levels among women with mild and severe preeclampsia, as well as with eclampsia and HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. Studies were also held eligible if they reported the diagnostic accuracy of serum uric acid in terms of sensitivity and specificity for preeclampsia detection or prediction of adverse perinatal outcomes (rate of cesarean section, fetal growth restriction, low birthweight, preterm birth, 5‐minute Apgar score <7, fetal or neonatal death).
Studies were excluded if they included cases with comorbidities, such as pre‐existing hypertension, diabetes mellitus, or autoimmune disease. Women with gestational hypertension were also not included in the present analysis. The control group consisted of healthy pregnant women, without evidence of any gestational complication. No date restrictions were applied, and thus, preeclampsia definition was not considered as a criterion for exclusion.
2.2. Literature search and data collection
Literature search was conducted using the MEDLINE, Scopus, Cochrane Central Register of Controlled Trials (CENTRAL), and Clinicaltrials.gov databases. The Google Scholar database, the full reference list of all the included articles (“snowball method”), and previous systematic reviews and meta‐analyses in the field were also screened in order to find out potential additional articles, not identified by the primary databases. Search was performed from inception to October 15, 2019, and was based on the use of the Medical Subject Headings (MeSH) terms "Pre‐Eclampsia"[Mesh] and "Uric Acid"[Mesh] combined with the following key terms: “urate”, “hyperuricemia”, “preeclampsia”, “eclampsia”, “gestational hypertension”. No language or date restrictions were applied.
2.3. Study selection
The process of study selection was consecutively performed in three stages. Firstly, the titles and abstracts of all electronic papers were screened to assess their potential eligibility. All studies that were presumed to meet the criteria were retrieved as full texts. Finally, all observational studies (prospective or retrospective), reporting the outcomes of interest, were considered to be eligible. Small case series (<10 patients), case reports, conference proceedings, and animal studies were excluded from the present systematic review. Study selection was independently performed by two researchers, while any potential disagreements regarding the retrieval of studies were resolved through the consensus of all authors.
2.4. Data extraction
The following parameters were planned to be extracted from each of the included studies: name of first author, year of publication, study design, preeclampsia definition, disease onset, severity and complications, type of sample (serum or plasma), trimester at sampling, number of patients, maternal age, serum uric acid values, and its sensitivity and specificity for prediction of preeclampsia or adverse perinatal outcomes. The process of data extraction was performed independently by two researchers, while any discrepancies were resolved by the consensus of all authors.
2.5. Data analysis
Statistical analysis of serum uric acid levels was performed in Review Manager 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) and Open Meta‐Analyst software, 22 as well as in R (3.6.1 version) programming environment using the “metafor” package. 23 Confidence intervals (CI) were set at 95%, while the DerSimonian‐Laird random‐effects model was implemented to provide pooled estimates mean difference (MD) and standard deviation (SD). Inter‐study heterogeneity was quantified by the estimation of between‐study variance (τ 2), while its influence was evaluated by calculating the 95% prediction intervals (PI), which indicate the effects to be expected by future studies in the field. The estimation of 95% PI was performed according to the equations proposed by IntHout et al. 24
Subgroup analysis was conducted on the basis of pregnancy trimester (1st, 2nd, or 3rd), preeclampsia onset (early or late), severity (mild or severe), and complications (eclampsia and HELLP syndrome). Sensitivity analysis was conducted by separately examining prospective cohort studies. Residual heterogeneity was explored by conducting meta‐regression analysis taking into account the following parameters: year of publication, sample size, region, Newcastle‐Ottawa Scale score, study design, type of sample, and preeclampsia definition. Meta‐regression was not performed for covariate levels with <3 studies. Publication bias was assessed by examining the possibility of small‐study effects through the visual inspection of funnel plots. The asymmetry of funnel plots was statistically evaluated using Egger's regression 25 and Begg‐Mazumdar's rank correlation 26 tests.
Diagnostic accuracy analysis was performed in R‐3.6.1 (“mada” 27 and “bamdit” 28 packages). A bivariate model was implemented, as it takes into account the possible correlation between sensitivity and specificity, due to the potential presence of threshold effect. Bivariate summary receiver operating characteristic (SROC) curves were constructed, and the areas under the curves (AUCs) were calculated. Diagnostic accuracy analysis was used to assess the efficacy of serum uric acid to detect preeclampsia during the 2nd trimester and 3rd trimester of pregnancy, as well as its ability to predict adverse perinatal outcomes. The “bamdit” package was also used as it provides a Bayesian approach not requiring the assumption of normality and it allows the estimation of marginal and joint posterior predictive distributions of sensitivity and specificity.
2.6. Quality assessment
The Newcastle‐Ottawa Scale (NOS) score 29 was selected to assess the methodological quality of all included studies. Evaluation of bias risk in case‐control studies was performed by evaluating the domains of selection of cases and controls, comparability of the two groups, ascertainment of exposure, and non‐response rate. Comparability was judged by taking into consideration whether studies controlled for maternal and gestational age. Cohort studies were evaluated concerning the risk of bias in the following domains: patient selection process, the comparability of the exposed and non‐exposed cohorts, the assessment of outcome, and the adequacy of the follow‐up period. Studies included in the diagnostic accuracy analysis were also evaluated with the QUADAS‐2 (Quality Assessment of Diagnostic Accuracy Studies‐2) tool 30 on the grounds of patient selection, index test, reference standard, flow, and timing. Risk of bias assessment was performed independently by two authors, while any disagreement was discussed with all authors and was resolved by their consensus.
Quality of evidence was evaluated under the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, 31 ranging from very low to high. More specifically, credibility of evidence was assessed by taking into account the following domains: study limitations, directness, consistency, precision, and publication bias. In particular, study limitations were evaluated based on risk of bias assessments (NOS score), while directness was judged using the PICOS (population, intervention, comparison, outcome, study type) approach. To assess consistency and precision, clinically important effects were defined as serum uric acid differences of ≥0.5 mg/dL, indicating a range of equivalence from −0.5 to +0.5 mg/dL. In this context, consistency referred to the agreement of 95% confidence and prediction intervals for each outcome in relation to clinically relevant effects, while precision assessment was made by taking into account whether 95% CI extended into the range of equivalence. Publication bias was evaluated by examining the potential presence of small‐study effects through the visual inspection of funnel plots.
3. RESULTS
3.1. Excluded studies
Eleven studies were excluded after being retrieved as full texts (Appendix S1, Figure S1). Three studies were excluded, since they were considered as partial duplicates of studies already included in the meta‐analysis. Moreover, one study was not included as it evaluated women with gestational diabetes, while in three studies the control group consisted of women diagnosed with gestational hypertension. The outcome of interest was not present in another 4 studies, since they did not provide serum uric acid values or enough data for the construction of the 2 × 2 tables (Appendix S2).
3.2. Included studies
The present meta‐analysis included a cohort of 196 observational studies, with a total of 39 540 women (Appendix S2). Serum uric acid was measured during the 1st trimester in 10 studies (18 620 women), the 2nd trimester in 10 studies (16 976 women), and the 3rd trimester in 155 studies (23 345 women). Diagnostic accuracy analysis was based on 27 studies; 22 of them evaluated the efficacy of serum uric acid in the detection of preeclampsia during the 3rd trimester, while the rest five assessed its predictive value during the 2nd trimester of pregnancy. The analysis of adverse perinatal outcome prediction (cesarean section, preterm birth, fetal growth restriction, low birthweight, 5‐minute Apgar score <7, fetal and neonatal death) comprised eight studies, including 925 preeclamptic women. A prospective design was followed by 46 studies, while the most common preeclampsia definition was the combination of hypertension and proteinuria (162 studies). Serum was used in 167 studies for uric acid measurement, while plasma concentrations were reported in 31 studies (Appendix S3, Table S1).
3.3. Data analysis
The outcomes regarding serum uric acid values are presented in Table 1. Preeclampsia was associated with significantly higher serum uric acid levels during the 1st (MD: 0.21 mg/dL, 95% CI: 0.06‐0.35) trimester, 2nd (MD: 1.41 mg/dL, 95% CI: 0.78‐2.05) trimester, and 3rd (MD: 2.26 mg/dL, 95% CI: 2.12‐2.40) trimester of pregnancy. Subgroup analysis revealed significantly elevated urate levels both in early preeclampsia (MD: 2.36 mg/dL, 95% CI: 1.42‐3.30) and in late‐onset preeclampsia (1.79 mg/dL, 95% CI: 0.66‐2.92). When the two forms of the disease were compared, early‐onset preeclampsia was linked to higher uric acid concentration (MD: 0.49 mg/dL, 95% CI: 0.26‐0.73).
TABLE 1.
Outcomes of serum uric acid values among women with normal pregnancy, preeclampsia, and its complications
| Subgroup | No. of studies | No. of patients | τ 2 | Mean difference | 95% confidence intervals | 95% prediction intervals | Quality of evidence |
|---|---|---|---|---|---|---|---|
| Trimester of pregnancy | |||||||
| 1st trimester | 10 | 18 620 | 0.04 | 0.21 | [0.06, 0.35]* | [−0.27, 0.69] |
⊕⊕◯◯ Low |
| 2nd trimester | 10 | 16 976 | 0.95 | 1.41 | [0.78, 2.05]* | [−0.91, 3.73] |
⊕⊕⊕◯ Moderate |
| 3rd trimester | 155 | 23 345 | 0.69 | 2.26 | [2.12, 2.40]* | [0.83, 3.91]* |
⊕⊕⊕◯ Moderate |
| PE severity | |||||||
| Mild PE vs. control | 29 | 3974 | 0.51 | 1.88 | [1.61, 2.16]* | [0.39, 3.37]* |
⊕⊕◯◯ Low |
| Severe PE vs. control | 36 | 4331 | 0.91 | 2.91 | [2.58, 3.24]* | [0.95, 4.88]* |
⊕⊕⊕◯ Moderate |
| Mild PE vs. severe PE | 33 | 4193 | 0.10 | −1.03 | [−1.17, −0.88]* | [−1.58, −0.48]* |
⊕⊕◯◯ Low |
| PE onset | |||||||
| Early‐onset PE vs. control | 11 | 1527 | 0.15 | 2.36 | [2.04, 2.69]* | [1.42, 3.30]* |
⊕⊕⊕◯ Moderate |
| Late‐onset PE vs. control | 10 | 2116 | 0.22 | 1.79 | [1.45, 2.12]* | [0.66, 2.92]* |
⊕⊕⊕◯ Moderate |
| Early‐onset PE vs. late‐onset PE | 17 | 2360 | 0.15 | 0.49 | [0.26, 0.73]* | [−0.37, 1.35] |
⊕⊕◯◯ Low |
| Eclampsia | |||||||
| Eclampsia vs. PE | 11 | 931 | 1.38 | 1.29 | [0.52, 2.07]* | [−1.47, 4.05] |
⊕⊕◯◯ Low |
| Eclampsia vs. control | 9 | 509 | 1.29 | 3.63 | [2.81, 4.45]* | [0.84, 6.42]* |
⊕⊕⊕◯ Moderate |
| HELLP syndrome | |||||||
| HELLP syndrome vs. PE | 4 | 811 | 0 | 0.67 | [0.33, 1.00]* | [0.13, 1.21]* |
⊕⊕⊕◯ Moderate |
Abbreviations: HELLP, hemolysis, elevated liver enzymes, low platelets; PE, preeclampsia.
P‐value <.05.
Concerning disease severity, both mild (MD: 1.88, 95% CI: 1.61‐2.16) trimester and severe (MD: 2.91 mg/dL, 95% CI: 2.58‐3.24) preeclampsia were associated with significantly higher uric acid levels compared with healthy pregnant controls, while women with the mild form of the disease presented significantly lower urate values (MD: −1.03 mg/dL, 95% CI: −1.17 to −0.88) compared to those with severe preeclampsia. Moreover, eclampsia was linked to higher serum uric concentration compared with both preeclamptic women (MD: 1.29 mg/dL, 95% CI: 0.52‐2.07) and healthy pregnant controls (MD: 3.63 mg/dL, 95% CI: 2.81‐4.45), while HELLP syndrome was also associated with elevated urate levels compared with uncomplicated preeclampsia (MD: 0.67 mg/dL, 95% CI: 0.33‐1.00 mg/dL) (Appendix S4, Figures S2‐S13).
Estimation of 95% prediction intervals indicated a significant outcome regarding serum uric levels during the 3rd trimester (95% PI: 0.83‐3.91), but not for measurements early in pregnancy. Statistical significance was also achieved when preeclamptic cases were subgrouped according to disease onset (95% PI: 1.42‐3.30 for early‐onset preeclampsia and 0.66‐2.92 for late‐onset preeclampsia) and severity (95% PI: 0.39‐3.397 for mild preeclampsia and 0.95‐4.88 for severe preeclampsia). In addition, eclampsia presented significant prediction intervals when compared to healthy (95% PI: 0.84‐6.42) but not to preeclamptic women (95% PI: −1.47 to 4.05). The comparison of women with HELLP syndrome and preeclampsia resulted also in significant prediction intervals (95% PI: 0.13‐1.21; Figure 1).
FIGURE 1.
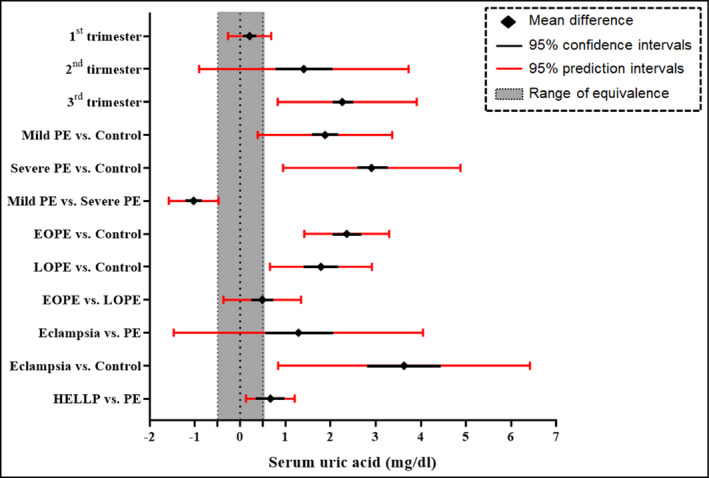
Forest plot summarizing the findings of the meta‐analysis. A serum uric acid difference of 0.5 mg/dL was considered as clinically important, defining thus a range of equivalence from −0.5 to +0.5 mg/dL. EOPE, early‐onset preeclampsia; HELLP, hemolysis, elevated liver enzymes, low platelets; LOPE, late‐onset preeclampsia; PE, preeclampsia
Meta‐regression analysis (Appendix S5, Table S2) revealed that the outcomes of serum uric acid measured during the 3rd trimester were not significantly influenced by year of publication, sample size, NOS score, study design, type of sample, or preeclampsia definition. On the other hand, slight, albeit statistically significant effects were noted for the covariates of preeclampsia definition (β = −0.287, standard error: 0.137, P‐value: .037) and year of publication (β = 0.018, standard error: 0.064, P‐value: .018) in the 1st‐trimester and 2nd‐trimester outcomes, respectively. Publication bias was suspected for the outcomes of 3rd‐trimester measurements, as well as for severity subgroups and eclampsia comparisons (Appendix S7, Table S3 & Figures S15‐S26).
The analysis of prospective cohort studies indicated that preeclampsia was associated with significantly higher serum uric acid levels during the 1st trimester (MD: 0.25 mg/dL, 95% CI: 0.15‐0.43), 2nd trimester (MD: 1.11 mg/dL, 95% CI: 0.15‐2.07), and 3rd trimester (MD: 2.13 mg/dL, 95% CI: 1.84‐2.42). Pooling of prospective studies also demonstrated significantly increased values in women with both mild (MD: 2.32 mg/dL, 95% CI: 1.39‐3.25) preeclampsia and severe (MD: 3.02 mg/dL, 95% CI: 2.18‐3.86) preeclampsia. Mild cases presented significantly lower uric acid concentration compared with severe ones (MD: −1.29 mg/dL, 95% CI: −1.80 to −0.78). Moreover, serum uric acid levels were estimated to be higher in both early‐onset (MD: 2.45 mg/dL, 95% CI: 1.98‐2.93) preeclampsia and late‐onset (MD: 1.55 mg/dL, 95% CI: 1.30‐1.81) preeclampsia compared with healthy pregnancy, while no difference was evident between these two forms of the disease (MD: 0.45 mg/dL, 95% CI: −0.28‐1.19). Prospective data regarding preeclampsia complications were limited, indicating that eclampsia is linked to significantly higher uric acid levels compared with healthy pregnancy (MD: 4.24 mg/dL, 95% CI: 2.26‐6.22) but not to preeclampsia (MD: 1.12 mg/dL, 95% CI: −1.27 to 3.51) (Appendix S6, Figure S14).
Diagnostic accuracy analysis (Appendix S9, Table S5, & Figures S27‐S31) demonstrated that evaluation of serum uric acid concentration during the 3rd trimester was able to detect preeclampsia with an estimated sensitivity of 76.7% (95% CI: 70.5‐81.9) and specificity of 79.6% (95% CI: 74.2‐84.1), while 2nd‐trimester measurements predicted preeclampsia with sensitivity of 78.6% (95% CI: 52.4‐92.4) and specificity of 61.6% (95% CI: 57.1‐65.8). Summary ROC curves are presented in Figure 2, indicating that evaluation of serum urate during the 3rd trimester resulted in higher accuracy than during the 2nd trimester (AUC: 84.8% vs. 65.5%). The outcomes of the analysis regarding adverse perinatal outcome prediction (Appendix S10, Table S6, & Figures S34‐S40) are summarized in Table 2. The mean sensitivity of the marker ranged from 67.3% to 82.7% and its specificity from 47.7% to 70.7%. Correspondingly, the estimated AUCs ranged from 69.9% for low birthweight prediction to 81.2% for the outcome of fetal death.
FIGURE 2.
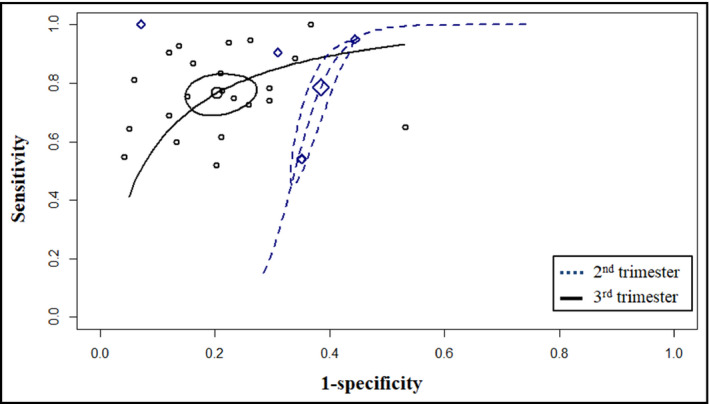
Summary receiver operating characteristic curves depicting the ability of serum uric acid to predict preeclampsia during the 2nd trimester and 3rd trimester of pregnancy
TABLE 2.
Outcomes of serum uric acid ability to predict adverse perinatal outcomes
| Pregnancy outcome | Patient no. | Sensitivity [95% CI] (%) | Specificity [95% CI] (%) | AUC (%) |
|---|---|---|---|---|
| Cesarean section | 388 | 71.9 [62.2, 79.9] | 63.9 [51.1, 75.1] | 73.8 |
| Fetal growth restriction | 599 | 76.5 [65.3, 85.0] | 62.7 [45.0, 77.5] | 77.0 |
| Low birthweight | 440 | 67.3 [59.5, 74.2] | 70.7 [41.9, 89.0] | 69.9 |
| Preterm birth | 545 | 68.6 [53.2, 80.8] | 67.9 [52.5, 80.2] | 73.4 |
| 5‐minute Apgar score <7 | 659 | 74.2 [66.4, 80.8] | 64.1 [49.2, 76.7] | 75.8 |
| Fetal death | 599 | 82.7 [67.7, 91.6] | 51.9 [42.8, 60.9] | 81.2 |
| Neonatal death | 480 | 81.7 [64.0, 91.8] | 47.7 [34.6, 61.2] | 71.3 |
Abbreviations: AUC, area under the curve; CI, confidence intervals.
3.4. Quality assessment
The outcomes of NOS score are exhibited in Table S2. More specifically, 8 (4.1%) studies were scored with 5 points, 62 (31.6%) studies with 6, 84 (42.9%) studies with 7, and 42 (21.4%) studies with 8 points, indicating an overall low‐to‐moderate risk of bias. Downgrading mainly was decided for studies with an inadequate description of patient recruitment process, as well as when confounding was suspected due to differentiation of the compared groups in terms of maternal or gestational age. The outcomes of QUADAS‐2 tool are presented in Appendices S9 and S10, indicating that the main sources of bias risk came from the domains of patient selection in case‐control studies and index test, as most studies used the optimal uric acid cutoff instead of a prespecified one. The results of quality of evidence assessment are provided in Appendix S8 (Table S4). The overall credibility of outcomes ranged from low to moderate (Table 1), with downgrading to be applied in the domains of consistency, precision, and publication bias.
4. DISCUSSION
The present meta‐analysis accumulated all current literature knowledge concerning the role of serum uric acid in preeclampsia and assessed its potential efficacy as a marker of severity and disease complications. The outcomes suggest that preeclampsia is associated with significantly increased serum uric acid concentration compared with normal pregnancy, a finding that was based on a large number of patients and applied for all disease phenotypes. Measurements during the first two gestational trimesters also demonstrated significantly higher values in women that later developed preeclampsia; however, the estimated prediction intervals did not reach statistical significance, and thus, the reproducibility of this result by future studies could not be ensured. The prognostic impact of serum uric was supported, since a significant elevation was noted in women with the severe form of the disease, as well as in those diagnosed with eclampsia and HELLP syndrome.
Diagnostic accuracy analysis indicated that third‐trimester evaluation of serum urate levels may serve as a useful tool to strengthen preeclampsia diagnosis, as a high sensitivity was achieved (76.7%). Its predictive efficacy during the 2nd trimester was also calculated to be promising, presenting high sensitivity (78.6%), although the limited available data along with the high variation of the reported cutoffs preclude its direct applicability in clinical practice for this purpose. The performance of uric acid levels for the prediction of adverse perinatal outcomes was proposed to be moderate (sensitivity: 67.3% to 82.7%) and was mainly based on the outcomes of small‐scale studies; nonetheless, it should be highlighted that conventional predefined threshold values were predominantly used, and thus, a more realistic estimate was obtained.
The value of uric acid has also been supported by studies incorporating its serum levels in combined models, indicating enhanced predictive efficacy when they were used in conjunction with clinical, biochemical, and ultrasound biomarkers. 32 , 33 From a pathophysiological point of view, it has been hypothesized that uric acid may not represent merely an innocent bystander, since it has been suggested to promote preeclampsia progression through a feed‐forward mechanism, by compromising spiral artery remodeling and stimulating the activation of oxidative and inflammatory cascades. 16 Nevertheless, limited data are available regarding the potential effects of treating hyperuricemia in women at risk of preeclampsia. More specifically, the use of allopurinol has been studied in a clinical trial which showed no benefit in terms of fetal and maternal complication reduction, although serum uric acid levels were not effectively reduced. 34 Similarly, another trial examining the effects of the uricosuric probenecid found no influence on preeclampsia progression, despite a significant improvement of platelet count. 35 As a result, future well‐designed trials are needed to evaluate whether serum uric acid reduction may prevent or ameliorate the clinical course of the disease.
4.1. Strengths and limitations of the study
The present meta‐analysis represents the largest one in the field, as it was based on a large cohort of studies, including 39 540 women. To achieve this, five independent literature databases were searched in a systematic manner, applying no date or language restrictions. Gray literature was also not excluded from our analysis in order to limit the effects of publication bias. 36 At the same time, the applied eligibility criteria were strict and studies available only as abstracts or conference proceedings were not included, as they lack essential information for the conduction of the planned analysis. No date restrictions were applied aiming to include all the available evidence in the field. Heterogeneity was expected not to be negligible, and thus, a multilevel approach was implemented to assess its impact. In particular, extensive subgroup and meta‐regression analyses were performed and the implementation of prediction intervals permits an estimation of the effects to be anticipated by future studies in the field. As a result, the effects of several confounders such as preeclampsia definition, study design, type of sample, and region were taken into account, obtaining stable outcomes. In addition, the estimated prediction intervals remained significant regarding serum uric acid during the 3rd trimester, suggesting that this observation is robust and should be expected by future populations. Similarly, prediction intervals indicated significant associations of uric acid levels with severe preeclampsia, eclampsia, and HELLP syndrome, supporting thus that the marker may serve as a useful marker of predicting disease complications. The robustness of the outcomes was strengthened as prospective cohort studies were separately pooled, obtaining similar outcomes. Moreover, the credibility of outcomes was evaluated under the GRADE framework, providing a pragmatic interpretation of the available evidence concerning the prognostic role of uric acid in preeclampsia.
On the other hand, the clinical usefulness of the presented results is limited by several factors. Concerning the use of serum uric acid levels as an index that would help determinate patients that would require early treatment with aspirin, the results of our analysis on studies involving women at the 1st trimester and 2nd trimester were associated with wide prediction intervals, hence raising the necessity of further evidence to ensure their validity. Significant differences were detected in the third trimester; however, the reliability of diagnostic accuracy analysis was challenged by the case‐control design in conjunction with the use of optimal thresholds, especially studies that involved women in the 3rd trimester of pregnancy. It should be also noted that evidence regarding the prediction of perinatal complications was limited, since the number of the available studies was small and the heterogeneity in outcome reporting was high.
4.2. Implications for current clinical practice and future research
The findings of this meta‐analysis corroborate the proposed link between hyperuricemia and preeclampsia, especially during the 3rd gestational trimester, while promising evidence is provided regarding the potential role of serum uric acid as a biomarker of disease complications. However, the heterogeneity of outcome reporting and use of optimal cutoffs hinder safe conclusions; thus, these outcomes need to be verified by large‐scale prospective cohort studies in order to define the optimal gestational age for sampling, as well as to determine the most appropriate cutoff value to be widely applied. Current evidence is of moderate grade, although the summary effect estimate is of clinical importance as the reported mean differences range between 2 and 3 mg/dL, which correspond approximately to 40% of the higher acceptable normal value (7.0 mg/dL); therefore, further research is of scientific significance in large‐scale studies. The present study may serve as a pilot which can guide future research as it provides substantial evidence that can be used in the design of future studies, particularly when it comes to the assessment of the predictive accuracy of the various thresholds which have been proposed to date. This way it will be possible to minimize the risk of bias and provide results that will be comparable, thus permitting generalization of findings. Moreover, serum uric acid should be evaluated in multivariate models in conjunction with other novel biomarkers, so as to construct algorithms of optimal predictive efficacy. In this line, the implementation of artificial intelligence could be of significant usefulness as novel statistical analyses, such as decision trees and neural networks, seem to provide a more sensitive approach that increases the accuracy of multivariate models. Finally, in the therapeutic setting, although evidence concerning the benefit of drugs against uric acid seems to be sparse to help reach firm conclusions, there is a significant amount of space to permit reasoning. This is why well‐designed clinical trials are needed to elucidate whether anti‐gout drugs such as allopurinol could be used in the third trimester of pregnancy to optimize maternal and neonatal outcomes among cases with preeclampsia.
5. CONCLUSIONS
The present meta‐analysis suggests that serum uric acid is significantly elevated in pregnancies complicated by preeclampsia, presenting moderate prognostic value in detecting disease severity and the occurrence of complications. The credibility of evidence was assessed to range from low to moderate, as several gaps were detected in the methodology of the existing literature in the field. Given this information, it is suggested that future well‐designed prospective studies are needed in order to shed light on populations that seem to exhibit the most prominent differences (patients with severe, early‐onset preeclampsia, and those that are prone to develop eclampsia). Moreover, the implementation of serum uric acid in predictive models in combination with established biomarkers could help determine its potential additive value in the prediction of the disease as well as the severity of accompanying complications.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
I. Bellos and V. Pergialiotis designed and conceived the study, drafted the article, and provided statistical expertise. I. Bellos, V. Pergialiotis, and G. Daskalakis analyzed and interpreted the data and collected and assembled the data. G. Daskalakis and D. Loutradis critically revised the article for important intellectual content. I. Bellos, V. Pergialiotis, G. Daskalakis, and D. Loutradis approved the final version of the article to be published.
ETHICAL APPROVAL
No ethical approval was required. The present systematic review and meta‐analysis are based on aggregated data that were retrieved from studies already retrieved. We did not collect individual patient data and did not have direct contact with patients included.
Supporting information
Appendix S1‐S11
ACKNOWLEDGMENTS
None.
Bellos I, Pergialiotis V, Loutradis D, Daskalakis G. The prognostic role of serum uric acid levels in preeclampsia: A meta‐analysis. J Clin Hypertens. 2020;22:826–834. 10.1111/jch.13865
DATA AVAILABILITY STATEMENT
Data are available in the appendix. Additional details are available from the corresponding author upon request.
REFERENCES
- 1. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre‐eclampsia. Lancet. 2016;387(10022):999‐1011. [DOI] [PubMed] [Google Scholar]
- 2. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1‐7. [DOI] [PubMed] [Google Scholar]
- 3. Redman CW. Early and late onset preeclampsia: two sides of the same coin. Pregnancy Hypertens. 2017;7:58. [Google Scholar]
- 4. Burton GJ, Redman CW, Roberts JM, Moffett A. Pre‐eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [DOI] [PubMed] [Google Scholar]
- 5. Veisani Y, Jenabi E, Delpisheh A, Khazaei S. Angiogenic factors and the risk of preeclampsia: a systematic review and meta‐analysis. Int J Reprod Biomed. 2019;17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellos I, Papantoniou N, Pergialiotis V. Serum ceruloplasmin levels in preeclampsia: a meta‐analysis. J Matern Neonatal Med. 2018;31(17):2342‐2348. [DOI] [PubMed] [Google Scholar]
- 7. Bellos I, Karageorgiou V, Kapnias D, Karamanli K‐E, Siristatidis C. The role of interleukins in preeclampsia: a comprehensive review. Am J Reprod Immunol. 2018;80(6):e13055. [DOI] [PubMed] [Google Scholar]
- 8. Tomimatsu T, Mimura K, Endo M, Kumasawa K, Kimura T. Pathophysiology of preeclampsia: an angiogenic imbalance and long‐lasting systemic vascular dysfunction. Hypertens Res. 2017;40(4):305‐310. [DOI] [PubMed] [Google Scholar]
- 9. Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613‐622. [DOI] [PubMed] [Google Scholar]
- 10. Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13‐22. [DOI] [PubMed] [Google Scholar]
- 11. De Kat AC, Hirst J, Woodward M, Kennedy S, Peters SA. Prediction models for preeclampsia: a systematic review. Pregnancy Hypertens. 2019;16:48‐66. [DOI] [PubMed] [Google Scholar]
- 12. Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174(1):288‐291. [DOI] [PubMed] [Google Scholar]
- 13. Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20(3):209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khaliq OP, Konoshita T, Moodley J, Naicker T. The role of uric acid in preeclampsia: is uric acid a causative factor or a sign of preeclampsia? Curr Hypertens Rep. 2018;20(9):80. [DOI] [PubMed] [Google Scholar]
- 15. Martin AC, Brown MA. Could uric acid have a pathogenic role in pre‐eclampsia? Nat Rev Nephrol. 2010;6(12):744‐748. [DOI] [PubMed] [Google Scholar]
- 16. Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29:67‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Braga TT, Forni MF, Correa‐Costa M, et al. Soluble uric acid activates the NLRP3 inflammasome. Sci Rep. 2017;7(1):39884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cnossen JS, Ter RG, Mol BW, et al. Are tests for predicting pre‐eclampsia good enough to make screening viable? A review of reviews and critical appraisal. Acta Obstet Gynecol Scand. 2009;88(7):758‐765. [DOI] [PubMed] [Google Scholar]
- 19. Koopmans CM, van Pampus MG, Groen H, Aarnoudse JG, van den Berg PP, Mol BWJ. Accuracy of serum uric acid as a predictive test for maternal complications in pre‐eclampsia: bivariate meta‐analysis and decision analysis. Eur J Obstet Gynecol Reprod Biol. 2009;146(1):8‐14. [DOI] [PubMed] [Google Scholar]
- 20. Taravati A, Tohidi F. Comprehensive analysis of oxidative stress markers and antioxidants status in preeclampsia. Taiwan J Obstet Gynecol. 2018;57(6):779‐790. [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1‐e34. [DOI] [PubMed] [Google Scholar]
- 22. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta‐analyst: software for meta‐analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Viechtbauer W. Conducting meta‐analyses in R with the metafor package. J Stat Softw. 2010;36(3):1‐48. [Google Scholar]
- 24. IntHout J, Ioannidis JPA, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta‐analysis. BMJ Open. 2016;6(7):e010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 27. Doebler P, Münster W, Holling H.Meta‐analysis of diagnostic accuracy with mada. https://cran.r‐project.org/web/packages/mada/vignettes/mada.pdf. Accessed March 19, 2018.
- 28. Verde PE. bamdit : an R package for Bayesian meta‐analysis of diagnostic test data. J Stat Softw. 2018;86(10):1‐32. [Google Scholar]
- 29. Wells G, Shea B, O'Connell J, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analysis. 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 05, 2019
- 30. Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529‐536. [DOI] [PubMed] [Google Scholar]
- 31. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. [DOI] [PubMed] [Google Scholar]
- 32. Zhou J, Zhao X, Wang Z, Hu Y. Combination of lipids and uric acid in mid‐second trimester can be used to predict adverse pregnancy outcomes. J Matern Neonatal Med. 2012;25(12):2633‐2638. [DOI] [PubMed] [Google Scholar]
- 33. Massé J, Forest J‐C, Moutquin J‐M, Marcoux S, Brideau N‐A, Bélanger M. A prospective study of several potential biologic markers for early prediction of the development of preeclampsia. Am J Obstet Gynecol. 1993;169(3):501‐508. [DOI] [PubMed] [Google Scholar]
- 34. Gulmezoglu AM, Hofmeyr GJ, Oosthuisen MMJ. Antioxidants in the treatment of severe pre‐eclampsis an explanatory randomised controlled trial. BJOG An Int J Obstet Gynaecol. 1997;104(6):689‐696. [DOI] [PubMed] [Google Scholar]
- 35. Schackis R. Hyperuricaemia and preeclampsia: is there a pathogenic link? Med Hypotheses. 2004;63(2):239‐244. [DOI] [PubMed] [Google Scholar]
- 36. Paez A. Grey literature: an important resource in systematic reviews. J Evid Based Med. 2017;10:233‐240. 10.1111/jebm.12265 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1‐S11
Data Availability Statement
Data are available in the appendix. Additional details are available from the corresponding author upon request.


